Abstract
Backgound
In cases of known aetiology, gastric duodenal metaplasia (GMD) is a reversible lesion. In cases of unknown aetiology, the fate of GMD remains elusive. GMD was recently found in a duodenal adenoma.
Aim
To audit the frequency of GMD occurring in a cohort of duodenal adenomas.
Methods
Filed H&E‐stained sections from 306 consecutive duodenal adenomas were investigated for the presence of GMD.
Results
68% of the adenomas (n = 208) were from patients with familial adenomatous polyposis (FAP), and the remaining 32% (n = 98) were sporadic. GMD was found in 31.7% (66/208) of the duodenal FAP adenomas and in 59.2% (58/98) of the duodenal sporadic adenomas (p<0.05). The causes for this difference are elusive.
Conclusions
As for other metaplasias of the gastrointestinal tract (intestinal metaplasia of the oesophagus and of the stomach, and metaplastic–hyperplastic polyposis of the colon, known to antedate neoplastic transformation), a subset of GMDs of unknown cause might be present in the duodenal mucosa before adenomatous changes ensue. That subset of GMD might have neoplastic proclivity similar to the metaplastic epithelium in other organs of the gastrointestinal tract. The known carcinogenic effect of high concentrations of bile acids and pancreatic juices bathing the duodenal mucosa carrying an irreversible subset of GDM might set aflame the adenomatous neoplastic transformation in these patients.
The normal mucosa of the duodenum is built of absorbing columnar enterocytes and secreting goblet cells. Goblet cells produce acid sialomucins and stain with periodic acid‐Schiff (PAS), and more specifically with the cationic dye alcian blue.1 The absorbing columnar enterocytes are unable to produce neutral mucins, or sulphomucins under normal conditions.1 In gastric metaplasia in the duodenum (GMD), the luminal aspect of the cytoplasm of absorbing columnar enterocytes has the property of secreting PAS‐positive neutral mucins and, occasionally, traces of alcian blue‐positive sialomucins.1 GMD is a histologically detectable mucosal change thought to evolve following an abnormally high production of gastric acid triggered by Helicobacter pylori infection.2 When that hypersecretion reaches the duodenum, the enterocytes of the villi react with apical mucin metaplasia to buffer the unwanted low pH of the microenvironment. Recent studies indicate, however, that GMD may be found in the absence of gastric H pylori infection2,3,4,5,6 in coeliac disease,7 in Crohn's disease extending to the duodenum8 and even in the absence of all these conditions. The cause(s) of GMD in the latter group of patients is elusive. Whereas GMD usually disappears following the eradication of the H. pylori,9 and following a gluten‐free diet in patients with coeliac disease, little is known about the evolution of GMD10 in patients without H. pylori infection and without coeliac disease.
Recently, while reviewing duodenal adenomas,11 we noticed that GMD could be detected in those neoplasias. The purpose of the present study was to investigate the frequency of GMD occurring in a consecutive cohort of adenomas of the duodenum.
Materials and methods
Between January 2000 and October 2005, 306 cases with duodenal adenomas were diagnosed at our department (Department of Pathology, Karolinska Institute and University Hospital, Stockholm, Sweden). Filed H&E‐stained sections were reviewed. In all, 20 consecutive adenomas with GMD and 20 duodenal adenomas without GMD were also stained with PAS and with PAS‐diastase.
