Abstract
Background
Involvement of the lateral resection margin (LRM) has been shown to be a reliable predictor of local recurrence of rectal cancer. Accurate determination of the LRM status is crucial in selecting patients for postoperative radiotherapy. However, variability in processing factors may affect the measurement of the LRM.
Aim
To investigate how formalin fixation and laboratory processing affects the measurement of the LRM.
Methods
For this study, rectal cancer specimens (n = 9) were fixed in formalin for 4 days and then sectioned transversally, and one half of the specimen was sent for processing. The effacing tumours were placed back in formalin for another 3 days. At day 7, the effacing tumour block (mirror image) was sent for processing. The longest and the shortest perpendicular resection margins for each of the day 4 and day 7 specimens were measured. In a second experiment, control tissue (colon; n = 40), length 10 (0.05) mm, was also processed from a normal sigmoid colon.
Specimens were retained in formalin for 24 h (n = 12), 48 h (n = 12), 72 h (n = 9) and 96 h (n = 7). The degree of tissue shrinkage was then recorded. Variations in the recorded LRM and length of colonic tissue are presented as a median (interquartile range) and data were compared using analysis of variance.
Results
In the cases of rectal cancer, the variation in measured LRM between day 4 and day 7 specimens was 3.2 (1.5–5) mm. In 30 of the 37 comparisons, the day 7 LRM increased in length, whereas in the remaining 7 it decreased. In the second experiment, control tissue of the original length 10 (0.05) mm increased in length to 10.9 (8.9–13.0) mm, p<0.01.
Conclusion
These results suggest that the fixation period/laboratory processes result in measurable differences in the reported LRM. This degree of variation has implications for the reliable reporting of the LRM, predicting local recurrence rates and planning subsequent adjuvant radiotherapy.
Achievement of local clearance during rectal cancer surgery improves local disease control and may also impact on long‐term disease‐free survival. Accurate determination of the resection margin status facilitates the selection of patients for postoperative radiotherapy.1,2,3,4,5
Resection specimens are fixed routinely in formalin (formaldehyde) immediately after surgery for subsequent histological examination. The fixation process allows formalin to penetrate through the entire tissue to preserve its architectural integrity. However, the period of fixation in this solution is subject to variation depending on the day of the week that surgery is undertaken and availability of individual pathologists or technical staff.
Although the effect of formalin fixation on longitudinal shrinkage (30%) has been demonstrated previously,6,7 the processing variability of the lateral resection margin (LRM) has not been addressed.
Accurate, reproducible reporting of the LRM forms the basis for predicting local recurrence and referral for postoperative radiotherapy in cases in which the margin is ⩽1 mm, as these would be considered to be involved with the tumour.2,3
The aim of this study was to examine what effect the period of formalin fixation has on reporting of the LRM. We also looked at its effect on the longitudinal measurements of the control colon.
Methods
The study was composed of two parts.
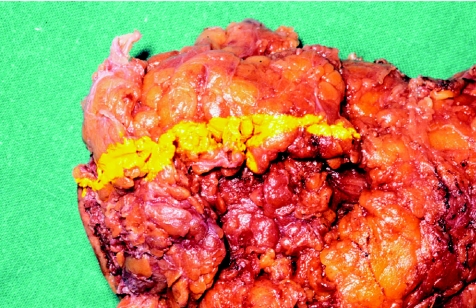
In the first part of the study, rectal cancer specimens (n = 9) were fixed, after resection, in 10% neutral buffered formalin for 4 days. The tumours were then removed from formalin, and linear tattoos were placed on the lateral margins of the resected specimens using indelible ink (n = 1, 2 or 3 sites; fig 1). The specimen was then sectioned transversally across the marked area. Blocks were taken from the marked area, tattooed and sent for processing, labelled as day 4 specimens (fig 2).
Figure 1 Linear tattoos on lateral margins of the resected tumour specimen.
Figure 2 Transversally sectioned specimen.
The effacing tumour mass was then placed back in formalin for a further 3 days.
At day 7, mirror‐image blocks were taken from adjacent areas (identical macroscopically) and processed and labelled as day 7 specimens.
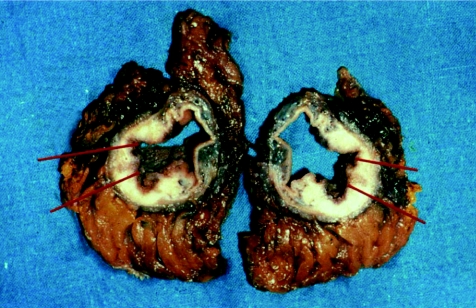
The specimens from day 4 and day 7 were then studied histologically, and the longest and the shortest perpendicular resection margins for each of the day 4 specimens were measured to 0.1 mm and compared with its day 7 effacing mirror image (fig 3). A total of 37 specimen pairs were used.
Figure 3 Resection margins for each of the day 4 specimens.
