Cerebrovascular accident (CVA) is the third leading cause of death in North America and Europe, accounting for approximately 10–12% of all deaths.w1 CVA may have several aetiologies but is generally characterised as being either thrombotic or haemorrhagic in origin. The thrombotic causes of CVA are multiple and can be divided into large vessel occlusion, small vessel occlusion, and embolisation. Large vessel occlusion from atherothrombosis of the carotid system is responsible for approximately 25% of those afflicted by a CVA. The treatment of carotid atherothrombosis is evolving and involves risk factor management and in selected patients may warrant either surgical carotid endarterectomy or percutaneous carotid stenting. This is a review of the current understanding of carotid atherothrombosis and data regarding percutaneous approaches for those with this condition.
Pathophysiology
Atherosclerosis is the most common pathologic process affecting the carotid system leading to a CVA. This complex inflammatory process is mediated by both environmental and genetic factors and is extensively reviewed elsewhere.1 In brief, atherosclerotic plaque develops in the intimal layer of the carotid system and has a predilection for the carotid bifurcation and regions near this area. Indeed, even those with increased intimal–medial thickness are at increased risk for a future cardiovascular event.2 Once present, the initial lesion in the carotid arterial system may grow due to lipid laden macrophage expansion in the setting of chronic inflammatory signals. Although the pathophysiology of carotid disease leading to a CVA is not completely understood, the unstable (or ulcerated plaque) may develop due to several types of mechanisms:
Plaque rupture—constituents of the plaque are exuded into the circulation, expressing tissue factor, resulting in thrombus formation in that region.
Erosion—A thrombus can occur on a de‐endothelialised (ulcerated), but otherwise intact, plaque. This may lead to vessel occlusion and haemodynamic compromise due to vessel occlusion, and may also occur without a plaque rupture.
Calcified nodule—It is also possible to have a thrombus occur on the nidus of a calcified nodule that projects into the circulation and may act as a “lightning rod” for thrombus formation, which might then embolise and cause a cerebrovascular event.
Intraplaque haemorrhage—Another possibility is when a thrombus forms within the plaque. This intraplaque haemorrhage is usually asymptomatic because it is walled off by the plaque cap (that is, there is no eruption of the plaque). However, this may lead to further carotid stenosis and haemodynamic compromise.
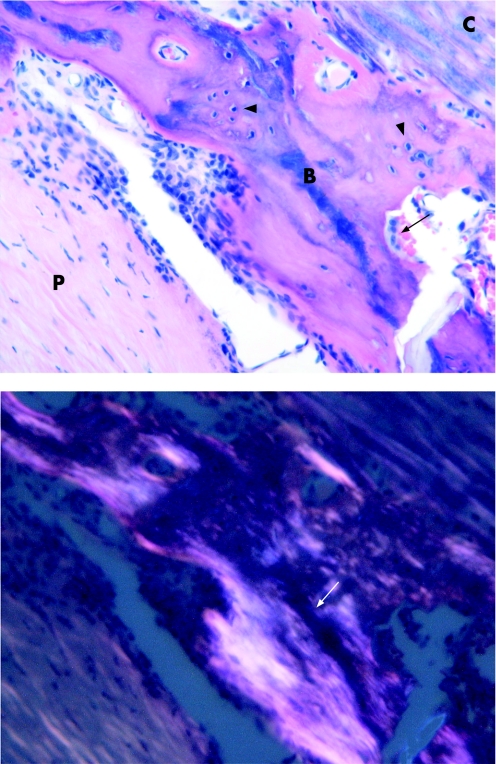
Other characteristics of the carotid plaque correlate with CVA, including those with a thin fibrous cap and a high degree of inflammation. The calcification to lipid ratio also affects carotid plaque stability as heavily calcified carotid plaques are associated with fewer neurological deficits than those with a majority of lipid and relatively little calcification.3 Carotid lesions may even develop sheets of calcification and bone formation that may also be protective in nature (fig 1).3
Figure 1 (Upper panel) Bone in specimen. This photomicrograph demonstrates mature lamellar bone in a plaque wall. The bone is in the centre of the field (B); it appears eosinophilic. The plaque substance, composed of fibrosis and smooth muscle hyperplasia, is to the lower left (P). In the upper right, there is a focus of calcification (C), which appears basophilic. Osteocytes (arrow heads) and an osteoclast in a resorption space (arrow) are present. (Lower panel) Bone in specimen with polarised light. This photomicrograph shows the same focus in the upper panel, but under polarised light microscopy. The ossified structure of mature bone shows up as linear, lamellated bright areas (arrow). Haematoxylin and eosin, 40× original magnification. Figure reproduced with permission from Hunt et al.3
Carotid endarterectomy
Carotid endarterectomy (CEA) was reported as early as 1954 by Eastcot et al.w2 They successfully treated a 66‐year‐old woman with 33 episodes of transient cerebral ischaemia. Despite relief of her symptoms and successful operations in others, the definitive role of CEA in the management of patients with carotid disease came into question in the late 1980s due to a lack of clinical trials showing efficacy. In the 1990s, large scale randomised trials were completed showing benefit of CEA in symptomatic patients with a stenosis of at least 50%.w3–5 The benefit of surgery was lost if the complication rate exceeded 6% for symptomatic patients.w4 w5
