Abstract
Background
Nasal T/natural killer (NK)‐cell lymphoma is an aggressive type of non‐Hodking's lymphoma associated with Epstein–Barr virus (EBV) and striking geographical variations worldwide.
Aim
To characterise nasal NK/T‐cell lymphoma associated with genotypes of EBV in Chile, a Latin American country, where multiple strains of EBV, including two new recombinant strains, in healthy individuals were recently found.
Methods
Cases with diagnosis of primary nasal lymphoma were selected for histological and immunohistochemical analysis (CD3, CD3e, CD4, CD8, CD79a, CD56, CD57 and TIA‐1) and in‐situ hybridisation, serology and genotyping analysis for EBV.
Results
Out of 22 cases, 9 (41%) cases fulfilled the World Health Organization criteria for nasal NK/T‐cell lymphoma; of these 7 (78%) cases were positive for EBV. Genotyping analysis revealed 6 cases of type 1 EBV and wildtype F at the BamHI‐F region, 4 cases type “i” EBV at the BamHI‐W1/I1 region; XhoI wild type was found in 2 and XhoI loss in 4 cases, respectively. Cosegregation analysis of the BamHI‐W1/I1 region and XhoI restriction site showed the new recombinant strain type “i”/XhoI loss in 3 cases and type “i”/XhoI wild‐type strain in 1 case. Most patients were treated with combined anthracycline‐containing regimens. Half of the cases attained complete remission.
Conclusion
Although nasal NK/T‐cell lymphomas from Chile share similar clinicopathological features, high association with EBV and unfavourable prognosis with those described elsewhere, genotype analysis shows that the new recombinant type “i”/XhoI loss strain might contribute to explain the intermediate incidence of nasal NK/T‐cell lymphomas in Latin America.
Nasal T/natural killer (NK)‐cell lymphoma is a disease entity that has been recognised since the 1990s, but was defined as a distinct clinicopathological entity highly associated with Epstein–Barr virus (EBV) only after a workshop held in Hong Kong in 1996.1 The World Health Organization (WHO) Classification listed this neoplasia in the category of mature T‐cell and NK‐cell neoplasms and defined it as an extranodal NK/T‐cell lymphoma, nasal type.2 Typically, the immunophenotype is CD2 and CD56 positive and surface CD3 is usually negative; cytoplasmic CD3 can be detected in paraffin wax sections, and clonal T‐cell receptor gene rearrangement is not found, indicating an NK cell origin.1 Histological features of this lymphoma are angiocentric infiltration by lymphoma cells and invasion of blood vessels, which results in notable ischaemic necrosis of normal and neoplastic tissues. Interestingly, tumour cells usually show evidence of clonal EBV, suggesting its aetiological role rather than a “silent passenger” in the pathogenesis of this lymphoma.3,4 Patients commonly present with nasal symptoms, such as nasal obstruction, facial mass and bleeding. The response of this lymphoma to therapy is inadequate even when radiotherapy (RT) and chemotherapy (CH) are combined, and therefore this lymphoma has a distinctly poor prognosis.5,6
Although nasal NK/T‐cell lymphoma is relatively uncommon worldwide, its incidence shows striking geographical variations. This disease is unusual in Western countries, accounting for <1% of lymphomas in Europe and North America.7,8,9,10 By contrast, it is relatively common in Asia, making up 6–8% of all lymphomas in China and Japan.3,11,12,13 Among Latin American countries, previous studies show an incidence in between that in Western and in Asian countries.14,15,16,17,18
Among the two major types of EBV, type 1 EBV is the predominant strain all over the world, with the exception of Africa, whereas type 2 EBV prevails.19,20,21,22 Regarding the BamHI‐F region, the prototype F has a worldwide distribution, but variant “f”, featured by the presence of an extra BamHI site, is found only in China, where it is associated with nasopharyngeal carcinoma.23 The presence of an extra BamHI site at the BamHI‐W1/I1 region (type “i” variant) and the presence of an XhoI restriction site at exon 1 of the LMP1 gene (XhoI wild‐type variant) define genotypes for healthy people and EBV‐associated diseases in Western countries.24,25,26,27,28,29 Conversely, the lack of this extra BamHI site at the BamHI‐W1/I1 region and the loss of XhoI restriction site at LMP1 gene define type I and XhoI loss, respectively. These genotypes prevail in healthy donors and EBV‐associated disease in Japan and China.27,28 These observations raise the possibility that EBV genotypes or variants might contribute to explain geographical variations of nasal NK/T‐cell lymphoma around the world. The aim of this study was to characterise the nasal NK/T‐cell lymphoma associated with genotypes and variants of EBV in Chile, a Latin American country, where we have recently found multiple EBV infections including two novel recombinant strains (type “i”/XhoI loss and type I/XhoI wild type) in healthy individuals.20
Materials and methods
Patients and clinical data
From 1989 to 2001, 22 patients were diagnosed with and treated for primary nasal lymphoma by the National Adult Program for Antineoplastic Drugs. After immunophenotypic analysis, only nine cases were found fulfilling the criteria of the WHO Classification for extranodal NK/T‐cell lymphoma, nasal type, which is the subject of this study.
