Abstract
Objective
To determine the association of preprocedural C reactive protein (CRP) levels with angiographic restenosis and adverse clinical events after drug‐eluting stent (DES) implantation.
Design
A prospective cohort analysis of preprocedural CRP levels as a predictor of serious ischaemic complications or binary restenosis in patients treated with DES.
Setting
Tertiary referral centre.
Patients
1650 consecutive patients who underwent successful DES implantation. Patients were grouped into tertiles according to preprocedural CRP values for data analysis.
Interventions
Successful DES implantation.
Main outcome measures
The primary end point was a major coronary event, defined as cardiac death or Q‐wave myocardial infarction.
Results
Baseline clinical and angiographic characteristics were similar between the tertile groups, except that more patients had multivessel disease and acute coronary syndrome with increasing tertiles of CRP levels. At 1‐year follow‐up, a primary end point occurred in 4 (0.7%) patients of the lowest tertile, in 3 (0.5%) patients of the middle tertile and in 16 (2.9%) patients of the highest tertile (p = 0.003). In multivariate analysis, the highest tertile of CRP levels was an independent predictor of a major coronary event (HR 4.68, 95% CI 1.91 to 11.44, tertile III vs tertiles I and II, p = 0.001). However, restenosis rates were similar in all three groups (9.1% vs 11.4% vs 11.6%, respectively, p = 0.3).
Conclusions
Preprocedural CRP levels are significantly associated with major coronary events after DES implantation. However, preprocedural CRP levels do not predict subsequent restenosis. Baseline CRP levels may be useful to guide adjunctive management for preventing serious ischaemic events in patients undergoing DES implantation.
C reactive protein (CRP) is recognised as an important marker of vascular wall inflammation and as a strong predictor of adverse cardiovascular events.1,2 In addition, serum CRP levels have been associated with both subsequent ischaemic events and restenosis in patients undergoing balloon angioplasty or bare metal stent (BMS) implantation.3,4 These observations suggest the important role of systemic inflammatory activity in vessel wall responses to injury elicited by coronary intervention.3,4,5
Drug‐eluting stents (DESs) markedly reduced restenosis and the need for repeat coronary revascularisation.6,7,8 Considering the fact that accurate risk stratification using only clinical and lesion characteristics for patients undergoing DES implantation is limited, it would be very helpful to establish systemic biomarkers for prediction of the adverse coronary events and restenosis. We, therefore, prospectively evaluated the predictive value of preprocedural CRP levels for clinical and angiographic outcomes in patients undergoing DES implantation.
Methods
Study population
The study enrolled 1650 consecutive patients who underwent successful DES implantation between March 2003 and November 2004. Sirolimus‐eluting stents (Cypher stent, Cordis/Johnson and Johnson, Miami, Florida, USA) and paclitaxel‐eluting stents (TAXUS stent, Boston Scientific Corporation, Boston, Massachusetts, USA) were used in 1332 patients (1804 lesions) and 318 patients (497 lesions), respectively. Exclusion criteria were cardiogenic shock, concurrent inflammatory conditions such as infection, inflammatory arthritis and connective tissue disease, malignancies, and recent (<2 months) surgery or major trauma. Written informed consent was obtained from all patients, and the study was approved by our clinical study institutional review board.
Stenting procedure
Stent implantation was performed as described previously.8,9 During the procedure, patients received heparin to maintain the activated clotting time at ⩾250 s. Glycoprotein IIb/IIa inhibitors were used at the physician's discretion. All patients were pretreated with aspirin and clopidogrel (75 mg/day at least 4 days before stenting, or 300 mg loading dose given at least 6 h before the procedure). A loading dose of clopidogrel (300 mg) was given to patients not previously taking the drug. Clopidogrel (75 mg/day) was prescribed for at least 6 months regardless of DES type, and aspirin (200 mg/day) administered indefinitely.
Laboratory analysis
In all patients, serum samples were collected immediately before the procedure. CRP was measured using a latex‐enhanced high‐sensitivity CRP immunoassay (COBAS INTEGRA; Roche Diagnostics GmbH, Roche, Germany).