Large clinical trials evaluating asymptomatic patients with carotid atherosclerosis and at least 60% stenosis showed a benefit of CEA at approximately 2 years after the surgery.w6 However, the benefit was lost if the complication rate exceeded 3% for the asymptomatic patients. A recent meta‐analysis of CEA for asymptomatic carotid stenosis concluded that the absolute risk reduction for CEA is about 3% over 3 years for the outcome of any stroke.w7 A subgroup analysis suggested that women may not benefit in the short to medium term as the spontaneous stroke rate in women with asymptomatic carotid disease is less than in men. This meta‐analysis also found that there is limited evidence on the effects of CEA among older asymptomatic individuals, as opposed to a greater benefit from surgery seen for symptomatic stenosis among older individuals compared to younger patients. These large, randomised trials did not include patients who were at high risk because non‐stroke deaths may have reduced the chance of detecting a benefit.
Carotid stenting
Percutaneous carotid artery revascularisation has emerged as an alternative therapy to surgical CEA for the treatment of extracranial carotid stenosis. This percutaneous approach has several obvious attractions, including being a less invasive approach, but several studies are still underway to determine efficacy in the symptomatic and asymptomatic patient populations. Carotid revascularisation with balloon angioplasty began in the early 1980s.w8 The Carotid Angioplasty (CAVATS) Study showed at 3 years that there was no difference in the rate of ipsilateral stroke or any disabling stroke compared to conventional carotid surgery.4 However, it has become clear that percutaneous procedures are not without risk as catheter manipulation is associated with morbidity and mortality, with the potential of dislodging plaque resulting in embolic stroke as described later in this review.
The relatively recent availability of stents has shifted the percutaneous carotid revascularisation approach from balloon angioplasty to carotid stenting. Since the initial deployment of the first carotid stent in 1989, technological advances have improved the durability and safety of this device.w9 The first balloon expandable stents were prone to extrinsic compression, but this issue was resolved with the use of the self‐expanding Wallstent and later by self‐expanding nitinol stents.5 There have been numerous types of stents with various diameters and lengths produced for the carotid artery (table 1). Initially, carotid stents were done “alone” without distal embolic protection. Stents were used as an adjunct to thrombolytic treatment to maintain vessel patency. Subsequent clinical studies evaluating embolic protection showed a significant benefit in reducing the perioperative risk of a CVA.w10
Table 1 Stent type and manufacturer.
| Stent type | Manufacturer | Name |
|---|---|---|
| Stainless steel | Boston Scientific | Wallstent |
| Open cell nitinol | Guidant | Acculink |
| Medtronic | Exponent | |
| Bard | Vivexx | |
| Ev3 | Protégé | |
| Cordis | Precise | |
| Closed cell nitinol | Endotex | NexStent |
| Abbott Vascular | Xact | |
| Medinol | Nirtinol |
In general, there are two types of embolic protection devices (EPDs): proximal and distal types. The proximal EPD involves transient occlusion of the common carotid artery proximal to the target lesion, using a balloon with a second balloon in the external carotid artery, resulting in stagnant or reverse flow in the internal carotid artery. The advantage of this proximal EPD is avoidance of embolisation during initial passage of the guidewire and throughout the procedure. The distal EPD involves balloon‐mediated occlusion of the distal cervical portion of the internal carotid artery. After the procedure is completed, embolic debris is removed by manual aspiration, followed by balloon deflation and removal of the protection system. Another similar approach of distal protection involves deployment of a filter device in the distal internal carotid artery. After completion of the procedure, the filter is captured and removed from the patient along with debris that accumulated.