Histology and immunohistochemistry
Paraffin‐wax‐embedded sections were stained with H&E, and all cases were classified according to the new WHO Classification.2 Immunohistochemical analysis was conducted using the following monoclonal antibodies: CD3 (Novocastra Laboratories, Newcastle upon Tyne, UK, dilution 1:100); CD3e (Dako, Glostrup, Denmark, dilution 1:50); CD4 (Novocastra Laboratories, dilution 1:40); CD8 (Dako, Denmark, dilution 1:25); CD79a (Dako, Copenhagen, Denmark, dilution 1:25); CD56 (Novocastra Laboratories, dilution 1:100); CD57 (Immunotech, Marseille, France, dilution 1:1); and TIA‐1 (Coulter Immunology, Hialeah, Florida, USA, dilution 1:2). The avidin–biotin‐peroxidase complex method was used (VECTASTAIN ABC KIT, Vector Laboratories, Burlingame, California, USA). The expression of each molecule was graded as follows: (1) − (0%), (2) + (1–49%) and (3) ++ (>50%), according to the proportion of positive cells. Replicate slides devoid of monoclonal antibodies were included as a negative control.
In‐situ hybridisation for EBV‐encoded small RNA type 1
EBV‐encoded small RNA type‐1 (EBER‐1) expression was detected with a complementary digoxigenin‐labelled 30‐base oligomer, using the procedure described previously.30 A case was considered to be EBER‐1 positive based on a positive signal under microscopy in at least 10% of tumour cells. Lymph node section from a patient with infectious mononucleosis was used as positive control, and a sense probe for EBER‐1 was used as negative control.
Genotype‐specific primer sets and probes for EBV
Genotypes of EBV were examined by PCR, restriction enzyme and Southern blot analysis as described previously.20 For distinguishing between type 1 and 2 EBV, we used primers and probes described by Sample et al31 to produce 153 and 246 bp fragments for type 1 and 2, respectively. The BamHI‐F region was amplified with primers described by Lung et al24,32 that yield a 198‐bp fragment for the prototype F and 127 and 71 bp fragments for “f” variant. The BamHI‐W1/I1 region was amplified by primer pair.24,32 Type I, 205 bp fragment, and type “i”, 130 and 75 bp fragments, were determined by BamHI restriction enzyme digestion. XhoI restriction site polymorphisms were performed with a set of primers described,33 and digestion with XhoI restriction enzyme resulted in 67 and 46 bp fragments for XhoI wildtype and undigested 113 bp PCR product for XhoI loss type. Cell line B95–8 served as positive control for type 1, prototype F, type I and XhoI wildtype virus. Cell lines AG786 and Akata served as positive controls for type 2 and XhoI loss virus, respectively. Cloned BamHI‐“f” and BamHI‐“i” DNA fragments served as positive controls for variant “f” and type “i”, respectively.
Serology for human T‐lymphotropic leukaemia virus type‐1 and HIV
We conducted serological analyses of human T‐lymphotropic leukaemia virus type‐1 (HTLV‐1) and HIV by Enzyme Immunoassay (Abbott, Abott Park, Illinois, USA).
Treatment
The treatment for the cases was anthracycline‐contained CH, such as cyclophosphamide, doxorubicin hydrochloride, oncovin and prednisolone or cyclophosphamide, doxorubicin hydrochloride, oncovin, prednisolone and bleomycin, or a combination therapy with RT at a dose of 40–50 Gy.
