Pure red cell aplasia (PRCA) is a rare haematological syndrome characterised by anaemia, reticulocytopenia and severe erythroid hypoplasia without alteration in megakaryocytic and myeloid maturation. Immune system irregularity can be mediated by the presence of autoantibodies against erythroid cells or against erythropoietin (Epo), or by hyperactivity of large granular lymphocytes with enhanced T cell or natural killer cell cytotoxicity.1,2,3 The association between PRCA and other autoimmune diseases such as autoimmune polyglandular syndrome (APS) I is rare.4
The second autoimmune disease, named APS I or autoimmune polyendocrinopathy‐candidiasis‐ectodermal dystrophy (APECED), is a rare hereditary autosomal recessive disorder. It is characterised by the presence of chronic mucocutaneous candidiasis, multiple endocrinopathies (hypoparathyroidism, adrenocortical failure, hypergonadotropic hypogonadism, type I diabetes mellitus and pan‐hypopituitarism), and various ectodermal manifestations (enamel dysplasia, nail dystrophy, alopecia, vitiligo and keratopathy).5,6 Clinically, APECED can be confirmed by appearances of at least two of the three features: candidiasis, hypoparathyroidism and adrenocortical failure.5,6,7 This is the first multiple autoimmune disorder shown to be caused by mutations of a single gene—the autoimmune regulator gene (AIRE).8 This gene was mapped at 22q22.3 and consisted of 14 exons.8 It is expressed in immune‐related organs, such as thymus, lymph nodes and fetal liver, indicating that it has a pivotal role in the immune function. Its main role is to act as a transcriptional factor. Over 40 different mutations of the AIRE gene have been identified (point mutations, insertions and deletions).8,9
Case report
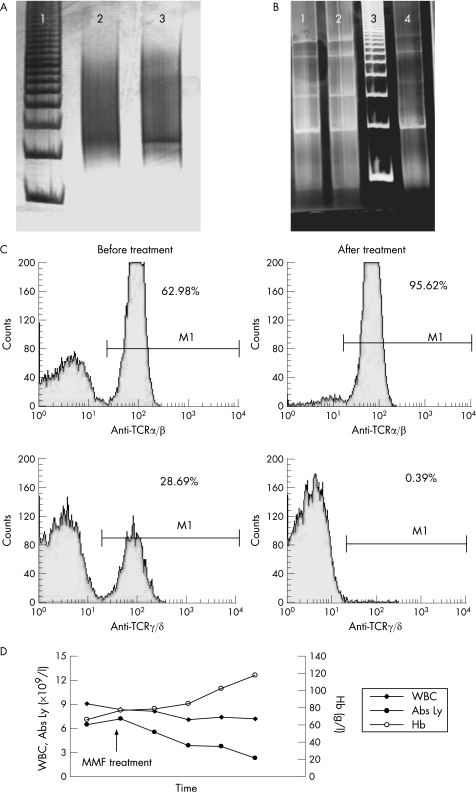
A 33‐year‐old woman was admitted to the Institute of Haematology, Belgrade, Serbia, in April 2001 for blood transfusion for the treatment of anaemia. The diagnosis of idiopathic hypoparathyroidism was made at age 7 years. At age 14 years, she developed idiopathic adrenal insufficiency and 2 years later, she developed mucocutaneous candidiasis. Since early infancy, she had had alopecia universalis. The patient had dysfunctional thyroid nodule, with positive anti‐tireoglobuline and anti‐microsomal antibodies. She had had euthyroid and amenorrhoea for the last 3 years. Her younger sister developed chronic mucocutaneous candidiasis, hypoparathyroidism, adrenal insufficiency, pernicious anaemia and lichen ruber planus. The DNA samples from the patient and her sister were additionally tested for a nonsense mutation in exon 6 (R257X) of the AIRE1 gene. PCR analysis and direct sequencing showed that both sisters were homozygotic for the R257X mutation. This change in arginine resulted in a truncated gene product. Physical examination revealed marked pallor, alopecia and oral candidiasis without lymphadenopathy or hepatosplenomegaly. MRI excluded the presence of thymoma. Laboratory findings revealed a haemoglobin level 60 g/l, red blood cells 1.4×1012/l, haematocrit 13.7%, mean corpuscular volume 115 fl, reticulocytes 0.0%, platelets 395×109/l and white blood cells (WBCs) 11.7×109/l (segmented neutrophils 15% and lymphocytes 85%). Morphologically, lymphocyte populations dominantly consisted of small lymphocytes (43%), partly small T lymphocyte with irregular nucleus (22%) and larger activated lymphocytes (20%). The Coombs test was negative. The increased haemolysis and paroxysmal nocturnal haemoglobinuria were ruled out. The