Abstract
Objectives
To assess the efficacy and safety of etoricoxib 60 mg once daily and naproxen 500 mg twice daily over a 138‐week treatment period in patients with osteoarthritis (OA).
Methods
Two 1‐year randomised, double blind, parallel group two‐part base studies (part I 12 weeks; part II 40 weeks), followed by an 86‐week extension, in patients with OA (hip or knee) were conducted at 80 clinical centres (19 countries). The studies had identical designs. Patients taking placebo in part I received etoricoxib or naproxen (1:1 ratio) in part II and the extension; patients taking etoricoxib or naproxen in part I continued to receive the same treatment throughout the entire length of the studies. Co‐primary efficacy end points were patient global assessment of disease status, and WOMAC questionnaire pain subscale and physical function subscale (100 mm VAS). Efficacy over 138 weeks was assessed by graphical analysis. Safety was assessed by observation of adverse experiences and laboratory and physical evaluations.
Results
997 patients entered (615 completed) the base studies. Of these patients, 463 patients entered the extensions. A total of 161 and 152 patients in the etoricoxib and naproxen groups, respectively, completed 138 treatment weeks. Etoricoxib and naproxen showed similar efficacy throughout the 138 weeks of treatment. For etoricoxib and naproxen, respectively, WOMAC pain assessments were 67 and 67 mm (baseline); 28 and 29 mm (1 year), and 34 and 33 mm (138 weeks). Results for the other efficacy end points were similar to those seen with the WOMAC pain assessments. Both etoricoxib and naproxen were generally well tolerated.
Conclusion
Both etoricoxib and naproxen demonstrated long‐term clinical efficacy for the treatment of OA. Etoricoxib and naproxen were generally well tolerated.
Keywords: COX‐2 selective inhibitors, NSAIDs, osteoarthritis, etoricoxib, naproxen
Osteoarthritis (OA) is a condition characterised by loss of articular cartilage in synovial joints, osteophyte formation, subchondral bone change and synovitis.1,2 Patients with OA experience symptoms such as pain, loss of physical function and, in the advanced stages, disability.1,3,4 The goal of treatment is to increase joint function and improve quality of life. Non‐pharmacological approaches, such as diet and exercise, as well as the use of paracetamol for the reduction of pain are recommended for patients with mild to moderate OA. For patients who require greater efficacy, non‐steroidal anti‐inflammatory drugs (NSAIDs) or selective cyclo‐oxygenase (COX)‐2 inhibitors are often prescribed. Although many treatments are available, selection of a therapeutic approach for patients is often difficult and involves weighing benefits of a particular treatment against its potential risks on an individual basis.
Traditional, non‐selective NSAIDs inhibit both isoforms of cyclo‐oxygenase: COX‐1 and COX‐2. These analgesic agents have demonstrated their value in the treatment of pain from OA, but, their use is associated with gastrointestinal (GI) adverse experiences (AEs) such as ulcers and GI bleeding because of their potent inhibition of the gastroprotective COX‐1 isoform.5,6 Selective COX‐2 inhibitors have demonstrated comparable efficacy in chronic and acute pain, with significantly improved GI tolerability compared with traditional NSAIDs.7
Etoricoxib is a COX‐2 selective inhibitor that has demonstrated efficacy in patients with OA.8 The objective of the current analysis was to assess the maintenance of efficacy and tolerability of etoricoxib 60 mg once daily and naproxen 500 mg twice daily in patients with OA in a combined analysis of two studies over 138 weeks of treatment. Recent studies have suggested that COX‐2 selective inhibitors are associated with an increased risk of thrombotic cardiovascular (CV) events in comparison with placebo.9,10 Data are also available that suggest that traditional NSAIDs are associated with increased CV risk.11 In this analysis, data on CV AEs were collected and adjudicated by an external safety monitoring committee; however, these studies were not powered or designed specifically to evaluate the CV safety profile of etoricoxib.
Methods
Two studies, protocols 018 and 019, were conducted at 47 centres in the United States and 33 centres internationally (United States, Europe, Canada and Australia), respectively. The protocol and consent forms were approved by institutional review boards or ethics review committees for each study site. Each patient provided written informed consent before entering the base studies and before starting the extension studies.