Results
Patients' characteristics
Table 1 summarises clinical data obtained from nine patients with extranodal nasal type lymphoma. There were five women and four men. Their median age was 43 years (range 24–62). Family names suggested that all nine patients were of Hispanic origin. The nasal cavity was the main site involved in all patients, but one, which presented with a middle‐line mass of the hard palate and invasion to adjoining tissues such as the nasopharynx, ethmoid and orbit. In all, 8 (88%) patients had a localised disease (stage I–II), except one (stage IV) with stomach involved.
Table 1 Clinical features of nasal natural killer/T‐cell lymphomas.
| Case | Age/sex | Tumour involvement | Clinical stage | Treatment | Response | Survival period, months | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 62/F | Nose | I | CT | Failure | 5 | Died |
| 2 | 24/F | Nose | I | CT and RT | Failure | 4 | Died |
| 3 | 52/F | Nose | I | CT | Failure | 22 | Alive |
| 4 | 40/M | Nose | I | CT | CR | 12 | Alive |
| 5 | 28/M | Nasopharynx, supraglotis, stomach | IV | CT | Failure | 1 | Died |
| 6 | 58/F | Nose | I | NA | NA | NA | NA |
| 7 | 53/F | Nose (at 46 months) | I | CT and RT | CR | 52 | Relapsed |
| 8 | 43/M | Hard palate, orbit, ethmoid | II | CT | CR | 14 | Died |
| 9 | 26/M | Nose | I | CT | CR | 7 | Died |
CR, complete remission; CT, chemotherapy; F, female; M, male; NA, not available; RT, radiotherapy.
Histology and immunohistochemistry
There was a broad cytologic spectrum of tumour cells, from atypical small cells, to a mixture of small and large cells to large cells, with frequent mitotic figures, admixing with a variable number of inflammatory cells, such as granulocytes, macrophages, plasma cells and small lymphocytes. Angiocentricity and angioinvasive pattern were reported in all cases. Necrosis was also found in all cases, but was most prominent in tissues with large cells. Table 2 summarises the immunophenotypic findings. Six patients met the criteria of the WHO Classification for nasal lymphoma (CD3e positive, CD56 positive, TIA‐1 positive and CD79a negative). Three patients were CD3e positive, TIA‐1 positive, CD79a negative but CD56 negative.
Table 2 Summary of immunohistochemical and Epstein–Barr virus RNA in‐situ hybridisation results in nasal natural killer/T‐cell lymphomas.
| Case | CD3 | CD4 | CD8 | CD79a | CD56 | CD57 | CD3e | TIA‐1 | EBER (%)* | Type | F/“f” | I/“i” | XhoI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ++ | +/− | ++ | −† | − | − | ++ | ++ | 80 | 1 | F | “i” | Loss |
| 2 | ++‡ | − | − | −† | ++ | − | ++ | ++ | 90< | 1 | F | NA | Wild type |
| 3 | −† | − | −§ | −† | ++ | − | −§ | ++ | 90< | 1 | F | NA | Loss |
| 4 | ++‡ | −† | −† | −† | ++ | −§ | ++ | ++ | 80 | NA | “f” | NA | NA |
| 5 | ++ | −§ | ++ | −† | − | −† | + | ++ | 70 | 1 | F | “i” | Loss |
| 6 | ++‡ | − | −† | − | ++ | −§ | ++ | ++ | 0 | NT | NT | NT | NT |
| 7 | −§ | − | −§ | −† | ++ | NT | ++ | ++ | 0 | NT | NT | NT | NT |
| 8 | −§ | − | + | − | + | NT | ++ | ++ | 90< | 1 | F | “i” | Loss |
| 9 | +‡ | − | ++ | −† | − | NT | ++ | ++ | 70 | 1 | F | “i” | Wild type |
EBER, Epstein–Barr virus‐encoded small RNA; NA, not amplified; NT, not tested.
*In‐situ hybridisation results were expressed as number of positive cells per medium‐powered field (×200). Cases were considered positive when a positive signal was observed in ⩾10% of the tumour cells.
†Background was also reacted.
‡Cytoplasmic expression was observed.
§Positive for reactive cells but not for neoplastic cells.
Serology to HTLV‐1 and HIV
Antibodies against HTLV‐1 and HIV were negative in four patients studied.
