Abstract
Background
A role for CXCR3, the receptor for chemokines Mig, IP‐10 and interferon‐inducible T cell α‐chemoattractant, in tumour cell migration during melanoma progression has been proposed.
Aims
To analyse CXCR3 expression in primary cutaneous malignant melanomas and its comparison with clinicopathological and prognostic factors.
Methods
A retrospective immunohistochemical study was carried out on formalin‐fixed paraffin‐wax‐embedded sections from 82 patients with primary invasive cutaneous melanomas, with a monoclonal antibody to CXCR3 (clone 49801.111; R&D Systems). Immunoreactivity was semiquantitatively evaluated: labelling intensity (0, absent; 1, weak; 2, moderate; 3, strong) multiplied by the percentage of cells in each of the four intensity categories. A positive staining was considered when the score was >100. Melanomas were categorised by age, sex, primary site, tumour thickness, growth phase, ulceration, lymphocytic infiltration, recurrence, lymph node and distant metastasis, and survival. Univariate and multivariate statistical analyses were carried out.
Results
Of the 82 patients, a positive CXCR3 staining was found in 26 (31.7%) patients, whereas 56 (68.3%) were negative. In univariate analysis, a significant association of CXCR3‐positive tumour cell immunostaining with tumour thickness >1 mm (p = 0.003), absence of lymphocytic infiltration (p = 0.04) and the presence of distant metastasis (p = 0.048) was found. Multivariate analysis found tumour thickness as the only independent factor with considerable association with distant metastases.
Conclusions
Our findings of a positive correlation of CXCR3 tumour cell immunoreactivity in human primary cutaneous melanoma with tumour thickness >1 mm and absence of intratumoral lymphocytic infiltration support the biological implication of CXCR3 in the tumour progression of cutaneous malignant melanoma.
The incidence of cutaneous malignant melanoma is increasing worldwide.1,2 The vertical growth phase of malignant melanoma is associated with a remarkable metastatic potential, which in turn is responsible for a high mortality rate. In this regard, 90% of patients with localised cutaneous melanoma stage I and II can be cured, whereas the mean survival of patients with loco‐regional extension (stage III) and distant metastasis (stage IV) is 2 years and 6 months, respectively.3
Although the overall increased incidence of malignant melanoma is mostly because of the increase in thin (⩽1 mm) tumours, melanoma‐related mortality seems to have stabilised.4 Recently, a few studies have detected a slight reduction in mortality in women and young men. It has been hypothesised that most of the thin melanomas, which are currently being detected in increasing numbers are devoid of metastatic potential, whereas the incidence of thick (⩾1 mm) potentially metastatic tumours remains essentially unaltered.4 Before testing this hypothesis, new molecular markers are needed, which with clinical and biological data, will serve to distinguish melanomas with and without metastatic potential.
A role for chemokines and their corresponding receptors in metastatic neoplastic dissemination has recently been proposed.5,6,7,8,9 In particular, the participation of CXCR3, the receptor for chemokines Mig, IP‐10 and interferon‐inducible T cell α‐chemoattractant in tumour cell migration during melanoma progression has been suggested. Furthermore, CXCR3 immunoreactivity has also been found in melanoma cell lines as well as primary and metastatic tumour cells.8,10
Our goal was to analyse CXCR3 expression in primary cutaneous malignant melanomas and its correlation with other clinicopathological and prognostic factors, with particular emphasis on the comparison of CXCR3 immunodetection in T1 tumours (Breslow ⩽1 mm) with that of T2–T4 tumours (Breslow >1 mm).