Patient inclusion/exclusion
Patients who entered the base studies were >40 years of age and had clinical symptoms or a clinical diagnosis of OA of the knee or hip, based on clinical and radiographic criteria, for more than 6 months before the start of the studies. Patients who entered the extension studies were required to have fulfilled eligibility requirements for the base studies and to have tolerated treatment during the previous treatment period. Patients were classified as American Rheumatism Association functional class I, II, or III. Other than OA, the patients were in general good health. Female patients of child‐bearing potential were instructed to use contraception and were excluded if they were pregnant. Patients included in the studies were regular users of either NSAIDs or paracetamol (ie, patients had used these analgesics for at least 25 of the previous 30 days before study enrolment). The number of paracetamol users enrolled at each study site was limited to 20%. Recent sustained use (ie, 6 consecutive days during the month before enrolment) of H2 receptor antagonists or proton pump inhibitors was not permitted. Proton pump inhibitors and H2 receptor antagonists were permitted at over the counter and prescription doses, as needed, after randomisation. Up to one‐third of patients were allowed to take low‐dose aspirin(⩽100 mg/day). Paracetamol (325 mg tablets) was available as rescue medication; rescue medication use was restricted (ie, it was not permitted during the initial 2 weeks of treatment) and recorded. All other analgesic drugs were not permitted. The following drugs were also not permitted during the studies: warfarin, ticlopidine, clopidogrel, anti‐epileptic drugs, digoxin, rifampin, dexamethasone, or lithium.
Prestudy NSAID users were required to demonstrate worsening of pain (flare) after a prespecified washout period based on the half‐life of the drug. The length of the washout period was based on the individual drug, but was at least 3 days and as many as 15 days. They were required to meet two flare criteria: (a) ⩾40 mm and an increase of 15 mm compared with screening values on question 1 of the Western Ontario and McMaster Universities OA Index (WOMAC),12 pain while walking on a flat surface (100 mm visual analogue scale (VAS)) and (b) a worsening on the investigator's global assessment of disease status by ⩾1 point on a 0–4 point Likert scale. At the flare visit, prestudy paracetamol users were required to have a response on the investigator's global assessment of disease status as fair, poor, or very poor and had to demonstrate reproducible disease activity compared with the screening visit: ⩾40 mm pain while walking on a flat surface (WOMAC 100 mm VAS) and the patient's global assessment of disease status (100 mm VAS).
Study design
The initial base studies, which had replicate study designs, randomised patients to receive placebo, etoricoxib 60 mg once daily, or naproxen 500 mg twice daily. Each base study consisted of a 12‐week placebo and active‐comparator controlled period (part I) followed by a 40‐week active‐comparator controlled period (part II); this was followed by an 86‐week active‐comparator controlled extension period. In part I, patients were randomly allocated (according to a computer‐generated allocation schedule) to once‐daily etoricoxib 60 mg or matching placebo or naproxen 1000 mg (500 mg twice daily, or matching placebo twice daily) in a blinded, double‐dummy fashion. In part II and the extensions, patients took etoricoxib 60 mg (once daily) or naproxen 1000 mg (500 mg twice daily) in a blinded, double‐dummy fashion. Patients taking placebo in part I were randomly assigned to take etoricoxib 60 mg (50%) or naproxen 1000 mg (50%) in part II and the extensions. Patients taking etoricoxib 60 mg or naproxen 1000 mg in part I continued to follow the same regimen throughout the base study and extension.
Study visits occurred at weeks 2, 4, 8, 12, 19, 26, 33, 39, 45, and 52 during the base studies. During the extension studies, study visits occurred at weeks 69, 86, 104, 121, and 138. If a patient stopped treatment, then a discontinuation visit was scheduled.
Efficacy measures
The primary efficacy end points were: the WOMAC pain subscale (100 mm VAS; 0 = no pain to 100 = extreme pain); the WOMAC physical function subscale (100 mm VAS; 0 = no difficulty to 100 = extreme difficult); and the patient's global assessment of disease status (100 mm VAS; 0 = “very well” to 100 = “very poor,” assessing the patient's overall wellbeing).