Quantitative coronary angiography analysis
Using the guiding catheter for magnification calibration and an on‐line quantitative angiographic analysis system (Xcelera Cath 1.1, Philips, The Netherlands), minimal luminal diameter (MLD), percent diameter stenosis and reference vessel diameter were measured before and after stenting, and at follow‐up, from the single, matched view showing the smallest lumen diameter. Angiographic restenosis was defined as a diameter stenosis of ⩾50% occurring in the segment inside the stent, or in the 5 mm segment proximal or distal to the stent at follow‐up angiography. Target‐vessel revascularisation was defined as repeat percutaneous or surgical intervention of the stented vessel. Acute gain was calculated as the difference between the MLD before and after the procedure. Late loss was defined as the difference between the MLD after the procedure and at follow‐up.
Follow‐up and definition of events
Clinical follow‐up data were obtained from outpatient record reviews or telephone interviews. All patients undergoing DES implantation at our institution (Asan Medical Center, Seoul, Korea) were requested to have a 6‐month follow‐up angiogram.
Death was classified as cardiac or non‐cardiac. Myocardial infarction (MI) was classified as (1) Q‐wave MI—the presence of new pathological Q waves in at least two contiguous leads associated with an increase in cardiac enzyme ⩾2 times the upper normal value—and (2) non‐Q‐wave MI—an increase in the myocardial band fraction of creatinine kinase ⩾3 times the upper normal value without new Q waves. An acute coronary syndrome was defined as either unstable angina or acute MI.
The prespecified primary end point was the occurrence of a major coronary event, defined as cardiac death or Q‐wave MI at 1‐year follow‐up. The end point of cardiac death or Q‐wave MI was assessed as time to first event, without double counting of clinical events within the same patients. The secondary end points were the rates of angiographic restenosis and any coronary event (any major coronary event and target‐vessel revascularisation).
Statistical analysis
Statistical analyses were performed with SPSS (v 12.0). A total sample size of 1700 observations was estimated to achieve 80% power at a two‐sided 0.05 significance level to detect a hazard ratio (HR) ⩾3.0 with a Cox regression of the log HR on a binary risk factor with ⩾25% prevalence. The sample size was adjusted for an expected event rate of 2.0%. CRP values, which showed a non‐Gaussian distribution by the Kolmogorov–Smirnov test, were divided into tertiles for data analysis. Categorical data are presented as frequencies and compared using χ2 analysis for trend or Fisher's exact test, as appropriate. Continuous variables are presented as mean (SD) and compared using one‐way parametric or non‐parametric (Kruskal–Wallis test) analysis of variance. Event‐free survival was analysed using the Kaplan–Meier method, and the corresponding p value was obtained from log‐rank test.
Multivariate logistic regression analysis using the forward stepwise selection process was undertaken to identify independent predictors of angiographic restenosis. Linear regression analysis with logarithmic transformation was also used to correlate preprocedural CRP levels and angiographic parameters of restenosis. Multivariate Cox's proportional‐hazards regression with the forward stepwise selection process was used to determine independent predictors of adverse coronary events. All significant parameters with p<0.05 at the univariate analyses and the clinical, procedural and angiographic variables with significant differences across the CRP tertiles were included in the multivariate analyses. Because of the small absolute number of clinical events, we performed an internal validation process to assess the goodness of fit of the final model and quantify overfitting.10 Additionally, a propensity analysis was carried out to determine the causal effect of CRP on clinical events.11 The balancing score for CRP levels (as a continuous variable with logarithmic transformation) was determined using multivariate logistic regression analysis.
A subgroup analysis of patients without acute MI at presentation was also performed to exclude the possible confounding effect of myocardial necrosis on preprocedural CRP levels. Two‐sided p values of <0.05 were regarded as statistically significant.
Results
Baseline characteristics
Patients were grouped into tertiles according to preprocedural CRP levels: tertile I (550 patients, CRP<1.2 mg/l), tertile II (550 patients, 1.2⩽CRP ⩽3.1 mg/l) and tertile III (550 patients, CRP>3.1 mg/l). Table 1 shows the baseline clinical and procedural characteristics.
