Abstract
Aims
To establish the number of histological levels necessary for the evaluation of breast needle core biopsy (NCB) specimens taken from areas of mammographic calcification in patients presenting via the UK National Health Service Breast Screening Programme.
Methods
Retrospective review of a series of breast NCB specimens initially examined routinely at nine levels. The presence of calcification within the histological sections in each of three sets of levels (levels 1–3, 4–6 and 7–9) and the (cumulative) diagnostic B category that would have pertained after each were assessed.
Results
Accurate diagnostic classification was possible after examination of three levels in 89% cases. Examination of a further three levels permitted accurate diagnosis in a further eight cases (total 97% cases). In only three cases were nine levels necessary for accurate classification. In only a single case (1%) was it likely that routine examination of six levels could have led to significant misclassification. In a significant group of patients (18%), nine levels were considered to provide additional useful information, although this information did not alter the diagnosis.
Conclusions
NCBs for screen‐detected mammographic calcification should be routinely examined at six levels. Further levels may be needed in occasional cases to identify more conclusively the associated pathological abnormality. Further levels may be of particular value when assessing atypical intraductal proliferative epithelial lesions.
The increasing use of needle core biopsy (NCB) performed as part of the triple assessment of mammographic abnormalities detected via the National Breast Screening Programme1 has major workload implications for histopathology laboratories throughout the UK. Progressive replacement of fine‐needle aspiration cytology (FNAC) and extension of the programme to include those aged between 65 and 70 years are likely to create significant additional demands on histopathological services over the next 5–10 years.
Examination of breast NCB specimens at multiple levels may be needed to identify the targeted calcification and to identify any associated histological change. This, however, creates additional work for both the laboratory and the pathologist, and may produce delays in the availability of a final pathology report if not performed routinely during the initial preparation of the specimen. National Institute for Health and Clinical Excellence guidelines require diagnostic breast specimens be reported in 3–5 days.2 The need for a rapid turnaround must be balanced against the requirement for diagnostic accuracy.
Here we present a retrospective audit of 100 NCB specimens performed for screen‐detected calcification. The purpose of this audit was to establish the optimum method of NCB assessment for our unit.
Methods
Breast NCB specimens from the Pennine Breast Screening Service are currently processed through an off‐site laboratory servicing the Teaching Hospitals of Bradford and Leeds. For the Pennine Service, the assessment clinics in Bradford are held on Wednesdays and Thursdays, with the NCB results discussed the following Monday. The majority of pathology reports are thus available within four working days or less of the biopsy. In 2005, the service screened 34 725 women and performed 918 NCBs, 560 (61%) of which were for mammographically detected calcification.
In cases where the mammographic abnormality is calcification, an average of 10 samples per case is taken and the specimens are routinely examined using x rays before being submitted directly to the off‐site laboratory. All samples are taken using a 14‐gauge core biopsy gun. Cores containing calcification are currently not identified separately. Cases are batched to ensure that the maximum number of cases can be processed the same day immediately on arrival at the laboratory. The pathologist usually receives the slides with a copy of the x ray the next day. All cases of calcification are currently examined at nine levels, with three levels present on each of three consecutive slides.
In this study, slides and reports from 112 consecutive NCBs performed for screen‐detected mammographic calcification from January to April 2005 were retrieved from the pathology files. Complete sets of slides were available for review in 100 cases. The slides were reviewed by two independent observers (PJC and VK). The presence of calcification on the specimen x ray and the presence of histological calcification on each of the levels 1–3, 4–6 and 7–9 were assessed. The (cumulative) diagnostic B category that would have pertained after each set of three levels was determined, and a comment was made as to the usefulness of each set. The reasons for the usefulness of the levels, the final NCB diagnosis and the results of any further surgery were recorded.