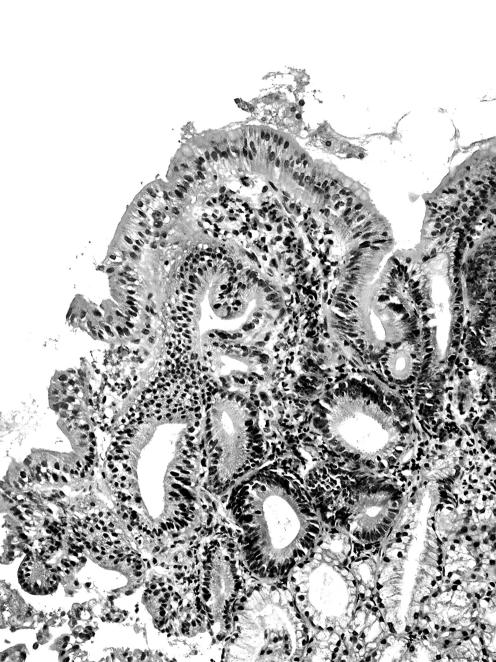
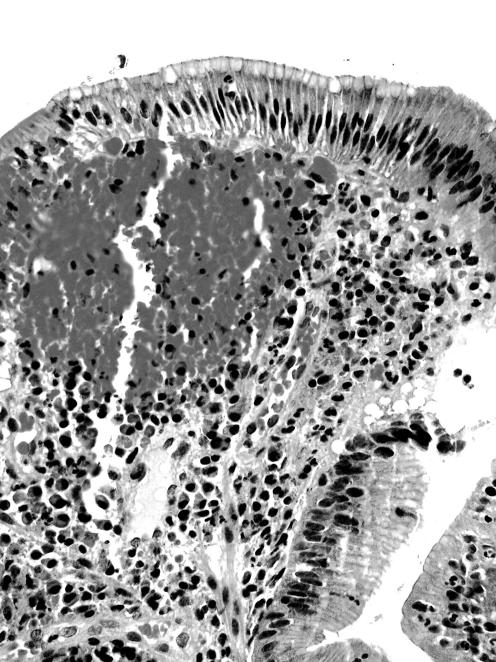
GMD in adenomas was found in the superficial (ie, luminal) non‐dysplastic epithelium of the adenoma (figs 1 and 2). Occasionally, it was also present in the luminal aspect dysplastic epithelium of the adenoma.
Figure 1 Duodenal adenoma showing gastric metaplasia in the duodenum in the apical cytoplasm of non‐dysplastic enterocytes at the lumen of the lesion (H&E magnification×20).
Figure 2 Another duodenal adenoma showing gastric metaplasia in the duodenum in the apical cytoplasm of non‐dysplastic enterocytes at the lumen of the lesion (H&E magnification×40).
Student's unpaired t test was used to compare the significance of the difference between the group means. Statistical significance was set at p<0.05.
Results
Patients with duodenal adenoma
Of the 306 patients with an adenoma in the duodenum, 68% (208) had a familial adenomatosis polyposis (FAP) and the remaining 32% (98) had a sporadic (ie, non‐FAP) duodenal adenoma.
Table 1 shows that females accounted for nearly two‐thirds of the FAP patients having duodenal adenomas, but for only one‐third of the patients with sporadic duodenal adenomas. The difference was significant (p<0.05).
Table 1 Genderwise break‐up of 306 patients with duodenal adenomas; with 208 patients having familial adenomatosis polyposis (FAP) and 98 patients having sporadic adenomas (non‐FAP).
| FAP cases | Sporadic cases | Total | |
|---|---|---|---|
| Male | 75 (36.1%) | 60 (61.2%) | 135 (44.1%) |
| Female | 133 (63.9%) | 38 (38,8%) | 171 (55.9%) |
| Total | 208 (100%) | 98 (100%) | 306 (100%) |
FAP, familial adenomatosis polyposis.
Younger patients (⩽59 years of age) accounted for 58.2% (121/208) of the FAP patients with duodenal adenomas, but only for 33.7% (33/98) of the patients with sporadic duodenal adenomas (p<0.05).
GMD in duodenal adenomas
GMD was recorded in one or more foci in 40.5% (124/306) of the adenomas.
GMD was found in 31.7% (66/208) of the duodenal FAP adenomas and in 59.2% (58/98) of the duodenal sporadic adenomas (p<0.05).
GMD was present in 72.7% (48/66) of the younger (⩽59 years) patients with an FAP adenoma and in 41.4% (24/58) of the younger patients with a sporadic adenoma (p<0.05). The gender‐wise distribution in patients with GMD was similar to that for the whole population (see above).
From table 2 it may be deduced that as many as 91.2% (47/51) of the patients having a duodenal adenoma with GMD and gastric biopsyspecimens had no H pylori infection, whereas only 7.8% (4/51) of the remaining patients with GMD in adenomas had H pylori infection (p<0.05). Gastric biopsy specimens were not taken from the remaining 58.9% (73/124) of the patients with duodenal adenomas having GMD.
Table 2 Gastric metaplasia in the duodenum in duodenal adenomas in 124 patients: in 66 patients having familiar adenomatosis polyposis (FAP), and in 58 sporadic cases (non‐FAP).
| GMD/FAP | GMD/Sporadic | |
|---|---|---|
| Helicobacter pylori infection | 2 | 2 |
| No Helicobacter pylori infection | 22 | 25 |
| Patients without gastric biopsy specimens | 42 | 31 |
| Total | 66 | 58 |
FAP, familial adenomatous polyposis; GMD, gastric metaplasia in the duodenum.