There are numerous clinical studies evaluating carotid stenting but only recently have large studies with cerebral protection been published allowing for modern assessment of this technique. Studies of carotid stents begin in 2001 and the complications range from a stroke and death rate as low as 0% to as high as 7.4%. This variation is likely related to age and comorbidities among the study participants. Three large registries were published outlining the risk in the multicentre population.6,7w11 Clinical trials comparing carotid stenting with CEA have yielded insights into the success rates and complication rates of both procedures. Presently, there are conflicting data regarding the efficacy of carotid stenting versus CEA. Two initial trials that did use embolic protection indicated a worse outcome with the percutaneous technique. One such study, the Wallstent Study, which was stopped prematurely, showed a 1 year ipsilateral stroke rate higher in the percutaneous group compared to surgery (12.2% vs 3.6%). Other trials, such as the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS)4 and a separate randomised trial in a community hospital,8 found that both treatments had similar major risks and effectiveness.
More recent trials have compared carotid stenting versus CEA for high risk patients. The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial9 was a large study employing distal protection in patients defined as having at least one of the high risk criteria listed in table 2.
Table 2 High risk criteria for carotid stenting.
| • Severe cardiovascular disease |
| ‐ congestive heart failure |
| ‐ myocardial infarction 1 day or 4 weeks before |
| ‐ coronary artery bypass grafting or open heart surgery within 6 months |
| ‐ angina at a low workload or unstable angina |
| • Severe pulmonary disease |
| • Previous carotid endarterectomy with recurrent stenosis |
| • Difficult surgical access |
| ‐ at or above the second cervical vertebra |
| ‐ inferior to clavicle |
| ‐ prior radiation therapy to the neck |
| • Contralateral carotid artery occlusion |
| • Contralateral laryngeal nerve palsy |
The entrance criteria included those who either had a symptomatic carotid artery stenosis of ⩾50% of the luminal diameter or an asymptomatic stenosis of at least 80%, or were being treated with carotid artery stenting or surgical endarterectomy. There were 334 patients enrolled in the study and the primary end point was a major cardiovascular event at 1 year, a composite end point of death, stroke or myocardial infarction within 30 days of the intervention, or an ipsilateral stroke within 31 days and at 1 year. The primary end point occurred in 20 patients randomised to undergo carotid stenting and in 32 patients randomised to undergo CEA. Of note, both of the groups in the SAPPHIRE study had a relatively high risk of complications at 30 days, which exceeded the ⩽3% recommended as a maximum rate according to the American Heart Association guidelines.10
There were two recently published randomised controlled trials that failed to establish the non‐inferiority of stenting when compared to CEA. One study called the SPACE trial is a multicentre European study comparing the efficacy of carotid artery stenting in the treatment of severe, symptomatic carotid disease.w12 A total of 1183 patients were randomised to either carotid stenting or CEA. Patients considered high risk with uncontrolled hypertension or severe concomitant disease and a poor prognosis were excluded from the study. This study was not successfully concluded due to recruitment and funding problems; however, after a second interim analysis, there was no evidence of a significant difference between carotid stenting and CEA in the primary composite outcome measure of death or ipsilateral ischaemic stroke at 30 days after the procedure. More recently, the EVA‐3S study involving multiple centres in France also evaluated patients with severe symptomatic carotid stenosis.11 A total of 527 patients were randomised to carotid artery stenting or CEA. Similarly, patients at high risk with unstable angina, uncontrolled diabetes or uncontrolled hypertension and patients with previous carotid revascularisation were not included in the study. After 30 days, the incidence of any stroke or death was significantly higher with stenting than with CEA (9.6% vs 3.9%, relative risk 2.5%).