Table 1 Baseline clinical, lesion and procedural characteristics.
| Tertile I (n = 550) | Tertile II (n = 550) | Tertile III (n = 550) | p Value | |
|---|---|---|---|---|
| Mean (SD) age (years) | 59 (10) | 61 (10) | 61 (11) | 0.04 |
| Male | 386 (70%) | 384 (70%) | 406 (74%) | 0.2 |
| Cardiac risk factors | ||||
| Hypertension | 280 (51%) | 275 (50%) | 314 (57%) | 0.08 |
| Diabetes mellitus | 155 (28%) | 148 (27%) | 172 (31%) | 0.3 |
| Hypercholesterolaemia | 108 (20%) | 154 (28%) | 129 (24%) | 0.1 |
| Cigarette smoking | 142 (26%) | 193 (35%) | 187 (34%) | 0.007 |
| Clinical presentation | <0.001 | |||
| Stable angina | 344 (63%) | 294 (54%) | 239 (44%) | |
| Unstable angina | 176 (32%) | 204 (37%) | 234 (43%) | |
| Acute MI | 30 (6%) | 52 (10%) | 77 (14%) | |
| Non‐ST segment elevation | 6 (1%) | 17 (3%) | 50 (9%) | |
| ST segment elevation | 24 (4%) | 35 (6%) | 27 (5%) | |
| Previous myocardial infarction | 30 (6%) | 45 (8%) | 57 (10%) | 0.003 |
| Previous bypass surgery | 15 (3%) | 13 (2%) | 8 (2%) | 0.2 |
| Multivessel disease | 292 (53%) | 314 (57%) | 352 (64%) | 0.04 |
| Renal insufficiency (creatinine ⩾2.0 mg/dl) | 14 (3%) | 12 (2%) | 19 (4%) | 0.4 |
| Peripheral vascular disease | 11 (2%) | 10 (2%) | 10 (2%) | 0.9 |
| Mean (SD) left ventricular ejection fraction (%) | 60 (8) | 59 (9) | 58 (9) | 0.06 |
| Coronary artery dilated | 0.3 | |||
| Left anterior descending | 334 (61%) | 293 (53%) | 303 (55%) | |
| Left circumflex | 50 (9%) | 68 (12%) | 74 (14%) | |
| Right | 113 (21%) | 130 (24%) | 123 (22%) | |
| Left main | 52 (10%) | 56 (10%) | 47 (9%) | |
| Grafted vessel | 1 (0.2%) | 3 (1%) | 3 (1%) | |
| Type B2 or C* | 451 (82%) | 442 (80%) | 452 (82%) | 0.8 |
| Ostial location | 72 (13%) | 55 (10%) | 55 (10%) | 0.2 |
| Bifurcation | 112 (20%) | 137 (25%) | 112 (20%) | 1.0 |
| In‐stent restenosis | 71 (13%) | 57 (10%) | 56 (10%) | 0.2 |
| Stent types | 0.2 | |||
| Sirolimus‐eluting stent | 453 (82%) | 444 (81%) | 435 (79%) | |
| Paclitaxel‐eluting stent | 97 (18%) | 106 (19%) | 115 (21%) | |
| Direct stenting | 102 (19%) | 81 (15%) | 76 (14%) | 0.031 |
| Mean (SD) maximal pressure (atm) | 16.2 (3.7) | 16.2 (3.8) | 16.0 (4.0) | 0.6 |
| Mean (SD) balloon to artery ratio | 1.3 (0.2) | 1.3 (0.2) | 1.2 (0.2) | 0.7 |
| Mean (SD) stents per lesion (n) | 1.5 (0.6) | 1.5 (0.7) | 1.5 (0.8) | 0.4 |
| Total stent length (mm) | 35.6 (18.7) | 34.8 (19.1) | 36.2 (20.3) | 0.5 |
MI, myocardial infarction.
All values are n (%) unless otherwise mentioned.
*Based on the modified American College of Cardiology/American Heart Association criteria.
With increasing tertiles of CRP levels, more patients had multivessel involvement and acute coronary syndrome on presentation. In addition, previous MI events were significantly frequent with increasing tertiles of CRP levels. The baseline procedural characteristics were similar among the three groups, except that direct stenting was more frequently performed in patients of the lowest tertile. The types of DES used were also similar among the groups.
Angiographic outcomes
Table 2 shows the angiographic measures used in the study.
