Abstract
Background
Poorly differentiated adenocarcinomas of the colon and rectum (Por) feature the worst prognosis among the various types of colorectal carcinomas. Por is highly associated with microsatellite instability (MSI), although MSI is associated with an improved prognosis in colorectal cancers.
Aim
To investigate the influence of MSI on clinicopathological features and survival of patients affected by Por.
Methods
53 patients affected by Por were investigated. DNA extracted from tumour sections and the corresponding normal tissue was analysed by PCR at five microsatellite loci: BAT25, BAT26, D2S123, D5S346 and D17S250. Tumours with alterations at two or more loci were classified as MSI‐Por. The others were classified as microsatellite stability (MSS)‐Por. The clinicopathological features and survival of patients with MSI‐Por and MSS‐Por were investigated.
Results
Of the 53 patients who were examined, 12 (22.6%) were MSI‐Por, whereas 41 (77.4%) were MSS‐Por. Significant differences were found between MSI‐Por and MSS‐Por regarding the following clinicopathological features: age, gender, lymph‐node metastasis (MSI‐Por: 4/12; MSS‐Por: 33/41), TNM stage (MSI‐Por: T1/T2/T3/T4 = 2/6/2/2; MSS‐Por: 3/3/19/16) and lymphatic invasion (MSI‐Por: 4/10; MSS‐Por: 27/35). Kaplan–Meier survival curves and log‐rank analysis showed that MSI‐Por was associated with better prognosis than MSS‐Por, although no significant difference was found.
Conclusions
Compared with MSS‐Por, MSI‐Por is significantly associated with a low incidence of lymph‐node metastases and a low stage. This indicates that MSI‐Por is a less aggressive subtype.
Adenocarcinomas of the colon and rectum are graded predominantly on the basis of the extent of glandular appearances, and are classified into well, moderately and poorly differentiated adenocarcinomas.1 Poorly differentiated adenocarcinomas of the colon and rectum (Por) exhibit glandular structures in 5–50% of the tumour,1 and account for 14–18% of all colorectal cancers.2,3,4 There have been many reports describing the clinicopathological features such as their predominant location in the proximal colon, more extensive invasiveness, greater frequency of advanced‐stage disease and poorer prognosis compared with other colorectal cancers that are specific to Por.2,3,4 However, extensive genetic analysis of Por has been difficult as the number of cases is too small.
Recently, the analysis of molecular genetic features of cancer cells has led to a better knowledge of colorectal carcinogenesis and to the identification of molecular alterations that could be used as markers for clinical evaluation; one of these alterations is microsatellite instability (MSI).5 MSI is a hallmark of hereditary non‐polyposis colorectal cancer and occurs in 10–15% of sporadic colorectal cancer cases.6,7,8,9 Previous studies indicated that colorectal cancers with MSI often show poor differentiation and that Por has a high frequency of MSI,6,7,9,10 although MSI is associated with an improved prognosis in colorectal cancers.6,7,9,11 This discrepancy makes it important to clarify the influence of MSI on clinicopathological features and survival of patients affected by Por, but, to the best of our knowledge, so far no studies have investigated this.
The aim of this study was to investigate the influence of MSI on clinicopathological features and survival of patients affected by Por. For this purpose, we evaluated the MSI status of the patients and classified Por based on their MSI status. We then investigated the clinicopathological features and survival of both groups.
Materials and methods
Patients
We studied 53 patients (29 men and 24 women) who underwent curative surgery for sporadic Por at the Department of Surgical Oncology, Tokyo University Hospital, Tokyo, Japan, between 1964 and 2004. Patients included in this study were considered to have sporadic tumours, because their clinical presentation and family history did not suggest a diagnosis of either familial adenomatous polyposis12 or hereditary non‐polyposis colorectal cancer.13 This study was approved by the Tokyo University Hospital Ethics Committee.