Materials and methods
A retrospective immunohistochemical study was carried out on formalin‐fixed paraffin‐wax‐embedded tissue sections from 82 patients with primary invasive cutaneous melanomas having a minimum follow‐up of 5 years. Approval from the Ethics Committee of the Hospital Clinico Universitario, Valencia, Spain and informed consent from every biopsied patient were obtained. A monoclonal antibody to CXCR3 (clone 49 801.111, R&D Systems, Minneapolis, Minnesota, USA) and the avidin–biotin complex immunoperoxidase technique with autoclave antigen retrieval (10 min at 1.5 atm in citrate buffer; pH 6) following previously reported protocols11,12 were used. The primary antibody (15 µg/ml) was applied for 30 min in a humidified chamber at 37°C. Slides were counterstained with Giemsa, which stains melanin green, to avoid potential misinterpretation as specific immunostaining of the melanin present in the cytoplasm of a variable number of tumour cells. Tumour cell immunoreactivity was semiquantitatively evaluated13 as follows: labelling intensity (0, absent; 1, weak; 2, moderate; 3, strong) multiplied by the percentage of tumour cells in each of the four intensity categories. A positive staining was considered when the final score was ⩾100. Immunostaining of a subset of perivascular lymphocytes and mast cells served as an internal positive control. The primary antibody was replaced with phosphate‐buffered saline in adjacent tissue sections as a negative control.
Melanomas were categorised by age, sex, primary site (extremities, trunk, head and neck), tumour thickness (⩾1, 1–4, >4 mm), growth phase (radial, vertical), ulceration, lymphocytic infiltration (dense, discontinuous or patchy, absent);14 local recurrence, lymph node metastasis, distant metastasis and survival.
Two‐tailed univariate statistical analysis was carried out using the Mann–Whitney U test and Kruskal–Wallis non‐parametric tests, for two or more independent variables respectively, and the χ2 test for linear trend for variables with natural ordering. A p value <0.05 was considered to be significant. Logistic regression was used for multivariate analysis to determine the effect of several risk factors on the development of distant metastasis. The risk factors were dichotomous (sex, ulceration, growth phase, local recurrence, tumour thickness), three‐level (primary site and lymphocytic infiltration) or continuous (age). The odds ratio (OR) was estimated with the coefficient exp(b); 95% confidence intervals (95% CIs) of ORs were also determined. The forward step (likelihood ratio) test was used to select the most parsimonious model among all risk factors.
Results
Of the 82 patients, 52.4% were women and 47.6% were men. The mean age of the patients was 54 years, ranging from 20 to 85 years. Clinical follow‐up ranged from 60 months (or the last available information on vital status before death) to 122 months (mean 77 months).
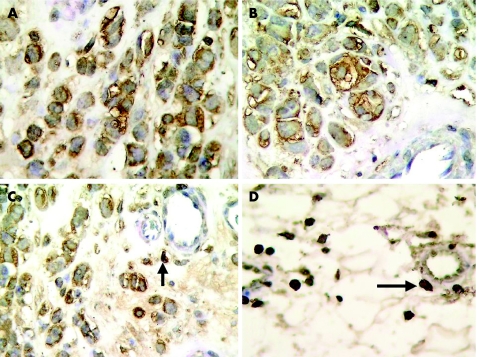
A positive CXCR3 immunostaining was found in 26 (31.7%) patients (fig 1 A, B), whereas 56 (68.3%) were negative. Immunoreactivity of isolated perivascular mononuclear inflammatory cells (fig 1 C, D) served as internal positive controls. Table 1 summarises the results.
Figure 1 CXCR3 immunostaining in invasive melanoma cells (A–C). Immunoreactivity of perivascular mononuclear inflammatory cells serves as internal positive control (C–D, arrows;) (immunoperoxidase, Giemsa counterstain, ×400).