Table 2 Angiographic measures used in the study.
| Tertile I (n = 550) | Tertile II (n = 550) | Tertile III (n = 550) | p Value | |
|---|---|---|---|---|
| Lesion length (mm) | 27.6 (15.0) | 27.2 (14.8) | 28.3 (16.2) | 0.5 |
| Reference diameter (mm) | 2.9 (0.5) | 2.9 (0.5) | 3.0 (1.0) | 0.3 |
| Minimal lumen diameter (mm) | ||||
| Pre‐intervention | 0.9 (0.6) | 0.9 (0.6) | 0.9 (0.6) | 0.8 |
| Post‐intervention | 2.9 (0.5) | 2.9 (0.5) | 2.8 (0.5) | 0.6 |
| Follow‐up | 2.5 (0.7) | 2.4 (0.8) | 2.4 (0.8) | 0.7 |
| Diameter stenosis (%) | ||||
| Pre‐intervention | 68.0 (17.5) | 68.6 (19.3) | 69.3 (17.4) | 0.5 |
| Post‐intervention | 0 (13.4) | 1.5 (14.6) | 2.0 (13.3) | 0.05 |
| Follow‐up | 11.4 (23.9) | 13.5 (26.5) | 11.7 (28.2) | 0.6 |
| Acute gain (mm) | 2.0 (0.6) | 1.9 (0.6) | 1.9 (0.6) | 0.8 |
| Late loss (mm) | 0.4 (0.6) | 0.5 (0.7) | 0.4 (0.70 | 0.6 |
| Restenosis, n (%) | 9.1% | 11.4% | 11.6% | 0.3 |
All values are mean (SD) unless otherwise mentioned.
Baseline and post‐stenting angiographic findings were similar in all three groups. Follow‐up angiography was performed in 1172 (71.0%) patients, at a mean of 6.4 (1.4) months (73.8% in tertile I, 70.4% in tertile II and 68.9% in tertile III, p = 0.2). There was no significant difference in the preprocedural CRP levels between patients with and those without angiographic follow‐up.
At 6‐month angiographic follow‐up, restenosis was found in 9.1% (37/406) of patients in tertile I, 11.4% (44/387) in tertile II and 11.6% (44/379) in tertile III, which did not show statistical difference (p = 0.3). Similarly, late‐lumen loss did not differ between the three groups (0.4 (0.6) in tertile I, 0.5 (0.7) in tertile II and 0.4 (0.7) in tertile III, p = 0.6). Further analysis was performed considering restenosis as a continuous variable; the correlation between preprocedural CRP levels and percent diameter stenosis, MLD and late loss at follow‐up was not statistically significant (r = 0.016, 0.022 and 0.028, respectively).
Clinical outcomes
All patients were assessed at in‐hospital stay and at 1‐year clinical follow‐up. Table 3 lists the adverse coronary events during follow‐up.
Table 3 Incidence of coronary events during in‐hospital and at 1‐year follow‐up.
| Tertile I (n = 550) | Tertile II (n = 550) | Tertile III (n = 550) | p Value | |
|---|---|---|---|---|
| In‐hospital events | ||||
| Death | 1 (0.2%) | 0 (0%) | 6 (1.1%) | 0.033 |
| Cardiac | 1 (0.2%) | 0 (0%) | 5 (0.9%) | 0.076 |
| Non‐cardiac | 0 (0%) | 0 (0%) | 1 (0.2%) | 0.7 |
| MI | ||||
| Non‐Q‐wave | 38 (6.9%) | 58 (10.5%) | 56 (10.2%) | 0.061 |
| Q‐wave | 1 (0.2%) | 0 (0%) | 2 (0.4%) | 0.7 |
| Urgent revascularisation | 1 (0.2%) | 0 (0%) | 4 (0.7%) | 0.2 |
| Cumulative events at 1‐year | ||||
| Death | 1 (0.2%) | 1 (0.2%) | 16 (2.9%) | <0.001 |
| Cardiac | 1 (0.2%) | 1 (0.2%) | 12 (2.2%) | <0.001 |
| Non‐cardiac | 0 (0%) | 0 (0%) | 4 (0.7%) | 0.025 |
| MI | ||||
| Non‐Q‐wave | 40 (7.3%) | 59 (10.7%) | 60 (10.9%) | 0.041 |
| Q‐wave | 3 (0.5%) | 2 (0.4%) | 4 (0.7%) | 0.7 |
| Target vessel revascularisation | 19 (3.5%) | 35 (6.4%) | 31 (5.6%) | 0.1 |
| Primary end point* | 4 (0.7%) | 3 (0.5%) | 16 (2.9%) | 0.003 |
| Any coronary event† | 22 (4.0%) | 36 (6.5%) | 44 (8.0%) | 0.006 |
MI, myocardial infarction.