Results
Diagnostic classification
In 17 of the 100 cases, it was not possible to identify representative calcification, and the core biopsy was classified as inadequate or normal (B1). In 64 cases, the calcification was identified and seen to be associated with a recognisable benign pathological abnormality (B2). Two cases were of uncertain malignant potential (B3), three were suspicious of malignancy (B4) and 14 were unequivocally malignant (B5). There were 11 cases of ductal carcinoma in situ (DCIS; B5a) and three invasive carcinomas (B5b).
The most common benign (B2) diagnoses were involutional change and microcystic/columnar cell change (table 1). Fat necrosis and benign fibrocystic changes (not otherwise specified) were the second most common diagnostic categories with a wide variety of diagnoses present as isolated examples.
Table 1 Range of benign (B2) diagnoses in 64 needle core biopsies.
| A | Involutional change | 21 |
| B | Microcystic/columnar change | 22 |
| C | Fat necrosis | 5 |
| D | Mucin‐filled cysts | 1 |
| E | Fibrocystic change | 6 |
| F | Vascular calcification | 2 |
| G | Fibroadenoma | 1 |
| H | Others (including cyst wall, cyst formation, periductal fibrosis, nodular adenosis, dilated duct and secretory change) | 6 |
In 89 of 100 cases, an accurate diagnosis was possible after examination of only 3 levels.
Changes in classification with deeper levels
In 11 cases, the diagnostic classification altered as deeper levels were examined. Three of these cases contained an atypical epithelial proliferation, either suspicious or diagnostic of DCIS (B4 or B5a). Eight cases contained a benign abnormality associated with the calcification and were classified B2.
In one of the cases (case 5) containing an atypical epithelial proliferation, a disrupted, distorted duct lined only partially by epithelium showing a marked crush artefact was seen on levels 1–3. This was insufficient for diagnosis (B4). DCIS (B5a) was confirmed after examination of levels 4–6.
In another case (case 4), levels 1–3 and 4–6 contained an atypical epithelial proliferation, but the final set of levels (7–9) significantly increased the suspicion of DCIS, where more marked pleomorphism and necrosis were seen. This significantly increased the index of suspicion and raised the B category from 3 to 4.
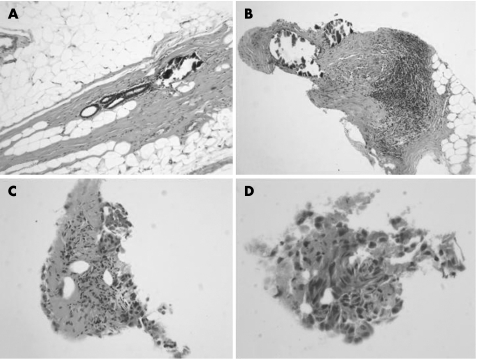
In the third case (case 3), an atypical intraductal proliferation suspicious of DCIS (B4) was not suggested until levels 7–9. A benign diagnosis (B2) was suggested after levels 4–6, as these contained a prominent, circumscribed focus of calcification associated with a benign duct structure without inflammation or epithelial proliferation (fig 1A). In levels 7–9, a few detached fragments of atypical cells with necrotic debris were seen, raising the B score from B2 to B4 (fig 1B–D).
Figure 1 (A) Microcalcification on levels 4–6 found to be associated with a benign duct structure (case 3). (B) Stromal microcalcification in levels 7–9 associated with chronic inflammation but no obvious duct structure (case 3). (C, D) Fragments of atypical epithelium suspicious of ductal carcinoma in situ elsewhere in levels 7–9 (case 3).
In eight (8%) benign (B2) cases, diagnostic features were not present in the initial three levels. In all but one of these, diagnostic changes were present in level 6. Levels 7–9 were needed in only one case where features diagnostic of involutional change with associated microcalcification were not seen in levels 1–3 or 4–6. In three cases, the examination of further levels showed more established diagnostic changes (involution, secretory change and cyst formation). In four cases, further levels were required to identify microcalcification, likely to be representative of that seen on the specimen radiograph.