Take‐home messages
Six out of seven Epstein–Barr virus (EBV)‐positive nasal natural killer (NK)/T‐cell lymphoma cases were type 1 EBV and wildtype F at the BamHI‐F region.
Among six EBV‐positive nasal NK/T‐cell lymphoma cases, four were type “i” EBV at the BamHI‐W1/I1 region.
Among six EBV‐positive nasal NK/T‐cell lymphoma cases, four showed XhoI loss and two had XhoI wild‐type restriction site.
Cosegregation analysis of the BamHI W1/I1 region and XhoI restriction site showed a novel recombinant strain type “i”/XhoI loss in three cases and type “i”/XhoI wildtype strain in one case.
Genotype analysis revealed that the novel recombinant strain type “i”/XhoI loss might contribute to explain the intermediate incidence of nasal NK/T‐cell lymphomas in Latin America.
EBV in‐situ hybridisation and genotyping analysis
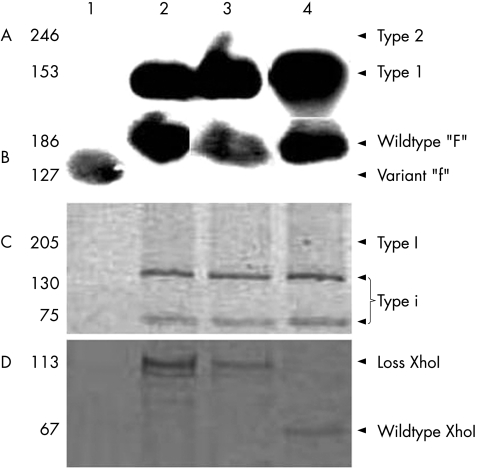
In total, 7 (78%) cases were considered EBV positive according to the expression of EBER‐1 by in‐situ hybridisation in ⩾10% of the tumour cells (table 2). Two cases were negative for EBER‐1 expression. Figure 1 shows the representative examples of EBV positive nasal lymphoma cases. Genotyping analysis revealed that six out of seven positive cases were type 1 EBV and wild‐type F at the BamHI‐F region. The remaining case (case 3) did not amplify for type 1 or 2 EBV, and was variant “f” at the BamHI‐F region. All four amplified cases of the BamHI‐W1/I1 region were type “i” EBV. In the remaining three cases, no amplification of this region could be obtained after several attempts. Amplification of XhoI restriction site was successful in six cases, two were XhoI wild type and four showed XhoI loss. Figure 2 shows the representative examples. As it is known that polymorphisms at the BamHI‐W1/I1 region cosegregate with XhoI restriction site polymorphisms,27 we analysed these two variants. Among four type “i” nasal lymphomas, three harboured a novel recombinant strain type “i”/XhoI loss (cases 4, 7 and 8) and one harboured type “i”/XhoI wildtype strain (case 9).
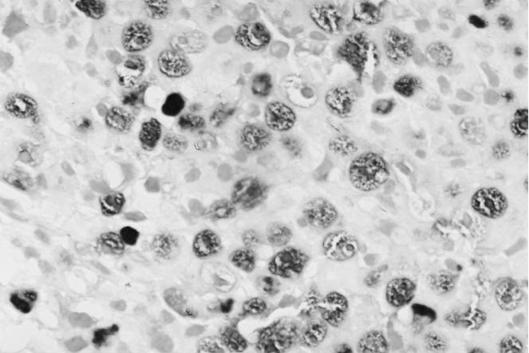
Figure 1 Identification of Epstein–Barr virus (EBV) in a case of nasal natural killer/T‐cell lymphoma (case 1) by EBV‐encoded small RNA type‐1 in situ hybridisation (×400).
Figure 2 Genotypes of Epstein–Barr virus in cases of nasal natural killer/T‐cell lymphomas. (A) Southern blot analysis after PCR amplification and hybridisation with specific probes for type 1 and 2 strains. (B) Southern blot analysis after PCR amplification, digestion with BamHI restriction enzyme and hybridisation with specific probes for polymorphims at the BamHI‐F region. (C) Polyacrylamide gel after PCR amplification and digestion with BamHI restriction enzyme at the BamHI‐W1/I1 region. (D) Polyacrylamide gel after PCR amplification and digestion with XhoI restriction enzyme at XhoI site polymorphisms. The second fragment after XhoI digestion (46 bp fragment) for the wild type is not seen in the gel.