*The primary end point was a major coronary event, defined as cardiac death or Q‐wave myocardial infarction.
†Major coronary events and target vessel revascularisation.
In‐hospital deaths occurred more significantly in patients with the highest tertile of CRP levels (p = 0.033). There was a trend towards more frequent periprocedural non‐Q‐wave MI in patients with the two upper tertiles (p = 0.061).
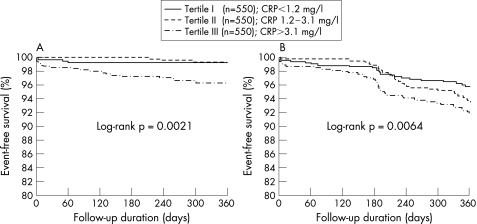
The primary end point at 1 year occurred in 4 (0.7%) patients of the lowest tertile, in 3 (0.5%) patients of the middle tertile and in 16 (2.9%) patients of the highest CRP tertile (p = 0.003). Kaplan–Meier survival curve showed that event‐free survival of major coronary events was significantly worse in patients with the highest tertile of CRP levels (fig 1). Coronary events occurred in 22 (4.0%) patients of the lowest tertile, in 36 (6.5%) patients of the middle tertile and in 44 (8.0%) patients of the highest CRP tertile, with significant differences across the three groups (p = 0.006; fig 1).
Figure 1 Cumulative survival curves for major coronary event (cardiac death or Q‐wave myocardial infarction) (A), and any coronary events (cardiac death, Q‐wave myocardial infarction and target vessel revascularisation) (B), according to tertiles of preprocedural CRP levels.
In the subgroup without acute MI at presentation, the incidence of primary end point was significantly higher in patients of the highest CRP tertile compared with those in the two lower tertiles (2.3% in tertile III vs 0.5% in tertiles I and II, p = 0.001). At least one of the coronary events occurred in 3.7% (19/520) patients in tertile I, 5.6% (28/498) in tertile II and 7.0% (33/473) in tertile III (p = 0.026).
Predictors of restenosis and coronary events
Table 4 lists the independent predictors of restenosis and adverse coronary events.
Table 4 Multivariate predictors of restenosis and coronary events.
| Variables | Risk (95% CI) | p Value |
|---|---|---|
| Restenosis | Odds ratio | |
| Paclitaxel‐eluting stent | 4.70 (2.84 to 7.78) | <0.001 |
| Postintervention MLD (mm) | 0.17 (0.09 to 0.31) | <0.001 |
| Stents per lesion (n) | 1.53 (1.08 to 2.16) | 0.016 |
| Major coronary event | Hazard ratio | |
| CRP tertiles | 0.018 | |
| Tertile II vs tertile I | 2.66 (0.28 to 25.65) | 0.4 |
| Tertile III vs tertile I | 9.94 (1.28 to 77.14) | 0.028 |
| Tertile III vs tertile I + II | 4.68 (1.91 to 11.44) | 0.001 |
| Acute MI | 3.52 (1.23 to 10.07) | 0.019 |
| Any coronary event | Hazard ratio | |
| CRP tertiles | 0.046 | |
| Tertile II vs tertile I | 1.83 (1.04 to 3.21) | 0.037 |
| Tertile III vs tertile I | 1.96 (1.13 to 3.43) | 0.017 |
| Tertile III vs tertile I + II | 1.56 (1.04 to 2.35) | 0.031 |
| Paclitaxel‐eluting stent | 3.28 (2.13 to 5.05) | <0.001 |
| Stents per lesion (n) | 1.38 (1.07 to 1.79) | 0.013 |
CRP, C reactive protein; MI, myocardial infarction; MLD, minimal luminal diameter.