Table 1 Correlation between CXCR3 immunoreactivity and clinicopathological parameters.
| Variable | No of patients (%) | p Value | |
|---|---|---|---|
| CXCR3− | CXCR3+ | ||
| Tumour thickness (mm) | 0.003 (MW)(⩽1, >1) | ||
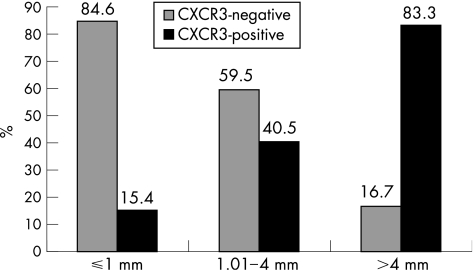
| ⩽1 | 33 (84.6) | 6 (15.4) | 0.015 (MW)(⩽1, (1.01–4)) |
| 1.01–4 | 22 (59.5) | 15 (40.5) | 0.001 (MW)(⩽1, >4) |
| >4 | 1 (16.7) | 5 (83.3) | |
| Ulceration | 0.448 (MW) | ||
| No | 47 (70.1) | 20 (29.9) | |
| Yes | 9 (60) | 6 (40) | |
| Growth phase | 0.096 (MW) | ||
| Radial | 26 (78.8) | 7 (21.2) | |
| Vertical | 30 (61.2) | 19 (38.8) | |
| Recurrence | 0.425 (MW) | ||
| No | 49 (70) | 21 (30) | |
| Yes | 7 (58.3) | 5 (41.7) | |
| Lymph node metastasis | 0.206 (MW) | ||
| No | 44 (72.1) | 17 (27.9) | |
| Yes | 12 (57.1) | 9 (42.9) | |
| Distant metastasis | 0.048 (MW) | ||
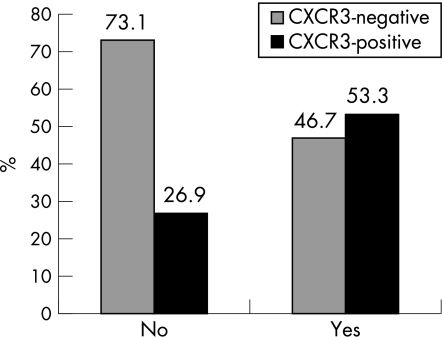
| No | 49 (73.1) | 18 (26.9) | |
| Yes | 7 (46.7) | 8 (53.3) | |
| 5‐year survival | 0.295 (MW) | ||
| Yes | 50 (70.4) | 21 (29.6) | |
| No | 6 (54.5) | 5 (45.5) | |
| Sex | 0.765 (MW) | ||
| Female | 30 (69.8) | 13 (30.2) | |
| Male | 26 (66.7) | 13 (33.3) | |
| Location | 0.533 (KW) | ||
| Extremities | 21 (61.8) | 13 (38.2) | |
| Trunk | 25 (71.4) | 10 (28.6) | |
| Head or neck | 10 (76.9) | 3 (23.1) | |
| Lymphocytic infiltration | 0.040 (Trend χ2) | ||
| Dense | 18 (81.8) | 4 (18.2) | |
| Ciscontinuous | 27 (69.2) | 12 (30.8) | |
| Absent | 11 (52.4) | 10 (47.6) | |
MW, Mann–Whitney U test; KW, Kruskal–Wallis test. Trend χ2: χ2 test for linear trend.
Cases with a tumour thickness of 1 mm could be differentiated from the remaining cases with respect to the variable CXCR3 immunostaining. CXCR3 and immunoreactivity showed a positive correlation with increased tumour thickness. The Mann–Whitney U test showed a significant association of CXCR3‐positive staining with tumour thickness >1 mm (p = 0.003). The highest percentage of CXCR3‐positive tumours was found in the group of T4 melanomas—that is, those thicker than 4 mm (p = 0.001; table 1 and fig 2). In addition, a linear trend was found between CXCR3 positive staining of tumour cells and absent intratumoral lymphocytic infiltration (p = 0.04, χ2 trend).
Figure 2 Correlation between CXCR3 immunoreactivity and tumour thickness (percentage of cases).