Pathological evaluation
Histological parameters were evaluated on H&E‐stained slides without the knowledge of MSI status. Por were diagnosed according to the World Health Organization (2000) classification when they exhibited glandular structures in 5–50% of the tumour.1 In this study, mucinous adenocarcinomas composed of cells in small irregular clusters were not classified as poorly differentiated, because grading became unreliable when there was excessive mucus and only a few remaining cellular elements. Age, gender, tumour location and distant metastasis were obtained from the pathology report. With regard to tumour location, two anatomical sites were considered: proximal colon (from cecum to transverse colon) and distal colon (from splenic flexure to rectum). Depth of tumour invasion and lymph‐node metastasis were assessed, and tumours were staged according to the TNM classification of malignant tumours, sixth edition (2002).14 The presence of lymphatic and venous invasion was noted.
Tissue samples
We investigated 53 pairs of matched normal colonic mucosa and tumour specimens from Por. Of them, 17 pairs were flash‐frozen in liquid nitrogen and stored at −80°C until analysis. The others were formalin‐fixed, paraffin‐wax‐embedded samples. In the fixed samples, tissues were microdissected as follows: several glands in the neoplastic lesions and the adjacent normal mucosa were carefully dissected microscopically from a 20 μm thick section of the paraffin‐wax‐embedded block, with reference to the adjacent H&E‐stained section. DNA was extracted from each specimen with DNAeasy kit (Qiagen, Tokyo, Japan) after deparaffinisation using sodium dodecyl sulphate and proteinase‐K.
Analysis of MSI
DNA extracted from tumour sections and the corresponding normal tissue was analysed by PCR at five microsatellite loci: BAT25, BAT26, D2S123, D5S346 and D17S250.15 Primer sequences for D5S346, D2S123 and D17S250 have been described previously.16 Primer sequences for other loci were obtained from the Genome Database and the database of the Whitehead Institute. All primer pairs were end‐labelled with fluorochromes FAM, HEX or NED. The PCR was performed in 10 μl reaction volumes containing 10×PCR Gold Buffer (Applied Biosystems, Tokyo, Japan), 2.5 mM MgCl2, 200 μM deoxynucleotide triphosphates mixture, 0.5 μM of each primer, 20–40 ng of extracted DNA and 0.4 units of Amplitaq Gold DNA polymerase (Applied Biosystems). The DNA was amplified in a thermal cycler (GeneAmp PCR system 9700, Perkin Elmer, Waltham, Massachusetts, USA) and PCR was performed according to the following protocol: 10 min at 95°C for polymerase activation; 40 cycles at 94°C for 30 s, 56°C for 1 min and 72°C for 1 min; then an additional 30 min at 70°C. After denaturation by heating at 95°C for 5 min, PCR products were electrophoresed and analysed on an automated sequencer (3100 ABI Genetic Analyzer, Applied Biosystems), and the fluorescent signals from the different‐sized alleles were recorded and analysed using GENESCAN V.3.1 and GENOTYPER V.2.1 software.
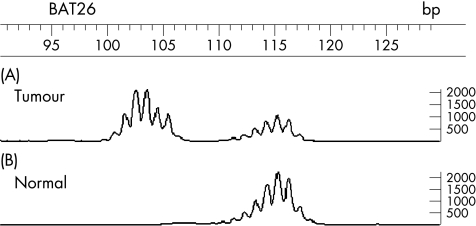
We classified tumours as MSI positive when the PCR product of tumour DNA revealed at least one peak that was not visible in the PCR product of the corresponding normal tissue DNA (fig 1). We used the criteria of the National Cancer Institute workshop to classify MSI and microsatellite stability (MSS) using the five primers that are commonly accepted in estimating MSI status. High‐frequency MSI is determined if two or more of the five markers exhibit instability, and low‐frequency MSI is determined if only one of the five markers does so.
Figure 1 An example of a case showing microsatellite instability at the locus BAT26. (A) The tumour DNA sample and (B) the corresponding normal DNA are shown. There are extra bands in the tumour. The horizontal axis represents base‐pair size.