serum Epo level was markedly increased (47.3 mIU/ml) in comparison to reference values (9.9 (2.9) mIU/ml; range 7.0–12.8 mIU/ml). Further studies revealed normal values for serum ferritin, transferin, vitamin B12 and folic acid. Tests for antibodies against human parvovirus B19, cytomegalovirus, HIV, hepatitis B, C, and Epstein–Barr virus were negative. Antinuclear antibody, rheumatoid factor and anti‐DNA antibody were not detectable. Bone marrow aspirate showed hypercellularity (>III), a lack of erythroid precursors (2% of bone marrow nucleated cells), normal granulocyte precursors (75%) and megakaryocytes, 16% of lymphocytes, 4% of plasmocytes and 3% of monocytes. Bone marrow biopsy revealed slight hypercellularity with normal maturation of the myeloid lineage and megakaryocytes, but <1% of the cells were erythroid precursors (including proerythroblasts). There was no increase in the blast count. The karyotype was normal. Immunophenotyping (Flow cytometry, Becton Dickinson, San Jose, USA) performed on peripheral blood cells showed that 91% (8.21×109/l) of all cells were mature T lymphocytes (CD2, CD3, CD5, CD7, CD4 or CD8, T cell receptor (TCR)α/β or TCRγ/δ)+. T cell subsets expressed 62.98% (5.15×109/l) of the TCR α/β+ T cells and 28.69% (3.06×109/l) of TCRγ/δ+ cells. A small subset, 9% (0.89×109/l), of mature phenotype B lymphocytes (CD19, CD20, CD24, SIg, κ or λ)+ was found. There was an inverse relationship between subsets of T lymphocytes (CD4/CD8 = 0.6) with complete absence of natural killer cells (CD16 = 0%). Rearrangements of TCR α/β or TCR γ/δ were examined by the PCR technique. PCR conditions used for the TCRδ region were as follows: denaturation step at 94°C for 10 min, followed by 35 cycles of denaturation at 94°C for 45 s, annealing at 65°C for 30 s and extension at 72°C for 1 min. Nested PCR (two rounds) was used for TCRγ rearrangement analysis. Primers TCRγ1 and TCRγ2 were used for the first PCR round, and primers TCRγ3 and TCRγ4 for the second PCR round (table 1). PCR conditions used for TCRγ region were as follows: denaturation step at 94°C for 10 min, followed by 30 cycles of denaturation at 94°C for 10 s, annealing at 58°C for 20 s and extension at 72°C for 1 min. PCR analysis was followed by electrophoresis on 8% polyacrylamide gel. We found rearranged TCRγ and TCRδ with PCR products (between 450 and 500 bp, respectively), but without IgH rearrangement (fig 1A,B). The bone marrow cell culture performed in standard semisolid methylcellulose medium showed normal numbers of erythroid and granulocytic progenitors. We also found 116 burst‐forming unit‐erythroid (BFU‐E) and 70 colony‐forming unit‐granulocyte macrophage (CFU‐GM) colonies growing spontaneously in comparison to reference values, which were 40–180 for BFU‐E and 38–120 for CFU‐GM, respectively. The number of Epo‐stimulated colonies (Epo‐CFU‐E) was 163 and 181 with 0.5 and 1.0 U of Epo, respectively. These findings indicated that the level of inhibition of Epo was between CFU‐E and proerythroblast. Different dilutions of the patient's serum showed significant inhibition of growth Epo‐stimulated BFU‐E colonies of healthy donors, in a dose‐dependent manner from 13.58% to 100%. Cocultures of separated T lymphocytes from the patient with allogenic bone marrow cells from healthy donors after Epo stimulation showed inhibition of BFU‐E (at 56% from reference values). These in vitro tests showed that both mechanisms (cellular and humoral) were involved in the inhibition of growth of Epo‐stimulated BFU‐E colonies.