The major determinants of angiographic restenosis were paclitaxel‐eluting stent, postintervention MLD and the number of stents implanted. Preprocedural CRP levels did not predict subsequent restenosis (p = 0.34). Stepwise multivariate analysis was performed to determine independent predictors of the primary end point. The following variables were tested (all with p<0.05 in univariate analysis, and the clinical and procedural variables with significant differences across the CRP tertiles): tertiles of CRP levels, age, cigarette smoking, unstable angina, acute MI, previous MI, multivessel disease, left ventricular ejection fraction, direct stenting and total stent length. In the multivariate analysis, the highest tertile of CRP levels was an independent predictor of the primary end point (HR 4.68, 95% CI 1.91 to 11.44, tertile III vs tertiles I and II; p = 0.001), as well as acute MI (HR 3.52, 95% CI 1.23 to 10.07; p = 0.019). Propensity analysis was performed to discriminate whether CRP is a risk factor or just a marker of adverse outcomes. The highest tertile of CRP levels was a significant risk factor of the primary end point (HR 4.57, 95% CI 1.21 to 17.24, tertile III vs tertiles I and II; p = 0.025) after the balancing score was introduced into the model. Also, tertiles of CRP levels (p = 0.046) were associated with a higher risk of any coronary event, in addition to paclitaxel‐eluting stent (p<0.001) and the number of stents implanted (p = 0.013).
When analysis was confined to patients without acute MI on presentation, tertiles of CRP levels were the only independent predictors of the primary end point (HR 4.82, 95% CI 1.67 to 13.96, tertile III vs tertiles I and II; p = 0.004). Tertiles of CRP levels (p = 0.034), paclitaxel‐eluting stent (p<0.001) and postintervention MLD (p = 0.022) were independent risk factors for any coronary event in this subgroup, similar to those for all patients. In the subgroup without acute coronary syndromes, tertiles of CRP levels were also the independent predictors of the primary end point after risk adjustment (HR 3.95, 95% CI 1.39 to 18.66, tertile III vs tertiles I and II, p = 0.011).
Discussion
Inflammatory risk stratification beyond classical risk assessment using clinical and lesion characteristics has been emphasised in patients undergoing coronary intervention.12,13 Several studies highlight the predictive value of CRP for recurrent ischaemic events among patients with coronary artery disease who did or did not undergo coronary intervention.1,2,3,4,14 However, data on the association between preprocedural CRP levels and recurrent coronary events or restenosis after DES implantation are lacking. Therefore, this study provides an insight into the role of preprocedural CRP levels in inflammatory risk assessment to identify high‐risk patients undergoing DES implantation.
It was reported that systemic inflammatory activity as measured by serum CRP may play an important role in the pathogenesis of neointimal hyperplasia after coronary stenting.15,16 Walter et al14 reported that increased preprocedural CRP levels were significantly associated with restenosis after BMS implantation. In contrast, other studies report no association between restenosis and increased CRP levels.4,17,18 Explanations for such discrepancies may include differences between the studies in terms of clinical and lesion subsets, rates of angiographic follow‐up and pharmacological treatments. There are only a few data on the relationship between preprocedural CRP levels and restenosis after DES implantation.19,20 Recently, Dibra et al19 showed that baseline CRP levels were not associated with increased restenosis after sirolimus‐eluting stent implantation. However, because high‐risk complex lesions (left main and restenotic lesions) were excluded and a relatively small number of patients were investigated, the impact of preprocedural CRP on restenosis after DES implantation in real practice could not be determined. The present study showed that preprocedural CRP was not associated with increased restenosis after DES implantation, in contrast with previous BMS studies showing a positive association between CRP and restenosis. A reason for the conflicting conclusions may be that the potent antiproliferative effects of DES alleviate the influence of local or systemic inflammatory activity on neointimal proliferation. The recent study indicates that the decreased restenosis after DES implantation is not related to the anti‐inflammatory properties of DES but rather possibly to decreased coronary sensitivity to inflammatory mediators.21 Also, it remains elusive that systemic biomarkers do not influence enhanced local susceptibility to restenosis, and that the reduction in restenosis after DES is not mediated by attenuation of systemic markers such as CRP. As with the use of DES, the process responsible for neointimal hyperplasia is rather delayed, and delayed occurrence of in‐stent restenosis was reported in patients treated with DES.22,23 Therefore, we cannot exclude the possibility that the conventional 6‐month angiographic follow‐up interval was too short to exclude an effect of CRP on late loss and in‐stent restenosis.