Treatment and outcome
The initial treatment was CH in eight cases, combined with RT in two cases and unknown for the remaining one. Complete remission was achieved in 4 (50%) of 8 cases whose treatment data were available. The median survival period of these four cases with complete remission was 13 months. All of the four patients who were unresponsive to the treatment died of progressive disease, except one patient (median survival period 4.5 months). Survival analysis could not be conducted because of the small number of patients.
Discussion
The present study shows that the clinical and phenotypic characteristics of nasal NK/T‐cell lymphoma in Chile are similar to those described in other Latin American as well as Asian and Western countries.3,7,8,9,10,11,12,13,14,15,17,18 Here, we also confirm that most nasal NK/T‐cell lymphomas were EBV positive,3,4 although we failed to show this association in two cases. Possible explanations may be the low quality of preservation of paraffin‐wax‐embedded blocks, or the lack of EBER‐1 expression, as has been shown in other tumours.34 Nevertheless, these negative cases strictly met the criteria for classification as nasal NK/T‐cell lymphomas, as they were CD56 positive, CD3e positive and TIA‐1 positive.2 Genotype analysis showed the presence of a new recombinant strain characterised by the presence of an extra BamHI site at the BamHI‐W1/I1 region, together with the loss of the XhoI restriction site at the LMP1 gene (type “i”/XhoI loss strain). Type “i”/XhoI loss strain has been recently identified in a proportion of 329 healthy adults in two Latin American countries, including Chile.20 In that study, type “i”/XhoI loss strain was found admixed with another recombinant strain, type I/XhoI wild‐type strain, and strains described previously in Western countries (type “i”/ XhoI wild type) and Asian countries (type I/XhoI loss).20,24,25,26,27,28,29 Therefore, these results provide the first evidence that type “i”/XhoI loss strain can be found in nasal NK/T‐cell lymphomas. Furthermore, the presence of type “i”/XhoI loss might contribute to explain the intermediate incidence of nasal NK/T‐cell lymphoma in Latin America, in between that in Western and in Asian regions.14,15,16,17,18 The frequency of other genotypes, type 1 and wild‐type F at the BamHI‐F region in our patients were similar to that of patients with nasal NK/T‐cell lymphoma described in Japan.35
Even early stages have an overall survival rate lower than that in other extranodal or nodal lymphomas. Besides being a resistant disease, relapses are also frequent.36 To date, there is no agreement on the best treatment. RT is effective in limited disease, whereas combined therapy seems to be more effective for advanced disease but does not ensure prolonged survival.5,6 Recent reports suggested that high dose CH with stem‐cell transplantation may be an effective treatment modality for refractory NK lymphomas.37 The survival benefit of this modality still needs to be shown.
In conclusion, we describe nasal NK/T‐cell lymphomas from Chile, with similar clinicopathological features as those described elsewhere, strong association with EBV and unfavourable prognosis. Genotype analysis revealed the presence of a novel recombinant strain (type “i”/XhoI loss) in most of the analysed cases, providing evidence that, although previously described in healthy donors, this strain can also be found in nasal NK/T‐cell lymphomas. Further research is necessary to clarify whether this finding is specific of Latin America or can be found in Western and Asian countries as well.
Acknowledgements
We thank Ms Yasuko Shinmura and Ms Yoshie Minakami for their skilful assistance in immunohistochemical analysis, and Professor Mel Greaves and Dr Estella Matutes for their valuable opinion of the manuscript. This study was supported by Grants‐in‐Aid for Scientific Research on Priority Areas of the Ministry of Education, Culture, Sports, Science and Technology of Japan (12218231), and partially by FONDECYT‐Chile (1030130).
Abbreviations
CH - chemotherapy
EBER‐1 - Epstein–Barr virus‐encoded small RNA type‐1
EBV - Epstein–Barr virus
HTLV‐1 - human T‐lymphotropic leukaemia virus type‐1
NK - natural killer
RT - radiotherapy
WHO - World Health Organization
Footnotes
Competing interests: None declared.