Ongoing clinical trials
There are several randomised, controlled clinical trials underway comparing the efficacy of carotid stenting versus endarterectomy. One such study is the Carotid Revascularization Endarterectomy versus Stent Trial (CREST).w13 A lead‐in study report from the CREST study detailed the clinical data from 465 patients, including symptomatic patients with ⩾50% carotid stenosis and asymptomatic patients with ⩾70% stenosis.w14 The 30 day morbidity and mortality results indicate that symptomatic patients had a composite stroke and death rate at 30 days of 5.6%, slightly lower than reported in the NASCET and the ECST studies. The asymptomatic patients had a composite 30 day stroke and death rate of 2.4%, slightly higher than the ACAS study, but slightly lower than the ACST clinical trials. The International Carotid Stent Study II (CAVATS‐II) will be evaluating patients who are at lower risk than those enrolled in the SAPPHIRE study and should provide important data comparing stenting versus surgery.
There are several ongoing registries of carotid angioplasty and stenting. The goal of these registries is to provide up‐to‐date clinical data on morbidity and mortality in patients undergoing this procedure, as technology changes rapidly and studies published several years ago may not reflect the current patient risk. One European study of 38 centres in Austria, Germany and Switzerland (Pro‐CAS) reported the results from 3267 patients who overwhelmingly had carotid stenting.w15 The neurologic deficit and combined mortality after the procedure was 2.8%. However, this neurologic deficit did not include minor strokes and inclusion of these events would have resulted in a combined death and stroke rate of 4.2%. Another registry of 581 patients (ARCHeR), who were symptomatic and asymptomatic and considered high risk for surgery, reported a 30 day rate of stroke and death of 6.9% with a 1 year composite end point of stroke, myocardial infarction and death of 8.3%.6 One issue that has arisen when trying to interpret clinical trials is whether a neurologist independently adjudicated the events. For example, in the Pro‐CAS registry described above, a neurologist was only involved in 63% of the cases.
Potential complications
Immediate complications caused by carotid stenting potentially include bradycardia, hypotension, dissection and minor or major stroke. The hyperperfusion syndrome is a rare phenomenon that can result after CEA; it manifests as headache, ipsilateral to the revascularised artery, and may be accompanied by focal, motor seizures and intracerebral haemorrhage.12 At present, there are few data regarding the prevalence of hyperfusion syndrome after carotid stenting. Other complications include acute and subacute in‐stent thrombosis beyond 30 days—restenosis. The estimated restenosis rate for CEA is 5–10% at 1 year.13w16 w17 A review of 34 carotid stent studies found an angiographic restenosis rate of 6% after 1 year, indicating that a carotid stent should not be expected to result in higher restenosis than CEA.w18
Medical treatment
All patients with carotid atherosclerosis should receive atherosclerotic risk factor modification. Several randomised controlled studies evaluating statin treatment showed a lower stroke rate.14 w19 One recent randomised study enrolling patients with history of transient ischaemic attack (TIA) or stroke showed a 5 year absolute reduction in the risk of major cardiovascular events of 3.5%.15 A prospective database of 180 patients receiving carotid stents found statin treatment appears to reduce the incidence of stroke, myocardial infarction and death within 30 days after procedure.16 Antiplatelet treatment is also essential to reduce cardiovascular events irrespective of whether or not revascularisation is done.w20 w21 Current evidence supports only utilising a single antiplatelet agent for patients with carotid atherosclerotic disease as duel therapy increases side effects, primarily bleeding, that offset effectiveness in preventing stroke.17 w22 However, duel therapy with aspirin and clopidogrel appears effective after carotid stent placement.18 Prospective clinical trials are needed to evaluate carotid stenting or CEA versus optimal medical treatment for asymptomatic patients with carotid stenosis.