Table 3 shows that, in patients with FAP, GMD was detected in 31.3% (35/112) of the tubular adenomas, in 31.3% (25/80) of the tubulovillous adenomas and in 37.5% (6/16) of the villous adenomas (p = 0.6). In patients with sporadic adenomas, GMD was found in 56.8% (25/44) of the tubular adenomas, in 60.1% (26/43) of the tubulovillous adenomas and in 63.6% (7/11) of the villous adenomas (p = 0.6). The difference between the frequency of GMD in the three histological phenotypes was significantly higher (p<0.05) in sporadic (59.2%) than in FAP adenomas (31.7%).
Table 3 Histological phenotype in 306 duodenal adenomas and gastric metaplasia in the duodenum in patients with familial adenomatosis polyposis (FAP) and in sporadic cases (ie, without FAP).
| Adenoma phenotype | GMD/FAP adenomas | GMD/sporadic adenomas | Total |
|---|---|---|---|
| Tubular | 35/112 (31.3%) | 25/44 (56.8%) | 60/156 (38.5%) |
| Tubulovillous | 25/80 (31.3%) | 26/43 (60.1%) | 51/123 (41.5%) |
| Villous | 6/16 (37.5%) | 7/11 (63.6%) | 13/27 (4.18%) |
| Total | 66/208 (31.7%) | 58/98 (59.2%) | 124/306 (40.5%) |
FAP, familial adenomatosis polyposis; GMD, gastric metaplasia in the duodenum.
PAS‐positive and PAS‐diastase‐positive stains were recorded in enterocytes in all 20 adenomas with GMD, but in none of the 20 adenomas without GMD.
Discussion
In the present study, we found a high frequency (namely 40.5%) of GMD in duodenal adenomas. The frequency of GMD was significantly higher in sporadic duodenal adenomas than in FAP duodenal adenomas. Although the reason(s) for this difference remains elusive, GMD is apparently not evoked by the genetic alterations connected with FAP.
GMD in adenomas was often detected among younger patients with FAP. This is not surprising, considering that young patients with FAP are subjected to surveillance programmes for the early detection of duodenal adenomas. GMD in adenomas was also found in older patients (⩾60 years of age), suggesting that GMD in adenomas may occur at all ages.
In the absence of duodenal adenomas, GMD is found in patients with gastric H pylori infection, with coeliac disease, with Crohn's disease extending to the duodenum, and even in the absence of these conditions. GMD is known to disappear after the eradication of the H.pylori infection,9 and of gluten from the diet in patients with coeliac disease.7 On the other hand, the fate of GMD in duodenal adenomas remains unclear. In this series of consecutive duodenal adenomas, the frequency of cases having gastric biopsy specimens with H pylori gastritis was very low, indicating that GMD in these adenomas was evoked by reason(s) other than the secondary effect of that bacterium. In the light of these results, one possibility is that two types of GMD could exist: one of known aetiology, that is reversible, and the other of unknown aetiology. Perhaps GMD is not one single lesion, but a superfamily of lesions having a similar histological appearance and histochemical reactions but different biological attributes. The possibility that the phenomenon reported herein reflects a subset of GMD of unknown aetiology should be entertained. That metaplastic subset might be a remnant mucosal alteration that has antedated the neoplastic transformation. This possibility seems to be validated by the various reports indicating that metaplasias in disparate mucosas in the gastrointestinal tract may precede neoplasias, such as in the oesophagus (intestinal metaplasia in Barrett's oesophagus–dysplasia–carcinoma sequence12), in the stomach (intestinal metaplasia–dysplasia–carcinoma sequence13) and in the colonic mucosa (metaplastic–hyperplastic polyposis coli–dysplasia–carcinoma sequence14,15).
Recently Sakurai et al16 found adenocarcinomas developing in duodenal Brunner gland hyperplasia. One of the carcinomas in the hyperplastic glands around dysplastic foci was associated with gastric foveolar metaplasia. Immunohistochemical profiles supported the concept of a continuous spectrum in carcinogenesis from gastric foveolar hyperplasia through atypical hyperplasia or dysplasia, and eventually to frank Brunner gland adenocarcinoma.