Requirement for levels 7–9
In only three cases were the final sets of levels (7–9) essential for accurate core biopsy classification. Two were B4, suspicious of DCIS, and one was B2, diagnostic of involutional change. In only one of these, however, was it likely that failure to request nine levels routinely could have resulted in a misclassification with the potential to influence patient management. In routine practice, the absence of calcification in levels 4–6 in the presence of calcification visible on the specimen radiograph in case 1 (table 2) would have prompted a request for further levels. In case 4, a diagnostic excision biopsy would have been performed whether the biopsy had been classified as B3 or B4. Only in case 3 would a benign (B2) classification have had the potential to return the patient to routine recall, and a diagnosis of DCIS would have been missed. In this case, however, the radiological findings were suspicious of malignancy, mandating further investigation whatever the biopsy result.
Table 2 Eleven cases not accurately classified after examination of three levels.
| Case number | Levels 1–3 | Levels 4–6 | Levels 7–9 | Diagnosis |
|---|---|---|---|---|
| 1 | B1 | B1 | B2 | Involutional change |
| 2 | B1 | B2 | B2 | Microcystic change/secretory change |
| 3 | B1 | B2 | B4 | Suspicious of DCIS |
| 4 | B3 | B3 | B4 | Suspicious of DCIS |
| 5 | B4 | B5a | B5a | DCIS |
| 6 | B1 | B2 | B2 | Fibrocystic change |
| 7 | B1 | B2 | B2 | Cyst formation |
| 8 | B1 | B2 | B2 | Involutional change |
| 9 | B1 | B2 | B2 | Fibrocystic change |
| 10 | B1 | B2 | B2 | Secretory change |
| 11 | B1 | B2 | B2 | Microcystic change/fibrocystic disease |
DCIS, ductal carcinoma in situ.
Although not essential, the additional three levels were believed to provide additional useful information in a further 18% of the cases, occasionally because microcalcification was more fully represented or because further levels confirmed the absence of representative calcification despite visible calcification on the specimen radiograph (n = 6) or occasionally because more established changes in the lesion became apparent (n = 2). Additional levels were thought to be of some value in excluding associated invasion or microinvasion in cases of DCIS (n = 5) and in fully assessing lesions known to be associated with atypia such as columnar cell change and mucocoele‐like lesions (n = 5; table 3).
Table 3 Usefulness of levels 7–9 in 18 additional cases.
| Number of cases | |
|---|---|
| More established diagnostic changes | 2 |
| More microcalcification seen | 5 |
| Microcalcification excluded | 1 |
| Exclusion of invasion/microinvasion | 5 |
| Full assessment of epithelial proliferation | 5 |
Discussion
Take‐home messages
Extending the age for breast screening will increase the work of pathology departments by increasing the number of core biopsies performed for mammographically detected microcalcification.
A total of 89% of core biopsy samples for screen‐detected microcalcification are accurately classified after examination of three levels and 97% are accurately classified after six levels.
Pathology departments should audit their individual practice to optimise the relationship between workload and diagnostic accuracy.
Non‐operative diagnosis of mammographically detected breast disease in the UK National Health Service Breast Screening Programme usually involves FNAC or NCB of the lesion. FNAC is simple and cheap, with a low complication rate, but NCB diagnosis is both more sensitive and specific and is used with increasing frequency.3 Preservation of tissue integrity allows architectural evaluation with direct visualisation and assessment of microcalcifications, and there is more chance of obtaining diagnostic material in lesions of low cellularity. NCB also yields more diagnostic material than FNAC when dealing with fibrotic lesions.4 NCB allows in situ and invasive cancer to be distinguished and immunohistochemistry is more easily performed, allowing assessment of predictive and prognostic factors—for example, oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 (HER2). The disadvantages of core biopsies include increased time, both for the procedure and for tissue processing.