A previous study indicated that MSS and low‐frequency MSI tumours have a common molecular background,17 so tumours that showed high‐frequency MSI were classified as MSI‐Por and the others were classified as MSS‐Por.
Statistical analysis
Fisher's exact test was used to test the statistical significance of the observed differences between the two groups. The Mann–Whitney U test was used to compare the age distribution. In the survival analysis, only death from colorectal cancers was considered; the starting point for survival time was the date of surgery. Survival curves were calculated by the Kaplan–Meier method, and the statistical significance between curves was tested by the log‐rank test. A value of p<0.05 was considered to be significant. Statistical analysis was performed using SAS V.5.0 software.
Results
Patient characteristics
Of the 53 patients who were examined, 12 (22.6%) were with MSI‐Por and 41 (77.4%) were with MSS‐Por. Table 1 shows the correlations between genomic instability and clinicopathological features. The mean (SD) age of patients with MSI‐Por and MSS‐Por was 67.9 (15.5) years and 65.8 (17.3) years, respectively (p = 0.026). MSI‐Por was found in 3 men and 9 women; MSS‐Por was found in 26 men and 15 women (p = 0.024).
Table 1 Correlation between genomic instability and clinicopathological features.
| MSI‐Por | MSS‐Por | p Value | |
|---|---|---|---|
| Mean (SD) age (years) | 67.9 (15.5) | 65.8 (17.3) | 0.026 |
| Gender | |||
| Male | 3 | 26 | 0.024 |
| Female | 9 | 15 | |
| Location | |||
| Proximal colon | 9 | 20 | 0.18 |
| Distal colon | 3 | 21 | |
| T classification | |||
| T1 | 1 | 2 | 0.98 |
| T2 | 1 | 1 | |
| T3 | 4 | 17 | |
| T4 | 6 | 21 | |
| Lymph‐node metastasis | |||
| + | 4 | 33 | 0.003 |
| – | 8 | 8 | |
| Distant metastasis | |||
| + | 2 | 16 | 0.18 |
| – | 10 | 25 | |
| TNM stage | |||
| I | 2 | 3 | 0.019 |
| II | 6 | 3 | |
| III | 2 | 19 | |
| IV | 2 | 16 | |
| Lymphatic invasion | |||
| + | 4 | 27 | 0.048 |
| – | 6 | 8 | |
| Venous invasion | |||
| + | 5 | 28 | 0.094 |
| – | 5 | 7 | |
| Total | 12 | 41 | |
MSI, microsatellite instability; MSS, microsatellite stability; Por, poorly differentiated adenocarcinomas of the colon and rectum.
Clinicopathological features of MSI‐Por and MSS‐Por
Two cases of MSI‐Por and six cases of MSS‐Por could not be evaluated according to lymphatic and venous invasion. Significant differences were found between MSI‐Por and MSS‐Por regarding the following clinicopathological features: lymph‐node metastasis (p = 0.003), TNM stage (p = 0.019) and lymphatic invasion (p = 0.048). MSS‐Por correlated more strongly with venous invasion than MSI‐Por, although no significant difference was found.
Survival of patients
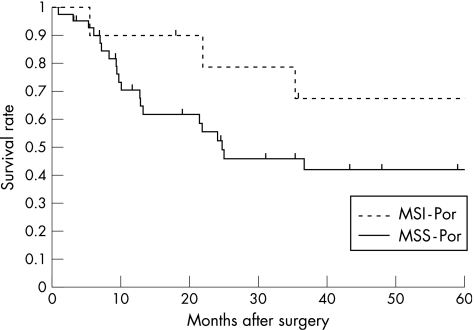
At the end of the study period (December 2005), 24 patients had died from cancers, and 9 patients were lost to follow‐up. At the time of analysis, the median (range) follow‐up time for all patients was 21.8 (1–278) months. Kaplan–Meier survival curves and log‐rank analysis showed that MSI‐Por was associated with better prognosis than MSS‐Por, although no significant difference was found (p = 0.15; fig 2).