Table 1 Primer sequences for PCR analyses.
| TCRδ1: 5′‐GAGTCATGTCAGCCATGAG‐3′ |
| TCRδ2: 5′‐AGGGAAATGGCACTTTTGCC‐3′ |
| TCRγ1: 5′‐CTGTGACAACAAGTGTTGTTCCAC‐3′ |
| TCRγ2: 5′‐GTGCTTCTAGCTTTCCTGTCTC‐3′ |
| TCRγ3: 5′‐GAGTACGCTGCCTACAGAGAGG‐3′ |
| TCRγ4: 5′‐CCACTGCCAAAGAGTTTCTT‐3′ |
TCR, T cell receptor.
Primer TCRδ1 amplifies from the V2 gene, TCRδ2 from the D3 sequences, TCRγ1 from J1/J2, TCRγ2 from V1–V8, TCRγ3 from V1–V8 and TCRγ4 from J1/J2.
Figure 1 (A) PCR analysis of T cell receptor (TCR) γ rearrangement: line 1, marker; line 2, healthy patient; and line 3, patient sample (monoclonal pattern between 450 and 500 bp). (B) PCR analysis of IgH rearrangement. Lines 1 and 2 are healthy controls, line 3 is marker and line 4 is patient (smear). (C) Flow cytometry data for α–β and γ–δ T cell subsets before and after treatment. (D) Clinical course of disease estimated by the determination of total white blood cell (WBC) count, absolute lymphocyte count (Abs Ly) and haemoglobin (Hb) concentration. MMF, mycophenolate‐mofetil.
On account of the patient's severe anaemia and frequent transfusions, treatment was started with prednisolone (40 mg/day) and ciclosporin A (CyA; 5 mg/kg/day) after obtaining informed consent of the patient. Anaemia was partially improved by this treatment, but was followed by a decrease in T lymphocyte count and severe renal dysfunction (acute nephrotoxicity). Therefore, we reduced CyA to 100 mg/d after 3 weeks of initial treatment. During the next 4 months, the patient received treatment along with continuing substitution for APECED (hydrocortisone, Vitamin αD3, fluconasole). Unfortunately, her Hb decreased maximally to 65 g/l. Therefore, we decided to start treatment with mycophenolate‐mofetil (MMF; CellCept, 2 g/day). After a period of 4 months, we observed complete haematological recovery with almost normal value of Hb (119 g/l) and lymphocytes 2.3×109/l (23% of WBC), without nephrotoxicity (fig 1D). After 3 years of immunosuppressive treatment, the patient had normal blood cell count and progression of other autoimmune phenomena—primary ovary insufficiency and exocrine pancreatic insufficiency. Repeated flow cytometric analysis showed prominently (significantly) a decreased number of γ/δ TCR+ lymphocytes. PCR analysis showed that these cells had monoclonality for TCRγ/δ. Additional double‐stained cells analysed by flow cytometry after treatment showed a significant decrease (1.25%) in the CD4+CD25+ T regulatory cells (T reg). The other cell subsets were 76.10% for CD38+CD3+ cells and 70.68% for human leucocyte antigen (HLA)‐DR+CD3+ cells. After treatment with MMF, we analysed the sensitivity of peripheral blood lymphocytes (PBLs) to apoptosis using annexin‐V and propidium iodide, flow cytometry as well as TUNEL (terminal deoxynucleotidyl transferase‐mediated dUTP‐biotin nick end‐labelling) assay (Roche, In Situ Cell Death Detection Kit, AP, Roche, Basel, Switzerland). Results showed significantly higher rates of apoptosis and late necrosis of patient's lymphocytes cultured for 24 h in the presence of different sera (55.74%, 55.07% and 47.76% for autologous, heterologous and fetal calf serum, respectively) in comparison with healthy controls (23.82%, 23.04% and 29.05%, respectively; p<0.05, Mann–Whitney U test). We obtained identical results by two different assays showing that total number of PBLs of patients have a high rate of apoptosis and necrosis (87.5%). To address the question about the high rate of apoptosis after treatment, we found that PBLs expressed a high level of FAS (CD95) antigen (60%) detected by immunocytochemical analysis using monoclonal antibody anti‐CD95 (Dako, Glostrup, Denmark).