The presence or absence of distant metastasis was significantly associated in univariate analysis with CXCR3 immunostaining. CXCR3‐negative tumours were found in 73.1% of patients without distant metastasis. By contrast, 53.3% of patients with distant metastases had CXCR3‐positive tumours (p = 0.048; fig 3). In multivariate analysis, the final model found tumour thickness as the only risk factor with independent significant prognostic value for the development of distant metastases. Estimated OR was 8017 (1677–38 327; p = 0.009). The predictive ability of CXCR3 positive immunoreactivity for the development of distant metastasis was near the significance level: OR of 3.1 (95% CI 0.9 to 9.8; p = 0.053); no significant predictive value for overall survival was found: OR of 1.98 (95% CI 0.54 to 7.22; p = 0.298).
Figure 3 Correlation between CXCR3 immunoreactivity and distant metastasis (percentage of cases).
Discussion
Chemokines are a family of low‐molecular‐weight cytokines, which provoke cellular migratory responses. They have been classified into four groups: CXC, CC, C and CX3C. The CXC family includes among others interleukin 8, which is recognised by CXCR1 and CXCR2 receptors; CXCL9 (Mig), CXCL10 (IP‐10) and CXCL11 (I‐TAC), all of which bind to the CXCR3 receptor, and CXCL12 (SDF‐1) whose receptor is CXCR4. Chemokine‐receptor binding on the cell surface leads to a complex series of cell responses with molecular and cell signal transduction activation, cytoskeletal, particularly actin, reorganisation and pseudopod formation. GTPases of the Rho family are involved in this process.6,8 These cellular changes permit cellular locomotion, an essential part of the metastatic cascade.8
Although the participation of chemokines in the immune response of the host against cancer cells has also been suggested,9 it is currently widely accepted that cytokines and chemokines in human tumours most probably contribute to tumour cell growth, neoplastic progression and immunosupression than to induce an effective antitumour response.15 Neutralisation of some chemokines and their receptors such as CXCL12 and CXCR4 in immunodeficient mice with breast cancer significantly reduces the development of lymph node and lung metastases.6 On the other hand, complementary effects of CXC chemokines, such as interleukin‐8, and MMP‐2 and MMP‐9 metalloproteinases, have been reported.16
Muller et al6 found CXCR4 over expression in melanoma cell lines. Since CXCR4 ligand (CXCL12) is preferentially detected in those organs (lymph nodes, lung liver, bone marrow), which are targets of the metastatic dissemination of malignant melanoma, as well as many other cancer types, these authors proposed a role for CXCR4 and other chemokines in the selective distribution of metastatic deposits in these patients.6 In support of this hypothesis, Robledo et al8 have detected CXCR3 and CXCR4 expression in subcutaneous and lymph node metastases of human malignant melanoma, as well as in melanoma cell lines, and showed that both receptors facilitate tumour cell adhesion and migration by interactions with their corresponding chemokine ligands. The same group has recently reported a study on CXCR3 immunoreactivity in 40 cases of human melanomas and found no significant association with poor prognosis.10 By contrast, Kawada and et al17 have showed that CXCR3 has an outstanding role in experimental B16F10 melanoma cell metastasis to lymph nodes in mice, and that a high level expression of CXCL9 and CXCL10 in lymph nodes may facilitate this process.
Our findings of a positive correlation of CXCR3 immunostaining with tumour thickness support the implication of this chemokine receptor in tissue invasion and metastasis of malignant melanoma as has been previously suggested. In addition, the fact that CXCR3 tumour cell immunoreactivity is found more often in tumours with no significant lymphocytic infiltration is also of interest given the poor prognostic implications of the absence of tumour infiltrating lymphocytes in primary cutaneous melanoma.14 The association of CXCR3 tumour cell immunostaining with the presence of distant metastasis found in univariate analysis did not retain this value in multivariate analysis, where only tumour thickness remained significant. Therefore, the association of CXCR3 immunoreactivity with the development of distant metastasis is dependant on its strong association with tumour thickness. Nevertheless, the predictive value of CXCR3 positive immunostaining for distant metastasis is near the significance level, which indicates that it may have some influence on the development of distant metastasis.