Figure 2 Survival curves of 53 patients with poorly differentiated adenocarcinomas of the colon and rectum, in relation to genomic instability (log‐rank test, p = 0.15). MSI, microsatellite instability; MSS, microsatellite stability; Por, poorly differentiated adenocarcinomas of the colon and rectum.
Discussion
Our study is the first to compare clinicopathological features of MSI‐Por with those of MSS‐Por and to show that MSI‐Por is a less aggressive subtype. In addition, our study is one of the largest series in which genetic alterations of Por have been analysed. To the best of our knowledge, there have been no studies that have classified Por based on its MSI status and investigated the clinicopathological features of MSI‐Por and MSS‐Por. Previous studies have indicated that Por is highly associated with MSI.6,7,9,10 MSI is associated with an improved prognosis in colorectal cancers,6,7,9 although Por features the worst prognosis among the various types of colorectal cancers.2,3,4 This discrepancy makes it important to clarify the influence of MSI on clinicopathological features and survival of patients affected by Por.
We showed that MSI‐Por shows less likelihood of lymph‐node metastases and advanced stage than MSS‐Por, which suggests that MSI‐Por is a clinically distinct and less aggressive subtype. These features are similar to those previously described in colorectal cancers with MSI.6 We also showed that lymphatic invasion is less likely with MSI‐Por than with MSS‐Por. This may underline the low frequency of lymph‐node metastases of MSI‐Por.
In our study, Kaplan–Meier survival curves and log‐rank analysis showed that MSI‐Por was associated with better prognosis than MSS‐Por, although no significant difference was found. These features are similar to those previously described in colorectal cancers with MSI.6,7,9 The survival of patients with colorectal cancers strongly correlates with the presence of lymph‐node metastases,5,18 so a further study with a large number of patients may show a significant difference.
Previous studies indicated that fluorouracil‐based adjuvant chemotherapy does not benefit patients with colorectal cancers with high‐frequency MSI tumours,19 but that high‐frequency MSI tumours have high sensitivity to irinotecan,20 and therefore genetic classification may be important in the clinical management of patients with colorectal cancers. Our study and previous studies have shown that Por has a high frequency of MSI (22–40%).6,7,8,9,10 This high frequency makes the genetic classification of Por more important than that of other colorectal cancers, which have a low frequency of MSI (about 10–15%).6,7,8,9
Take‐home messages
Poorly differentiated adenocarcinomas of the colon and rectum (Por) are highly aggressive adenocarcinomas with poor prognosis and show a high frequency of microsatellite instability (MSI), although MSI is associated with an improved prognosis in colorectal cancers.
Compared with Por exhibiting microsatellite stability, Por exhibiting MSI (MSI‐Por) is associated with a low incidence of lymph‐node metastases and a low stage.
This indicates that MSI‐Por is a less aggressive subtype.
MSI status can be determined prior to surgery by PCR using preoperative biopsy samples of matched normal colonic mucosa and tumour. As this study indicated that MSI‐Por has much less likelihood of lymph‐node metastases than MSS‐Por, the prediction of lymph‐node metastases in cases of Por prior to surgery can be improved by MSI analysis using preoperative biopsy samples. Therefore, in cases of Por, preoperative MSI analysis may be of increasing value in decision‐making in the multimodal treatment of patients, such as preoperative chemotherapy and less invasive surgical treatment.
In summary, we showed that MSI status has a large influence on clinicopathological features and survival of patients affected by Por. MSI‐Por shows less likelihood of lymph‐node metastases and advanced stage than MSS‐Por. This indicates that MSI‐Por is a clinically distinct and less aggressive subtype. MSI‐Por had better prognosis than MSS‐Por, although Kaplan–Meier survival curves and log‐rank analysis did not show significant differences. MSI status of Por may be more important than that of other colorectal cancers as Por has a high frequency of MSI.