It is, therefore, not unconceivable that, as for other metaplasias of the gastrointestinal tract (intestinal metaplasia of the oesophagus, of the stomach and metaplastic–hyperlastic polyposis of the colon) known to antedate neoplastic transformation, a subset of GMD of unknown cause might be present in the duodenal mucosa before adenomatous changes ensue. That subset of GMD might have a neoplastic proclivity similar to the metaplastic epithelium in other organs of the gastrointestinal tract. The known carcinogenic effect of high concentrations of bile acids and pancreatic juices bathing the duodenal mucosa carrying an irreversible subset of GDM might set aflame the adenomatous neoplastic transformation in these patients.
Abbreviations
FAP - familial adenomatous polyposis
GMD - gastric metaplasia in the duodenum
PAS - periodic acid‐Schiff
Footnotes
Competing interests: None declared.
References
- 1.Filipe M I. Mucins in the human gastrointestinal tract. A review. Invest Cell Pathol 19792195–216. [PubMed] [Google Scholar]
- 2.Harris A, Gummett P, Walter J.et al Relation between gastric output, Helicobacter pylori, and gastric metaplasia in the duodenal bulb. Gut 199639513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McColl K. Helicobacter pylori, gastric acid, and duodenal gastric metaplasia. Gut 199639615–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voutilainen M, Juhola M, Farkkila M.et al Gastric metaplasia and chronic inflammation at the duodenal bulb mucosa. Dig Liver Dis 20033594–98. [DOI] [PubMed] [Google Scholar]
- 5.Van De Bovenkamp J H, Korteland‐Van Male A M, Buller H A.et al Metaplasia of the duodenum shows a Helicobacter pylori‐correlated differentiation into gastric‐type protein expression. Hum Pathol 200334156–165. [DOI] [PubMed] [Google Scholar]
- 6.Heikkinen M, Pikkarainen P, Vornanen M.et al Prevalence of gastric metaplasia in the duodenal bulb is low in Helicobacter pylori positive non‐ulcer dyspepsia patients. Dig Liver Dis 200133459–463. [DOI] [PubMed] [Google Scholar]
- 7.Shaoul R, Marcon M A, Okada Y.et al Gastric metaplasia: a frequently overlooked feature of duodenal biopsy specimens in untreated celiac disease. J Pediatr Gastroenterol Nutr 200030397–403. [DOI] [PubMed] [Google Scholar]
- 8.Kushima R, Borchard F, Hattori T. A new aspect of gastric metaplasia in Crohn's disease: bidirectional (foveolar and pyloric) differentiation in so‐called ‘pyloric metaplasia' in the ileum. Pathol Int 199747416–419. [DOI] [PubMed] [Google Scholar]
- 9.Ciancio G, Nuti M, Orsini B.et al Regression of duodenal gastric metaplasia in Helicobacter pylori positive patients with duodenal ulcer disease. Dig Liver Dis 20023416–21. [DOI] [PubMed] [Google Scholar]
- 10.Rubio C A. A simple method to demonstrate duodenal gastric metaplasia. J Clin Pathol 200255520–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubio C A. Serrated adenoma of the duodenum. J Clin Pathol 2004571219–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubio C A, Lagergren J. Histological features pertinent to local tumour progression in Barrett's adenocarcinoma. Anticancer Res 2003233015–3018. [PubMed] [Google Scholar]
- 13.Rubio C A, Jónasson J G, Nesi G.et al Extensive intestinal metaplasia in gastric carcinoma and in other lesions requiring surgery. A study of 3421 gastrectomy specimens from dwellers of the Atlantic and the Pacific Basins. J Clin Pathol 2005581271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snover D C, Jass J R, Fenoglio‐Preiser C.et al Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept [review]. Am J Clin Pathol 2005124380–391. [DOI] [PubMed] [Google Scholar]
- 15.Rubio C A, Stemme S, Jaramillo E.et al Hyperplastic polyposis coli syndrome and colorectal carcinoma. Endoscopy 200638266–270. [DOI] [PubMed] [Google Scholar]
- 16.Sakurai T, Sakashita H, Honjo G.et al Gastric foveolar metaplasia with dysplastic changes in Brunner gland hyperplasia: possible precursor lesions for Brunner gland adenocarcinoma. Am J Surg Pathol 2005291442–1448. [DOI] [PubMed] [Google Scholar]