A 65‐year‐old woman was referred, after the discovery of pan‐hypogammaglobulinaemia during investigation for recurrent sinus and chest infections (IgG 0.6 g/l; IgA 0.16 g/l; IgM 0.44 g/l). Other causes of immunodeficiency were excluded and a diagnosis of common variable immunodeficiency (CVID) was made. Treatment with intravenous immunoglobulin (IVIG) was commenced, and the sinus and chest symptoms markedly improved. At 11 months after the diagnosis, she presented with a purpuric rash affecting her legs and blood blisters in her mouth. Examination was otherwise unremarkable, and a full blood count demonstrated severe thrombocytopenia (platelets 4×109/l; haemoglobin 13.1 g/l; white cell count 5.0×109/l; neutrophils 1.15×109/l). Bone marrow examination revealed a slightly hypocellular marrow that seemed to be consistent with her age, but with slightly increased numbers of megakaryocytes. A diagnosis of immune thrombocytopenic purpura (ITP) was made. She received a platelet transfusion and was treated with high‐dose IVIG (Octagam, 30 g daily for 5 days). Her platelet count improved, and, a month later, was found to be 93×109/l. Shortly afterwards, a second episode of thrombocytopenia occurred, which again responded to high‐dose IVIG. There was no corresponding improvement in her neutropenia, but she remained clinically well by treatment with replacement dose IVIG and prophylactic amoxicillin.
Her thrombocytopenia recurred 3 years later (platelets 3×109/l). She also remained neutropenic (0.8×109/l). Steroids were not tried at any point in the treatment of her ITP, as she refused to take steroids because of fears of side effects. On this occasion, high‐dose IVIG failed to provide a lasting improvement to her platelet count; treatment was continued with 30 g of Octagam twice weekly, and she required frequent platelet transfusions. She was referred for splenectomy, which was performed 3 months later. In the lead up to the splenectomy, she received granulocyte colony stimulating factor, which successfully reversed her neutropenia during the perioperative period. The operation was performed without complication, and she made a good recovery. However, no improvement in platelet count occurred following splenectomy, and she remained platelet transfusion dependent, despite continued treatment with 30 g Octagam twice weekly.
High levels of platelet‐bound immunoglobulin were demonstrated by direct platelet immunofluorescence using flow cytometry. The presence of platelet autoantibodies was therefore likely, although the target antigen was not delineated, as her serum was negative when assayed against GP IIb/IIIa and GP Ia. Granulocyte‐specific IgG was also present in the patient's serum against three out of three donor panel cells.
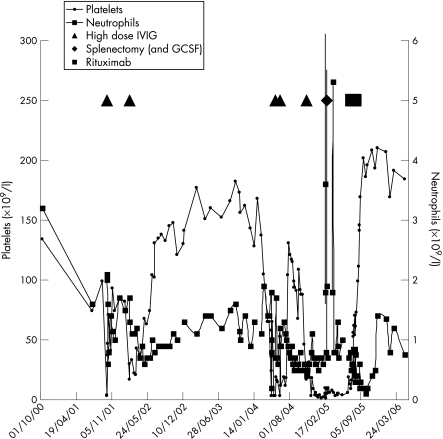
She received a course of rituximab (375 mg/m2 once weekly for 4 weeks), which was tolerated without any ill effects. Within a month of completion of the rituximab course, she was transfusion independent and had a platelet count of 170×109/l. The frequency of IVIG was gradually decreased to 30 g every 3 weeks, and currently, a year later, her platelet count is around 200×109/l. The neutrophil count initially fell after rituximab treatment, and then rose back to a similar level as in the pre‐rituximab condition. The most recent neutrophil count is 0.76×109/l. Repeat platelet immunology is now normal, although granulocyte‐specific IgG persists. Figure 1 summarises the platelet and neutrophil counts over a 6‐year period.
Figure 1 Graph demonstrating the platelet and neutrophil counts over time with the use of high‐dose intravenous immunoglobulin (IVIG), splenectomy and rituximab marked. High‐dose IVIG represents the periods when Octagam was administered at a dose of 30 g daily. From November 2004 until the administration of rituximab, IVIG was given at a dose of 30 g twice weekly. GCSF, granulocyte colony stimulating factor.