Clinical implications and conclusions
The American Heart Association (AHA) and the American Stroke Association (ASA) Council on Stroke recommendations for revascularisation for symptomatic patients are listed in table 3.10 A recently published multi‐society expert consensus document on carotid stenting affirms these recommendations.19
Table 3 American Heart Association/American Stroke Association recommendations for revascularisation in symptomatic patients.
| • For patients with recent TIA or ischaemic stroke within the last 6 months and ipsilateral severe (70–99%) carotid artery stenosis, CEA is recommended by a surgeon with a perioperative morbidity and mortality rate of <6%. Class I, level A |
| • For patients with recent TIA or ischaemic stroke and ipsilateral moderate (50–69%) carotid stenosis, CEA is recommended, depending on patient‐specific factors such as age, gender, comorbidities, and severity of initial symptoms. Class I, level A |
| • When degree of stenosis is <50%, there is no indication for CEA. Class III, level A |
| • When CEA is indicated, surgery within 2 weeks rather than delayed surgery is suggested. Class IIa, level B |
| • Among patients with symptomatic severe stenosis (>70%) in whom the stenosis is difficult to access surgically, medical conditions are present that greatly increase the risk for surgery, or when other specific circumstances exist such as radiation‐induced stenosis or restenosis after CEA, CAS is not inferior to CEA and may be considered. Class IIb, level B |
| • CAS is reasonable when performed by operators with established periprocedural morbidity and mortality rates of 4–6%, similar to that observed in trials of CEA and CAS. Class IIa, level B |
CAS, carotid artery stenting; CEA, carotid endarterectomy; TIA, transient ischaemic attack.
Modified with permission from Sacco et al.10
The current data favour carotid stenting with embolic protection for high risk patients where anaesthesia and surgical repair would pose excess risks, such as congestive heart failure, uncontrolled angina pectoris, and severe obstructive pulmonary disease.19 Other anatomic characteristics that increase the risk of CEA, such as previous radiation therapy to the neck, previous radical neck dissection, carotid restenosis after endarterectomy, and contralateral laryngeal palsy are other indications where stenting would be advocated.19 In the USA, government insurance reimbursement is currently only given for carotid stenting for patients at high risk for CEA, as outlined above, and symptomatic stenosis of ⩾70%.
The management of asymptomatic patients with carotid plaque is less clear. The AHA guidelines for asymptomatic patients recommend CEA for 60–99% stenosis, if the perioperative risk is <3%.10 Although clinical trial data support CEA in asymptomatic patients with carotid stenosis of 60–79%,w6 w7 some clinicians delay revascularisation until there is an 80% stenosis or greater, especially if comorbid conditions exist that shorten life expectancy. The American Academy of Neurology noted in 2005 guidelines that the optimal patient for carotid revascularisation is ⩽75 years with a life expectancy of 5 years.20
There remain several unanswered questions regarding carotid stenting. The overriding question is whether carotid stenting with embolic protection is equivalent to CEA. It is unclear if specific patient populations will benefit from carotid stent implantation—for example, are older patients (>80 years) at a higher risk of stroke or death from carotid stent implantation compared to younger patients? Some studies suggest that patients older than 80 years of age are at higher risk, but more data are needed.5 w14 Is there a role for carotid stenting in lower risk asymptomatic patients? The ongoing CREST and CAVATAS‐II studies should provide more definitive answers to patient selection for carotid stenting.
Additional references appear on the Heart website— http://heart.bmj.com/supplemental
Interactive multiple choice questions
This Education in Heart article has an accompanying series of six EBAC accredited multiple choice questions (MCQs).
To access the questions, click on BMJ Learning: Take this module on BMJ Learning from the content box at the top right and bottom left of the online article. For more information please go to: http://heart.bmj.com/misc/education.dtl Please note: The MCQs are hosted on BMJ Learning—the best available learning website for medical professionals from the BMJ Group.
If prompted, subscribers must sign into Heart with their journal's username and password. All users must also complete a one‐time registration on BMJ Learning and subsequently log in (with a BMJ Learning username and password) on every visit.
Additional references appear on the Heart website—http://heart.bmj.com/supplemental
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
Additional references appear on the Heart website—http://heart.bmj.com/supplemental
References
- 1.Mohler ER I I I, Schafer A I. Atherothrombosis: disease initiation, progression and treatment. In: Lichtman MA, Kipps TJ, Kaushansky K, et al eds. Williams hematology. New York: McGraw‐Hill, 20062067–2088.This book chapter provides a comprehensive review of atherothrombosis.