Discussion
Acquired PRCA is mostly considered to be of an autoimmune aetiology because of antibody‐mediated or T cell‐mediated inhibition of erythropoiesis.4 Involvement of the immune system in the pathogenesis of PRCA has been documented previously. Here, we reported that T cells mediated some immune phenomenon in PRCA associated with APECED based on the predominant expression of T cell subsets (23.60%) with γδ+ TCR rearrangements. Further analyses showed that these lymphocytes had clonal molecular abnormality confirmed by DNA analysis. The TCRαβ+ lymphocyte subset was also significantly expanded (60.98%) in peripheral blood but without monoclonality. Some reports indicated that most cytotoxic γ/δ T cells express killer‐cell inhibitory receptors, suggesting that they survey the body for missing self HLA‐class I alleles.10 It might be that γ/δ T cells can destroy erythroid progenitors in vivo by downregulation of HLA class I antigens in the erythroid lineage.
The clinical course in this patient also showed a strong correlation between the decreased number of total lymphocyte count and recovery from PRCA. This observation suggested that the γδ TCR+ cell subset that is not MHC restricted can be functional in vivo and be involved in the pathogenesis of PRCA.4 They can exert a suppressive effect on erythropoiesis mainly through a soluble product rather than by direct cell‐to‐cell interaction. In our patient with PRCA, selective suppression of erythropoiesis by both T cells and cell supernatants has been demonstrated.
We also showed that after oral immunosuppressive treatment with the new drug, MMF, depletion of T cells was achieved by induction of apoptosis. MMF is a new immunosuppressive drug, primarily used for renal transplantation,11 which inhibits de novo purine synthesis. MMF as a prodrug of mycophenolic acid (MPA) is an inhibitor of inositol monophosphate dehydrogenase. MMF and MPA have a more potent cytostatic effect on T and B lymphocytes than on other cell subsets. We, like others, showed that their effects can be mediated by the induction of apoptosis in activated T lymphocytes, and by elimination of reactive cell clones.12,13 MPA can suppress glycosylation and decrease expression of adhesion molecules by depleting guanosine nucleotides; thus, MPA decreases the activity of tetrahydrobiopterin, a cofactor for the inducible form of nitric oxide synthase, and suppresses primary, but not secondary, antibody responses. MMF does not inhibit early TCR‐mediated activation events, such as expression of CD25 and synthesis of interleukin2.13
Also, MMF can induce apoptosis of activated T lymphocytes, which may eliminate clones of cells responding to antigenic stimulation.13 In patients with autoimmune lymphoproliferative syndrome, apoptosis of T lymphocytes is achieved through activation of mutating FAS (CD95) receptor.14
The rate of apoptosis induced by MMF in our investigation is similar to other data showing that therapeutic levels of MMF, ranging in concentrations from 3 to 7 μg/ml, increased apoptosis rates from 56% to 67%.15 In our patients with PRCA and APSI, we found a significantly decreased T reg lymphocyte population (1.25%). This cell population has an important role in immune tolerance to self‐antigens as well as in homeostasis of the immune system.16 Interestingly, in patients with autoimmune thyroiditis, the level of T reg lymphocytes is decreased to 5.3% in comparison with healthy controls who have a level of about 16%.16 Diverse regulatory T cell subsets exist in the peripheral blood of patients with autoimmune diseases. Some of them have disturbed suppressive function, whereas other lymphocytes, although showing regularity, were without functional effects, probably owing to resistance of the appropriated target cells. Another interesting possibility is that once the immune tolerance is broken, and the inflammatory destructive phenomenon is ongoing, the activity of effector T cells would overcome the suppressive effect of T reg cells.
Engelan et al11 and Arbeiter et al17 mentioned a few cases of red blood cell aplasia after using MMF for patients who had undergone renal transplantation. MMF treatment in our patient induced complete recovery of PRCA. Furthermore, this drug did not alter the course of at least one of the endocrinopathies, as progression to primary ovarian failure and exocrine pancreatic insufficiency ensued over the 3 years of treatment. These data are in agreement with the results of Ward et al.9 They treated the patient having APECED with oral CyA, and succeeded in treating gastrointestinal dysfunction, alopecia universalis and keratoconjunctivitis, and the progression of primary ovarian and adrenal failure.
The present case of a young woman, with associated autoimmune disorders (acquired PRCA and APECED homozygotic for the common R257X mutation of AIRE gene), showed excellent response of anaemia to MMF treatment, without toxicity and with complete haematological recovery, but unfortunately with progression of endocrinopathy.