References
- 1.Nava V E, Jaffe E S. The pathology of NK‐cell lymphomas and leukemias. Adv Anat Pathol 20051227–34. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe E, Harris N, Stein H.et alPathology and genetics of tumors of haematopoietic and lymphoid tissues. Lyon: IARC Press, 2001
- 3.Harabuchi Y, Imai S, Wakashima J.et al Nasal T‐cell lymphoma causally associated with Epstein‐Barr virus: clinicopathologic, phenotypic, and genotypic studies. Cancer 1996772137–2149. [DOI] [PubMed] [Google Scholar]
- 4.Kanavaros P, Briere J, Emile J F.et al Epstein‐Barr virus in T and natural killer (NK) cell non‐Hodgkin's lymphomas. Leukemia 199610(Suppl 2)s84–s87. [PubMed] [Google Scholar]
- 5.Egger G, Liang G, Aparicio A.et al Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004429457–463. [DOI] [PubMed] [Google Scholar]
- 6.Oshimi K. NK cell lymphoma. Int J Hematol 200276(Suppl 2)118–121. [DOI] [PubMed] [Google Scholar]
- 7.Kanavaros P, Lescs M C, Briere J.et al Nasal T‐cell lymphoma: a clinicopathologic entity associated with peculiar phenotype and with Epstein‐Barr virus. Blood 1993812688–2695. [PubMed] [Google Scholar]
- 8.Garcia‐Cosio M, Santon A, Mendez M C.et al Nasopharyngeal/nasal type T/NK lymphomas: analysis of 14 cases and review of the literature. Tumori 200389278–284. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez J, Romaguera J E, Manning J.et al Nasal‐type T/NK lymphomas: a clinicopathologic study of 13 cases. Leuk Lymphoma 200039139–144. [DOI] [PubMed] [Google Scholar]
- 10.Gaal K, Sun N C, Hernandez A M.et al Sinonasal NK/T‐cell lymphomas in the United States. Am J Surg Pathol 2000241511–1517. [DOI] [PubMed] [Google Scholar]
- 11.Ho F C, Todd D, Loke S L.et al Clinico‐pathological features of malignant lymphomas in 294 Hong Kong Chinese patients, retrospective study covering an eight‐year period. Int J Cancer 198434143–148. [DOI] [PubMed] [Google Scholar]
- 12.Ng C S, Chan J K, Lo S T.et al Immunophenotypic analysis of non‐Hodgkin's lymphomas in Chinese. A study of 75 cases in Hong Kong. Pathology 198618419–425. [DOI] [PubMed] [Google Scholar]
- 13.Aozasa K, Ohsawa M, Tajima K.et al Nation‐wide study of lethal mid‐line granuloma in Japan: frequencies of Wegener's granulomatosis, polymorphic reticulosis, malignant lymphoma and other related conditions. Int J Cancer 19894463–66. [DOI] [PubMed] [Google Scholar]
- 14.Arber D A, Weiss L M, Albujar P F.et al Nasal lymphomas in Peru. High incidence of T‐cell immunophenotype and Epstein‐Barr virus infection. Am J Surg Pathol 199317392–399. [PubMed] [Google Scholar]
- 15.Aviles A, Diaz N R, Neri N.et al Angiocentric nasal T/natural killer cell lymphoma: a single centre study of prognostic factors in 108 patients. Clin Lab Haematol 200022215–220. [DOI] [PubMed] [Google Scholar]
- 16.Calderon‐Garciduenas L, Delgado R, Calderon‐Garciduenas A.et al Malignant neoplasms of the nasal cavity and paranasal sinuses: a series of 256 patients in Mexico City and Monterrey. Is air pollution the missing link? Otolaryngol Head Neck Surg 2000122499–508. [DOI] [PubMed] [Google Scholar]
- 17.Altemani A, Barbosa A C, Kulka M.et al Characteristics of nasal T/NK‐cell lymphoma among Brazilians. Neoplasma 20024955–60. [PubMed] [Google Scholar]
- 18.Elenitoba‐Johnson K S, Zarate‐Osorno A, Meneses A.et al Cytotoxic granular protein expression, Epstein‐Barr virus strain type, and latent membrane protein‐1 oncogene deletions in nasal T‐lymphocyte/natural killer cell lymphomas from Mexico. Mod Pathol 199811754–761. [PubMed] [Google Scholar]
- 19.Zimber U, Adldinger H K, Lenoir G M.et al Geographical prevalence of two types of Epstein‐Barr virus. Virology 198615456–66. [DOI] [PubMed] [Google Scholar]