The National Health Service Breast Screening Programme guidelines5 recommend that core biopsy specimens taken for microcalcification should be examined using rays to determine the presence of calcium. It is helpful if, whenever possible, a radiological comment regarding the presence of representative microcalcification is provided to the reporting pathologist along with a copy of the specimen x ray. Further levels should be examined if the calcification is not immediately apparent on histological examination, and previous authors have described how such an approach can improve the diagnostic yield.6 A minimum of three levels are recommended, but many laboratories, including our own, routinely examine these biopsy specimens in greater detail to maximise the chance of achieving a confident diagnosis in as short a time as possible. With a laboratory processing facility several miles distant from the screening assessment unit and the pathology department, the need to request additional laboratory investigations can introduce significant delays in the diagnostic process. We performed this audit to review our practice in the light of current guidelines regarding turnaround times and in the context of a progressively increasing workload.
Our results indicate that routine examination of six levels, with further levels when necessary, is sufficient for the assessment of NCBs performed for screen‐detected calcification in our unit. A total of 97% of cases were accurately classified after six levels, and thus could be reported for the next multidisciplinary team discussion; 89% of cases were accurately classified after three levels, with a further 8% requiring a second set of levels. Only 3% required further levels for accurate diagnosis, and in only one case (case 3) was it likely that omitting examination of levels 7–9 would have created the potential for misclassification. Review at multidisciplinary meetings in the context of suspicious radiological findings, however, would have prevented misdiagnosis and a return to routine recall.
Additional levels may be needed when calcification is present on the specimen x ray but is not seen on histological sections, when calcification corresponding to that seen radiologically is not identified, when the lesion associated with calcification is unclear, and when more of the lesion needs to be examined in order to better characterise it. Additional levels may be particularly useful when difficult or atypical epithelial proliferations are seen. If calcification is seen as rounded profiles within ducts without an obvious associated abnormality and without associated epithelium, the possibility of DCIS nearby should always be considered. Periductal inflammation and/or fibrosis may heighten the suspicion of an atypical intraductal epithelial proliferation elsewhere.
Routine examination of multiple levels can minimise diagnostic delay and maximise diagnostic accuracy. The need for both a timely and an accurate diagnosis must, however, be balanced against the risks associated with an increased workload both for the reporting pathologist and the laboratory staff preparing the sections. Individual departments should audit their own practice to establish the most appropriate process for their local breast screening service.
Acknowledgements
We thank Caroline Hilary for her invaluable assistance in undertaking this work.
Abbreviations
DCIS - ductal carcinoma in situ
FNAC - fine‐needle aspiration cytology
NCB - needle core biopsy
Footnotes
Competing interests: None.
References
- 1.National Breast Screening P r o g r a, Association of Breast Screening at B A S O.An audit of screen‐detected breast cancer for the year April 2003 to March 2004. West Midlands: NHS Breast Cancer Screening Publication, 2005
- 2.National Institute for Health and Clinical Excellence Guidance on cancer services: improving outcomes in breast cancer—manual update. London: NICE, 2002
- 3.Britton P D, McCann J. Needle biopsy in the NHS Breast Screening Programme 1996/1997: how much and how accurate? Breast 199985–11. [Google Scholar]
- 4.Berner A, Davidson B, Sigstad E.et al Fine‐needle aspiration cytology vs. core biopsy in the diagnosis of breast lesions. Diagn Cytopathol 200329344–348. [DOI] [PubMed] [Google Scholar]
- 5.National Health Service Breast Screening Programme, Guidelines for non‐operative diagnostic procedures and reporting in breast cancer screening NHSBSP Publication Number 50. Sheffield: NHSBSP, 2001
- 6.Karageorge L S, Hogge J P. Does exhaustive search for microcalcifications improve diagnostic yield in stereotactic core needle breast biopsies? Mod Pathol 200114350–353. [DOI] [PubMed] [Google Scholar]