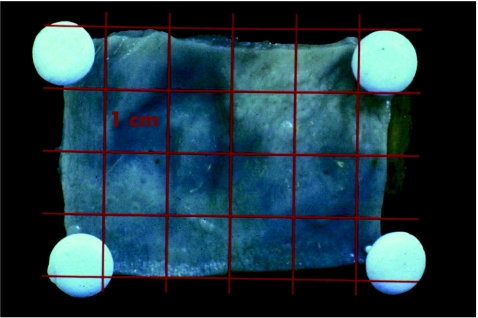
In the second part of the study, fresh control tissue obtained from the normal sigmoid colon of 4 patients was sectioned into 40 specimens with lengths of 10 (0.5) mm (fig 4).
Figure 4 Fresh control tissue obtained from the normal sigmoid colon sectioned into specimens of 10 mm.
Specimens were retained in formalin for 24 h (n = 12), 48 h (n = 12), 72 h (n = 9) and 96 h (n = 7). The specimens were then measured to 0.1 mm.
The variation in measurements of length in both experiments was calculated (median (interquartile range) and results were compared using analysis of variance.
Results
In cases of rectal cancer, the variation between the day 4 and day 7 specimens was 3.2 (1.5–5) mm.
In 30 of 37 comparisons, the day 7 LRM increased in length, whereas in the remaining 7 it decreased.
In the second experiment, control normal sigmoid colon, of original length 10 (0.5) mm, increased in length to 10.9 (8.9–13.0) mm, p value <0.01. There was no difference in measured length between the four time periods.
Discussion
Failure to clear the LRM during rectal cancer surgery will increase the likelihood of local recurrence and may affect long‐term survival. Accurate assessment of lateral margin involvement is important in tailoring postoperative adjuvant chemoradiotherapy.6
Our results suggest that the fixation period/laboratory processing time results in a measurable difference in the reported LRM.
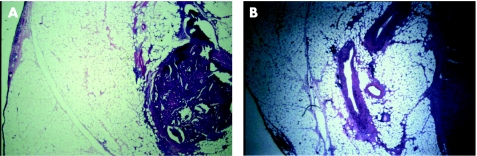
If we accept a processing variability of 10%, only 8 of the 37 specimens met this standard. Figure 5A and B shows an example of an effacing pair of segments used in the study from day 4 and its “mirror image” from day 7, respectively.
Figure 5 Effacing pair of segments used and its mirror image.
As can be seen, there is a significant difference that explains the difficulty in comparing areas of tissue, which are essentially microns apart.
These variations demonstrated in our study have implications for the reliable reporting of LRM, predicting local recurrence and planning subsequent adjuvant treatment. In addition, although macroscopic shrinkage of control tissue has been reported previously, changes that can occur during the histological processing of tissue have not been well documented. During the fixation period, formalin dehydrates the specimen and causes shrinkage. Rehydration and therefore tissue expansion occurs during the dissolving of supporting blocks in preparation for mounting on a slide. The degree to which this affects observed measurements has not been reported before.
Although the preparation of rectal cancer specimens by pathologists has now been standardised, laboratory procedures within and between departments may vary.1,4
Our study demonstrated that variations in fixation period/laboratory processing time results in significant measurable differences in reporting LRM and has the potential to under/overstage some tumours. This has potentially serious implications on the tailoring of postoperative adjuvant treatment and rates of local recurrence.
Footnotes
Competing interests: None declared.
References
- 1.Quirk P, Durdey P, Dixon M F.et al Local recurrence of rectal adenocarcinoma due to inadequate surgical resection —a histopathological study of lateral tumour spread and surgical excision. Lancet 19861996–999. [DOI] [PubMed] [Google Scholar]
- 2.Adam I J, Mohamdee M O, Martin I G.et al The role of circumferential resection margin involvement in the local recurrence of rectal cancer. Lancet 1994344707–711. [DOI] [PubMed] [Google Scholar]
- 3.De Haas‐Kok D F M, Baeton C G M, Jager J J.et al Prognostic significance of radial margins of clearance in rectal cancer. Br J Surg 199683781–785. [DOI] [PubMed] [Google Scholar]
- 4.Scott N, Jackson P, al‐Jaberi T.et al Total mesorectal excision and local recurrence: a study of tumour spread in the mesorectum distal to rectal cancer. Br J Surg 1995821031–1033. [DOI] [PubMed] [Google Scholar]
- 5.Wibe P R, Rendedal E, Svensson J.et al Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. B J Surg 200289327–334. [DOI] [PubMed] [Google Scholar]
- 6.Kwok S P Y, Lau W Y, Leung K L.et al Prospective analysis of the distal margin of clearance in anterior resection for rectal cancer. Br J Surg 199683969–972. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein N S, Soman A, Sacksner J. Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements. The effects of surgical resection and formalin fixation on organ shrinkage. Am J Clin Pathol 1999111349–351. [DOI] [PubMed] [Google Scholar]