- 20.Corvalan A, Ding S, Koriyama C.et al Association of a distinctive strain of Epstein‐Barr virus with gastric cancer. Int J Cancer 20061181736–1742. [DOI] [PubMed] [Google Scholar]
- 21.Young L S, Murray P G. Epstein‐Barr virus and oncogenesis: from latent genes to tumours. Oncogene 2003225108–5121. [DOI] [PubMed] [Google Scholar]
- 22.Young L S, Yao Q Y, Rooney C M.et al New type B isolates of Epstein‐Barr virus from Burkitt's lymphoma and from normal individuals in endemic areas. J Gen Virol 198768(Pt 11)2853–2862. [DOI] [PubMed] [Google Scholar]
- 23.Lung M L, Lam W P, Sham J.et al Detection and prevalence of the "f" variant of Epstein‐Barr virus in southern China. Virology 199118567–71. [DOI] [PubMed] [Google Scholar]
- 24.Lung M L, Chang G C. Detection of distinct Epstein‐Barr virus genotypes in NPC biopsies from southern Chinese and Caucasians. Int J Cancer 19925234–37. [DOI] [PubMed] [Google Scholar]
- 25.Lung M L, Chang R S, Huang M L.et al Epstein‐Barr virus genotypes associated with nasopharyngeal carcinoma in southern China. Virology 199017744–53. [DOI] [PubMed] [Google Scholar]
- 26.Lung M L, Chang R S, Jones J H. Genetic polymorphism of natural Epstein‐Barr virus isolates from infectious mononucleosis patients and healthy carriers. J Virol 1988623862–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdel‐Hamid M, Chen J J, Constantine N.et al EBV strain variation: geographical distribution and relation to disease state. Virology 1992190168–175. [DOI] [PubMed] [Google Scholar]
- 28.Khanim F, Yao Q Y, Niedobitek G.et al Analysis of Epstein‐Barr virus gene polymorphisms in normal donors and in virus‐associated tumors from different geographic locations. Blood 1996883491–3501. [PubMed] [Google Scholar]
- 29.Young L S, Murray P G. Epstein‐Barr virus and oncogenesis: from latent genes to tumours. Oncogene 2003225108–5121. [DOI] [PubMed] [Google Scholar]
- 30.Wu M S, Huang S P, Chang Y T.et al Association of the −160 C ‐‐> a promoter polymorphism of E‐cadherin gene with gastric carcinoma risk. Cancer 2002941443–1448. [DOI] [PubMed] [Google Scholar]
- 31.Sample J, Young L, Martin B.et al Epstein‐Barr virus types 1 and 2 differ in their EBNA‐3A, EBNA‐3B, and EBNA‐3C genes. J Virol 1990644084–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lung M L, Chang G C, Miller T R.et al Genotypic analysis of Epstein‐Barr virus isolates associated with nasopharyngeal carcinoma in Chinese immigrants to the United States. Int J Cancer 199459743–746. [DOI] [PubMed] [Google Scholar]
- 33.Sandvej K, Gratama J W, Munch M.et al Sequence analysis of the Epstein‐Barr virus (EBV) latent membrane protein‐1 gene and promoter region: identification of four variants among wild‐type EBV isolates. Blood 199790323–330. [PubMed] [Google Scholar]
- 34.Jaffe E S, Chan J K, Su I J.et al Report of the workshop on nasal and related extranodal angiocentric T/natural killer cell lymphomas. Definitions, differential diagnosis, and epidemiology. Am J Surg Pathol 199620103–111. [DOI] [PubMed] [Google Scholar]
- 35.Sidagis J, Ueno K, Tokunaga M.et al Molecular epidemiology of Epstein‐Barr virus (EBV) in EBV‐related malignancies. Int J Cancer 19977272–76. [DOI] [PubMed] [Google Scholar]
- 36.Lee H K, Wilder R B, Jones D.et al Outcomes using doxorubicin‐based chemotherapy with or without radiotherapy for early‐stage peripheral T‐cell lymphomas. Leuk Lymphoma 2002431769–1775. [DOI] [PubMed] [Google Scholar]
- 37.Au W Y, Lie A K, Liang R.et al Autologous stem cell transplantation for nasal NK/T‐cell lymphoma: a progress report on its value. Ann Oncol 2003141673–1676. [DOI] [PubMed] [Google Scholar]