This paper showed the significant role of γδTCR+ cells in PRCA, with a possibility that these cells were involved in pathogenesis of the disease. A significant decrease in the γδTCR+ cell count after 2 years of MMF treatment was associated with normal haematological findings, but without elimination of their monoclonal pattern.
Acknowledgements
This study was supported in part by grant 145061 from the Republic Ministry of Science of Serbia.
Footnotes
Competing interests: None declared.
References
- 1.Handgretinger R, Geiselhart A, Moris A.et al Pure red cell aplasia associated with clonal expansion of granular lymphocytes expressing killer‐cell inhibitory receptors. N Engl J Med 1999340278–284. [DOI] [PubMed] [Google Scholar]
- 2.Charles R J, Sabo K M, Kidd P G.et al The pathophysiology of pure red cell aplasia: implications for therapy. Blood 1996874831–4838. [PubMed] [Google Scholar]
- 3.Casadevall N, Dupry E, Mohlo‐Sabatier P.et al Autoantibodies against erythropoetin in a patient with pure red cell aplasia. N Engl J Med 1996334630–633. [DOI] [PubMed] [Google Scholar]
- 4.Hara T, Mizuno Y, Nagata M.et al Human γδ T‐cell receptor positive cell‐mediated inhibition of erythropoesis in vitro in a patient with type I autoimmune polyglandular syndrome and pure red cell aplasia. Blood 199075941–950. [PubMed] [Google Scholar]
- 5.Ahonen P, Myllarniemi S, Sipila I.et al Clinical variation of autoimmune polyendocrinopathy‐candidiasis‐ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med 19903221829–1836. [DOI] [PubMed] [Google Scholar]
- 6.Betterle C, Greggio N A, Volpato M. Autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab 1998831049–1055. [DOI] [PubMed] [Google Scholar]
- 7.Myhre A G, Halonen M, Eskelin P.et al Autoimmune polyendocrine syndrome type 1 (APS I) in Norway. Clin Endocrinol 200154211–217. [DOI] [PubMed] [Google Scholar]
- 8.Nagamine K, Peterson P, Scott H S.et al Positional cloning of the APECED gene. Nat Genet 199717393–397. [DOI] [PubMed] [Google Scholar]
- 9.Ward L, Paquette J, Seidman E.et al Severe autoimmune polyendocrinopathy‐candidiasis‐ectodermal dystrophy in an adolescent girl with a novel AIRE mutation: response to immunosuppressive therapy. J Clin Endocrinol Metab 199984844–852. [DOI] [PubMed] [Google Scholar]
- 10.Phillips J H, Gumperz J E, Partham P.et al Superantigen‐dependent, cell mediated cytotoxicity inhibited by MHC class I receptors on T lymphocytes. Science 1995268403–405. [DOI] [PubMed] [Google Scholar]
- 11.Engelen W, Verpooten G A, Van der Planken M.et al Four cases of red blood cell aplasia in association with the use of mycophenolate mofetil in renal transplant patients. Clin Nephrol 200360119–124. [DOI] [PubMed] [Google Scholar]
- 12.Allison A C, Eugui E M. Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation 200580181–190. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura M, Ogawa N, Shalabi A.et al Positive effect on T‐cell regulatory apoptosis by mycophenolate mofetil. Clin Transplant 20011536–40. [DOI] [PubMed] [Google Scholar]
- 14.Rao V K, Dugan F, Dale J K.et al Use of mycophenolate mofetil for chronic, refractory immune cytopenias in children with autoimmune lymphoproliferative syndrome. Br J Haematol 2005129534–538. [DOI] [PubMed] [Google Scholar]
- 15.Andrikos E, Yavuz A, Bordoni V.et al Effect of cyclosporine, mycophenolate mofetil, and their combination with steroids on apoptosis in a human cultured monocytic U937 cell line. Transplan Proc 2005373226–3229. [DOI] [PubMed] [Google Scholar]
- 16.Marazuela M, Garcia‐Lopez M A, Fugueroa‐Vega N.et al Regulatory T cells in human autoimmune disease. J Clin Endocrinol Metab 2006913639–3646. [DOI] [PubMed] [Google Scholar]
- 17.Arbeiter K, Greenbaum L, Balzar E.et al Reproducible erythroid aplasia caused by mycophenolate‐mofetil. Pediatr Nephrol 200014195–197. [DOI] [PubMed] [Google Scholar]