- 2.Roman M J, Naqvi T Z, Gardin J M.et al Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr 200619943–954.These guidelines provide the rationale and methods for carotid intimal–medial thickness measurement as a means of cardiovascular risk assessment. [DOI] [PubMed] [Google Scholar]
- 3.Hunt J L, Fairman R, Mitchell M E.et al Bone formation in carotid plaques: a clinicopathological study. Stroke 2002331214–1219.A prospective evaluation of carotid plaque morphology and risk of TIA and stroke. [DOI] [PubMed] [Google Scholar]
- 4.CAVATAS Investigators Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet 20013571729–1737. [PubMed] [Google Scholar]
- 5.Roubin G S, New G, Iyer S S.et al Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis: a 5‐year prospective analysis. Circulation 2001103532–537. [DOI] [PubMed] [Google Scholar]
- 6.Gray W A, Hopkins L N, Yadav S.et al Protected carotid stenting in high‐surgical‐risk patients: the ARCHeR results. J Vasc Surg 200644258–268.This is a report of a carotid stent registry of high risk patients who received a carotid embolic protection device during the procedure. [DOI] [PubMed] [Google Scholar]
- 7.Safian R D, Bresnahan J F, Jaff M R.et al Protected carotid stenting in high‐risk patients with severe carotid artery stenosis. J Am Coll Cardiol 2006472384–2389. [DOI] [PubMed] [Google Scholar]
- 8.Brooks W H, McClure R R, Jones M R.et al Carotid angioplasty and stenting versus carotid endarterectomy for treatment of asymptomatic carotid stenosis: a randomized trial in a community hospital. Neurosurgery 200454318–324. [DOI] [PubMed] [Google Scholar]
- 9.Yadav J S, Wholey M H, Kuntz R E.et al Protected carotid‐artery stenting versus endarterectomy in high‐risk patients. N Engl J Med 20043511493–1501.The SAPPHIRE study is the first multicentre, randomised study comparing the two procedures in high risk patients. [DOI] [PubMed] [Google Scholar]
- 10.Sacco R L, Adams R, Albers G.et al Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation 2006113e409–e449. [PubMed] [Google Scholar]
- 11.Mas J L, Chatellier G, Beyssen B.et al Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med 20063551660–1671.This randomised trial compared carotid stenting with carotid endarterectomy in patients with symptomatic disease. After 30 days the incidence of stroke or death was higher in the stent group, but an embolic protection device was recommended later in the study which may have skewed the findings. [DOI] [PubMed] [Google Scholar]
- 12.bou‐Chebl A, Yadav J S, Reginelli J P.et al Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting: risk factors, prevention, and treatment. J Am Coll Cardiol 2004431596–1601. [DOI] [PubMed] [Google Scholar]
- 13.McCabe D J, Pereira A C, Clifton A.et al Restenosis after carotid angioplasty, stenting, or endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS). Stroke 200536281–286. [DOI] [PubMed] [Google Scholar]
- 14.Mohler ER I I I, Delanty N, Rader D J.et al Statins and cerebrovascular disease: plaque attack to prevent brain attack. Vasc Med 19994269–272. [DOI] [PubMed] [Google Scholar]
- 15.The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006355549–559.16899775This randomised study showed that statin treatment lowered TIA and stroke rate in patients with previous TIA and stroke. [Google Scholar]
- 16.Groschel K, Ernemann U, Schulz J B.et al Statin therapy at carotid angioplasty and stent placement: effect on procedure‐related stroke, myocardial infarction, and death. Radiology 2006240145–151. [DOI] [PubMed] [Google Scholar]
- 17.Diener H C, Bogousslavsky J, Brass L M.et al Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial. Lancet 2004364331–337. [DOI] [PubMed] [Google Scholar]
- 18.Bhatt D L, Kapadia S R, Bajzer C T.et al Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J Invasive Cardiol 200113767–771.This study showed the importance of using two antiplatelet drugs after carotid artery stent placement. [PubMed] [Google Scholar]
- 19.Bates E R, Babb J D, Casey D E., Jret al ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (ACCF/SCAI/SVMB/SIR/ASITN Clinical Expert Consensus Document Committee on Carotid Stenting). J Am Coll Cardiol 200749126–170. [DOI] [PubMed] [Google Scholar]
- 20.Chaturvedi S, Bruno A, Feasby T.et al Carotid endarterectomy–an evidence‐based review: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 200565794–801. [DOI] [PubMed] [Google Scholar]