In conclusion, our findings of an association of CXCR3 tumour cell immunostaining in cutaneous melanoma with two well known clinicopathological prognostic factors, tumour thickness and absent lymphocytic infiltration, support the biological implication of this chemokine receptor in melanoma progression. CXCR3 immunostaining, however, does not contribute additional independent prognostic information to tumour thickness, a parameter that is easily and routinely evaluated. Therefore, assessment of CXCR3 immunoreactivity should be considered of biological rather than practical diagnostic value in primary cutaneous malignant melanoma.
Acknowledgements
Supported with grants BM‐019/2002 (to CM), from the Consellería de Sanidad, Generalidad Valenciana, and FIS 2003‐PI30512 (to CM) from Fondo de Investigación Sanitaria, Spain.
Footnotes
Competing interests: None.
Ethical approval: Approval from the Ethics Committee of the Hospital Clinico Universitario, Valencia, and informed consent from every patient who underwent biopsy were obtained.
References
- 1.Lens M B, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol 2004150179–185. [DOI] [PubMed] [Google Scholar]
- 2.Kim C H, Reintgen D S, Balch C M. The new melanoma staging system. Cancer Control 200299–15. [DOI] [PubMed] [Google Scholar]
- 3.Balch C M, Reintgen D S, Kirkwood S M.et al Cutaneous melanoma. In: De Vita VT, Hellman S, Rosenberg SA, eds. Cancer: principles and practice of oncology Philadelphia: Lippincott, 19971935–1993.
- 4.Lipsker D M, Hedelin G, Heid E.et al Striking increase of thin melanomas contrasts with stable incidence of thick melanomas. Arch Dermatol 19991351451–1456. [DOI] [PubMed] [Google Scholar]
- 5.Liotta L. An attractive force in metastasis. Nature 200141024–25. [DOI] [PubMed] [Google Scholar]
- 6.Muller A, Homey B, Soto H.et al Involvement of chemokine receptors in breast cancer metastasis. Nature 200141050–56. [DOI] [PubMed] [Google Scholar]
- 7.Murphy P M. Chemokines and the molecular basis of cancer metastasis. N Engl J Med 2001345833–835. [DOI] [PubMed] [Google Scholar]
- 8.Robledo M M, Bartolomé R A, Longo N.et al Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem 200127645098–45105. [DOI] [PubMed] [Google Scholar]
- 9.Wang J M, Deng X, Gong W.et al Chemokines and their role in tumor growth and metastasis. J Immunol Methods 19982201–17. [DOI] [PubMed] [Google Scholar]
- 10.Longo‐Imedio M I, Longo N, Treviño I.et al Clinical significance of CXCR3 and CXCR4 expression in primary melanoma. Int J Cancer Published Online First: 24 June 2005. doi 10.1002/ijc.21269 [DOI] [PubMed]
- 11.Agostini C, Calabrese F, Rea F.et al CXCR3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol 20011581703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saetta M, Mariani M, Panina‐Bordignon P.et al Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 20021651404–1409. [DOI] [PubMed] [Google Scholar]
- 13.McCarty K S, Miller L S, Cox E B.et al Estrogen receptor analyses. Arch Pathol Lab Med 1985109716–721. [PubMed] [Google Scholar]
- 14.Tuthill R J, Unger J M, Liu P Y.et al Risk assessment in localized primary cutaneous melanoma. Am J Clin Pathol 2002118504–511. [DOI] [PubMed] [Google Scholar]
- 15.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow. Lancet 2001357539–545. [DOI] [PubMed] [Google Scholar]
- 16.Van den Steen P E, Proost P, Wuyts A.et al Neutrophil gelatinase B potentiates interleukin‐8 tenfold by aminoterminal processing, whereas it degrades CTAP‐III, PF‐4, and GRO‐alfa and leaves RANTES and MCP‐2 intact. Blood 2000962673–2681. [PubMed] [Google Scholar]
- 17.Kawada K, Sonoshita M, Sakashita H.et al Pivotal role of CXCR3 in melanoma metastasis to lymph nodes. Cancer Res 2004644010–4017. [DOI] [PubMed] [Google Scholar]