Abbreviations
MSI - microsatellite instability
MSS - microsatellite stability
Por - poorly differentiated adenocarcinomas of the colon and rectum
Footnotes
Competing interests: None declared.
References
- 1.Hamilton S R, Aaltonen L A.Pathology and genetics; tumors of the digestive system. Lyon: IARC, 2000
- 2.Chung C K, Zaino R J, Stryker J A. Colorectal carcinoma: evaluation of histologic grade and factors influencing prognosis. J Surg Oncol 198221143–148. [DOI] [PubMed] [Google Scholar]
- 3.Umpleby H C, Bristol J B, Rainey J B.et al Survival of 727 patients with single carcinomas of the large bowel. Dis Colon Rectum 198427803–810. [DOI] [PubMed] [Google Scholar]
- 4.Bjerkeset T, Morild I, Mork S.et al Tumor characteristics in colorectal cancer and their relationship to treatment and prognosis. Dis Colon Rectum 198730934–938. [DOI] [PubMed] [Google Scholar]
- 5.Compton C C, Fielding L P, Burgart L J.et al Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000124979–994. [DOI] [PubMed] [Google Scholar]
- 6.Gryfe R, Kim H, Hsieh E T.et al Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 200034269–77. [DOI] [PubMed] [Google Scholar]
- 7.Gafa R, Maestri I, Matteuzzi M.et al Sporadic colorectal adenocarcinomas with high‐frequency microsatellite instability. Cancer 2000892025–2037. [PubMed] [Google Scholar]
- 8.Goel A, Arnold C N, Niedzwiecki D.et al Characterization of sporadic colon cancer by patterns of genomic instability. Cancer Res 2003631608–1614. [PubMed] [Google Scholar]
- 9.Ward R, Meagher A, Tomlinson I.et al Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut 200148821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawabata Y, Tomita N, Monden T.et al Molecular characteristics of poorly differentiated adenocarcinoma and signet‐ring‐cell carcinoma of colorectum. Int J Cancer 19998433–38. [DOI] [PubMed] [Google Scholar]
- 11.Hemminki A, Mecklin J P, Jarvinen H.et al Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology 2000119921–928. [DOI] [PubMed] [Google Scholar]
- 12.Kinzler K W, Vogelstein B. Lessons from hereditary colorectal cancer. Cell 199687159–170. [DOI] [PubMed] [Google Scholar]
- 13.Vasen H F, Watson P, Mecklin J P.et al New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 19991161453–1456. [DOI] [PubMed] [Google Scholar]
- 14.Sobin L H. TNM, sixth edition: new developments in general concepts and rules. Semin Surg Oncol 20032119–22. [DOI] [PubMed] [Google Scholar]
- 15.Boland C R, Thibodeau S N, Hamilton S R.et al A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998585248–5257. [PubMed] [Google Scholar]
- 16.Umetani N, Sasaki S, Watanabe T.et al Diagnostic primer sets for microsatellite instability optimized for a minimal amount of damaged DNA from colorectal tissue samples. Ann Surg Oncol 20007276–280. [DOI] [PubMed] [Google Scholar]
- 17.Laiho P, Launonen V, Lahermo P.et al Low‐level microsatellite instability in most colorectal carcinomas. Cancer Res 2002621166–1170. [PubMed] [Google Scholar]
- 18.Cserni G. Nodal staging of colorectal carcinomas and sentinel nodes. J Clin Pathol 200356327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribic C M, Sargent D J, Moore M J.et al Tumor microsatellite‐instability status as a predictor of benefit from fluorouracil‐based adjuvant chemotherapy for colon cancer. N Engl J Med 2003349247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallik D, Borrini F, Boige V.et al Microsatellite instability is a predictive factor of the tumor response to irinotecan in patients with advanced colorectal cancer. Cancer Res 2003635738–5744. [PubMed] [Google Scholar]