Our study demonstrated that preprocedural CRP was the independent predictor for future coronary events after DES implantation. Recently, Palmerini et al24 reported that the highest tertiles of preprocedural CRP levels, leucocyte count and acute coronary syndrome were associated with an increased risk of death and death/MI after left main stenting, in which DES was used in half of the overall number of patients. Consistent with this report, the current study found that the highest tertiles of CRP levels and acute MI were independent predictors of major coronary events after DES implantation. Therefore, our study may extend previous findings24 on patients with left main disease treated with DES to the unselected large population.
Additionally, in a subgroup analysis of patients without acute MI to exclude the possible confounding effect of myocardial necrosis on preprocedural CRP levels, the highest tertile of CRP levels was found to be the only independent predictor, and the hazard for a major coronary event in this subgroup was similar to that for all patients. This may exclude the confounding bias that patients with acute MI are more frequently distributed in higher tertiles of CRP levels.
Limitations
This is a prospective analysis from a single centre. Our study consisted of various clinical (stable angina vs acute coronary syndromes) or lesion (de novo vs in‐stent restenosis) subsets. However, a uniform relationship between CRP and clinical events in a subgroup with or without acute coronary syndromes or in‐stent restenosis may suggest that the strong correlation between preprocedural CRP levels and adverse events after DES implantation may be applicable in various clinical and lesion situations. Sirolimus‐eluting stents were used in most lesions, and the relationship between preprocedural CRP levels and DES type was not evaluated. Due to the limited angiographic follow‐up (71%), we cannot exclude a potential selection bias related to incomplete angiographic follow‐up. However, such a bias is unlikely to be clinically relevant, because the relationship between CRP levels and clinical events was similar for patients with and without angiographic follow‐up. The ratio between postprocedural and pre‐procedural concentrations of CRP expressing individual susceptibility to inflammatory noxae could not be evaluated, because postprocedural data were not available. Finally, quantitative coronary angiography analysis was not performed in a blinded independent core laboratory.
Conclusions
The current study demonstrates that systemic inflammation as determined using preprocedural CRP levels is an independent predictor of serious ischaemic events after DES implantation, but does not predict restenosis risk. Therefore, preprocedural CRP levels may be useful for inflammatory risk stratification beyond classical risk assessment prior to DES implantation, and may be helpful in guiding further management strategies for preventing serious ischaemic events after DES implantation.
Abbreviations
BMS - bare metal stent
CRP - C reactive protein
DES - drug‐eluting stent
MI - myocardial infarction
MLD - minimal luminal diameter
Footnotes
Funding: This study was partly supported by the Cardiovascular Research Foundation, Seoul, Korea, and a grant of the Korea Health 21 R&D Project, Ministry of Health and Welfare, Korea (0412‐CR02‐0704‐0001).
Competing interests: None declared.
References
- 1.Ridker P M, Rifai N, Rose L.et al Comparison of C‐reactive protein and low‐density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 20023471557–1565. [DOI] [PubMed] [Google Scholar]
- 2.Ridker P M. Clinical application of C‐reactive protein for cardiovascular disease detection and prevention. Circulation 2003107363–369. [DOI] [PubMed] [Google Scholar]
- 3.Buffon A, Liuzzo G, Biasucci L M.et al Preprocedural serum levels of C‐reactive protein predict early complications and late restenosis after coronary angioplasty. J Am Coll Cardiol 1999341512–1521. [DOI] [PubMed] [Google Scholar]
- 4.Zairis M N, Ambrose J A, Manousakis S J.et al GENERATION Study Group. The impact of plasma levels of C‐reactive protein, lipoprotein (a) and homocysteine on the long‐term prognosis after successful coronary stenting: The Global Evaluation of New Events and Restenosis After Stent Implantation Study, J Am Coll Cardiol 2002401375–1382. [DOI] [PubMed] [Google Scholar]