Discussion
CVID is a diagnosis of exclusion, and describes patients with hypogammaglobulinaemia in whom secondary immunodeficiencies and primary immunodeficiencies have been excluded.1 In addition to the increase in infections due to the immunodeficiency, there are a number of associated complications, including autoimmune diseases, of which ITP is one of the most common. In one study of 248 patients with CVID, 56 patients were found to have autoimmune disease. Of these, 22 had ITP and 2 had autoimmune neutropenia.2
ITP results from accelerated platelet destruction due to the presence of autoantibodies against platelet glycoproteins. Treatments for ITP include corticosteroids, and immunosuppressive agents such as azathioprine. High‐dose IVIG first established itself as an immunomodulatory agent in the treatment of ITP, although its use is usually limited to patients in whom corticosteroids have failed or who have clinical features of haemorrhage.3 Splenectomy is often effective and results in cure in up to 75% of patients.4
Rituximab is an anti‐CD20 humanised monoclonal antibody that causes temporary B cell depletion.5 The mechanisms of action include complement activation, antibody‐dependent cell‐mediated cytotoxicity and direct effects on B cells mediated via CD20 binding.6 Rituximab is licensed for the treatment of B cell non‐Hodgkin's lymphoma, where it is used at a dose of 375 mg/m2 weekly for four consecutive weeks. Increasing interest is being shown in the use of rituximab for the treatment of autoimmune diseases, where B cells/autoantibodies are suspected to have a role in the pathogenesis.7
With regard to ITP, a number of studies have investigated the potential benefit of rituximab. The largest study of 57 adults achieved a response in 31 patients (18 patients had a complete response, defined as a platelet count >150×109/l, and 13 had a partial response). Most of those who had a complete response maintained the response at 1 year, but only 2 of the 13 who had a partial response maintained their response.8 The duration of response over a longer term was assessed in a study of 24 paediatric patients with chronic ITP. In all, 15 patients had a complete response, 6 of whom relapsed at time points varying from 3 to 18 months. The other 9 patients still had ongoing complete responses (6 had responses lasting for ⩾1 year, 2 had continued responses at 24 and 30 months).9 A case report of two patients with autoimmune thrombocytopenia and neutropenia who were treated with rituximab demonstrated sustained remission of thrombocytopenia in both patients, but only one patient had resolution of neutropenia.10 There are two case reports of rituximab being used to treat ITP associated with CVID. In the first, rituximab resulted in only a partial response and a rise in platelet count to around 40×109/l.11 The second patient had both ITP and neutropenia in association with CVID. After rituximab treatment, the platelet count rose to 130×109/l and the neutrophil count normalised. However, treatment was continued with steroids at a reduced dose of 10 mg/day.12
This case report describes the successful use of rituximab to treat ITP in a patient with CVID. The platelet count remains at >150×109/litre 1 year after treatment, although the neutropenia has persisted, with neutrophil counts of around 0.8×109/litre. The anti‐platelet autoantibodies have resolved, but granulocyte specific IgG is persisting. Why rituximab treatment led to the clearance of anti‐platelet but not anti‐granulocyte antibodies is not clear. Neutropenia has been associated with rituximab treatment,13 but in this case our patient's neutropenia predated rituximab treatment by some years and antigranulocyte antibodies were present.
It is becoming increasingly apparent that rituximab has a place in the treatment of ITP, including when it is present in association with other conditions. The exact position of rituximab in treatment protocols is still to be determined, but for now it can probably be used when other treatment options have failed. Monoclonal antibodies are expensive, and, while rituximab is no exception, this has to be balanced against the cost of regular platelet transfusions, and the health and fiscal costs which result from the long‐term use of steroids. A number of other questions remain, such as whether the full dose of 375 mg/m2 used to treat B cell non‐Hodgkin's lymphoma is also required for ITP, or whether a lower dose would still provide the same clinical benefit. The literature suggests that recurrence of ITP treated with rituximab should be expected, although after a variable time period lasting months to years. Whether re‐treatment with rituximab at this stage is effective requires evaluation.
Footnotes
Competing interests: None declared.
References
- 1.Conley M E, Notarangelo L D, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan‐American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol 199993190–197. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham‐Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol 19999234–48. [DOI] [PubMed] [Google Scholar]
- 3.Jolles S, Sewell W A C, Misbah S A. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol 20051421–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, Diehn F E, Gertz M A.et al Splenectomy for immune thrombocytopenic purpura: long‐term results and treatment of postsplenectomy relapses. Ann Hematol 200281312–319. [DOI] [PubMed] [Google Scholar]
- 5.Reff M E, Carner K, Chambers K S.et al Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 199483435–445. [PubMed] [Google Scholar]
- 6.Leget G A, Czuczman M S. Use of rituximab, the new FDA‐approved antibody. Curr Opin Oncol 199810548–551. [DOI] [PubMed] [Google Scholar]
- 7.Kazkaz H, Isenberg D. Anti B cell therapy (rituximab) in the treatment of autoimmune diseases. Curr Opin Pharmacol 20044398–402. [DOI] [PubMed] [Google Scholar]
- 8.Cooper N, Stasi R, Cunningham‐Rundles S.et al The efficacy and safety of B‐cell depletion with anti‐CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol 2004125232–239. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Wiley J M, Luddy R.et al Chronic immune thrombocytopenic purpura in children: assessment of rituximab treatment. J Pediatr 2005146217–221. [DOI] [PubMed] [Google Scholar]
- 10.Faurschou M, Hasselbalch H C, Nielsen O J. Sustained remission of platelet counts following monoclonal anti‐CD20 antibody therapy in two cases of idiopathic autoimmune thrombocytopenia and neutropenia. Eur J Haematol 200166408–411. [DOI] [PubMed] [Google Scholar]
- 11.Carbone J, Escudero A, Mayayo M.et al Partial response to anti‐CD20 monoclonal antibody treatment of severe immune thrombocytopenic purpura in a patient with common variable immunodeficiency. Ann N Y Acad Sci 20051051666–671. [DOI] [PubMed] [Google Scholar]
- 12.Mahevas M, Le Page L, Salle V.et al Efficiency of rituximab in the treatment of autoimmune thrombocytopenic purpura associated with common variable immunodeficiency. Am J Hematol 200681645–646. [DOI] [PubMed] [Google Scholar]
- 13.Chaiwatanatorn K, Lee N, Grigg A.et al Delayed‐onset neutropenia associated with rituximab therapy. Br J Haematol 2003121913–918. [DOI] [PubMed] [Google Scholar]