- 5.Inoue T, Kato T, Uchida T.et al Local release of C‐reactive protein from vulnerable plaque or coronary arterial wall injured by stenting. J Am Coll Cardiol 200546239–245. [DOI] [PubMed] [Google Scholar]
- 6.Morice M C, Serruys P W, Sousa J E.et al RAVEL Study Group. A randomized comparison of a sirolimus‐eluting stent with a standard stent for coronary revascularization. N Engl J Med 20023461773–1780. [DOI] [PubMed] [Google Scholar]
- 7.Moses J W, Leon M B, Popma J J.et al SIRIUS Investigators. Sirolimus‐eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 20033491315–1323. [DOI] [PubMed] [Google Scholar]
- 8.Stone G W, Ellis S G, Cox D A.et al TAXUS‐IV Investigators. A polymer‐based, paclitaxel‐eluting stent in patients with coronary artery disease. N Engl J Med 2004350221–231. [DOI] [PubMed] [Google Scholar]
- 9.Park S J, Kim Y H, Lee B K.et al Sirolimus‐eluting stent implantation for unprotected left main coronary artery stenosis: comparison with bare metal stent implantation. J Am Coll Cardiol 200545351–356. [DOI] [PubMed] [Google Scholar]
- 10.Harrell F E, Jr, Lee K L, Mark D B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 199615361–387. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum P R, Rubin D B. The central role of the propensity score in observational studies for causal effects. Biometrika 19837041–55. [Google Scholar]
- 12.Chew D P, Bhatt D L, Robbins M A.et al Incremental prognostic value of elevated baseline C‐reactive protein among established markers of risk in percutaneous coronary intervention. Circulation 2001104992–997. [DOI] [PubMed] [Google Scholar]
- 13.Dibra A, Mehilli J, Braun S.et al Association between C‐reactive protein levels and subsequent cardiac events among patients with stable angina treated with coronary artery stenting. Am J Med 2003114715–722. [DOI] [PubMed] [Google Scholar]
- 14.Walter D H, Fichtlscherer S, Sellwig M.et al Preprocedural C‐reactive protein levels and cardiovascular events after coronary stent implantation. J Am Coll Cardiol 200137839–846. [DOI] [PubMed] [Google Scholar]
- 15.Skowasch D, Jabs A, Andrie R.et al Progression of native coronary plaques and in‐stent restenosis are associated and predicted by increased pre‐procedural C reactive protein. Heart 200591535–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dibra A, Mehilli J, Braun S.et al Inflammatory response after intervention assessed by serial C‐reactive protein measurements correlates with restenosis in patients treated with coronary stenting. Am Heart J 2005150344–350. [DOI] [PubMed] [Google Scholar]
- 17.Gomma A H, Hirschfield G M, Gallimore J R., Jret al Preprocedural inflammatory markers do not predict restenosis after successful coronary stenting. Am Heart J 20041471071–1077. [DOI] [PubMed] [Google Scholar]
- 18.Segev A, Kassam S, Buller C E.et al Pre‐procedural plasma levels of C‐reactive protein and interleukin‐6 do not predict late coronary angiographic restenosis after elective stenting. Eur Heart J 2004251029–1035. [DOI] [PubMed] [Google Scholar]
- 19.Dibra A, Ndrepepa G, Mehilli J.et al Comparison of C‐reactive protein levels before and after coronary stenting and restenosis among patients treated with sirolimus‐eluting versus bare metal stents. Am J Cardiol 2005951238–1240. [DOI] [PubMed] [Google Scholar]
- 20.De la Torre‐Hernandez J M, Sainz‐Laso F, Burgos V.et al Comparison of C‐reactive protein levels after coronary stenting with bare metal versus sirolimus‐eluting stents. Am J Cardiol 200595748–751. [DOI] [PubMed] [Google Scholar]
- 21.Gaspardone A, Versaci F, Tomai F.et al C‐reactive protein, clinical outcome, and restenosis rates after implantation of different drug‐eluting stents. Am J Cardiol 2006971311–1316. [DOI] [PubMed] [Google Scholar]
- 22.Virmani R, Farb A, Guagliumi G.et al Drug‐eluting stents caution and concerns for long‐term outcome. Coron Artery Dis 200415313–318. [DOI] [PubMed] [Google Scholar]
- 23.Valgimigli M, Malagutti P, van Mieghem C A.et al Persistence of neointimal growth 12 months after intervention and occurrence of delayed restenosis in patients with left main coronary artery disease treated with drug‐eluting stents. J Am Coll Cardiol 2006471491–1494. [DOI] [PubMed] [Google Scholar]
- 24.Palmerini T, Marzocchi A, Marrozzini C.et al Preprocedural levels of C‐reactive protein and leukocyte counts predict 9‐month mortality after coronary angioplasty for the treatment of unprotected left main coronary artery stenosis. Circulation 20051122332–2338. [DOI] [PubMed] [Google Scholar]