Safety measures
Patients were monitored for clinical or laboratory AEs by physical examinations, vital signs, electrocardiograms, and routine haematology, blood chemistry, and urine analysis at each study visit. Investigators were instructed to report all AEs occurring while patients received treatment and for 14 days after study drug discontinuation. Serious AEs (life‐threatening experiences, those resulting in, or prolonging, hospitalisation, those causing permanent incapacity, those requiring significant medical intervention to prevent hospitalisation, incapacity or death, or a malignancy) were identified by investigators. Additionally, before initiation of the studies, blinded, external adjudication committees were organised to evaluate any potential serious thrombotic CV or upper GI perforations, ulcers, or bleeding events (PUBs) that occurred during the trial.
Safety was evaluated by various means, including an examination of patients exceeding predefined limits for laboratory values of interest (eg, consecutive decreases in haemoglobin and packed cell volume, increased aminotransferase values, or increases in serum creatinine), common events associated with NSAIDs or selective COX‐2 inhibitors (eg, hypertension and lower extremity oedema), and clinical review of tabulated data.
Power and determination of sample size
Power and sample size were calculated for the efficacy evaluation during part I of the base study based on the variability seen in a previous etoricoxib dose‐ranging study.13 With 200 patients in each active treatment group and 50 in the placebo group, the detectable differences vs placebo (with 95% power, α = 0.05, two‐tailed) ranged from 12.8 to 14.1 mm for the primary end points. Prespecified clinical comparability between etoricoxib 60 mg and naproxen 1000 mg was demonstrated if the 95% confidence interval (CI) for the mean differences between the two groups in the time‐weighted average response fell within ±10 mm on a 100 mm VAS for all three primary end points (primary variables). With this equivalence range, the sample size of 200 patients for each treatment group has greater than 95% power to demonstrate equivalence if the true (not observed) mean difference between the etoricoxib group and the naproxen group is 0 for all three primary end points. For all evaluations, lower values were consistent with improvement. All statistical tests for differences were two tailed with α = 0.05; p⩽0.05 was considered significant.
Statistical analysis
In part I, the primary analysis for each end point was the time‐weighted average response over the 12‐week treatment period. The time‐weighted average response is calculated by taking the time between adjacent observations divided by the time from the randomisation visit to the last observation in the treatment period, and using it as the weight for computation of the average. Analysis of covariance was used to assess time‐weighted average changes from baseline for each efficacy end point, with treatment and primary study joint as the main effect and baseline as covariate. Analysis of variance with terms for treatment and primary study joint was employed for most end points without relevant baseline measurements. For the patient and investigator global response to therapy end points, the patient global assessment of disease was used as the covariate.
In part II and the extensions, the treatment response was assessed through graphical presentation and tabulation of mean change from baseline at each study visit. The comparability of etoricoxib and naproxen was examined by the time‐weighed average change from baseline over 52 weeks as described for part I, and the analysis was limited to patients who received the same treatment in parts I and II.
For the extensions, efficacy results were assessed over time within each of the treatment groups by least squares means changes from baseline obtained from an analysis of covariance model similar to that used for part I, with appropriate 95% CIs. No formal hypothesis testing was carried out owing to the non‐randomised, self‐selected nature of the patient population in the extension studies. Only visual examination of the summary statistics through tables and graphs was performed.
Results
Patient demographics
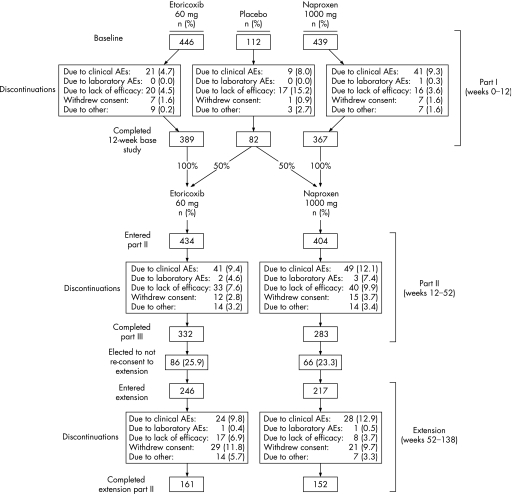
Of the 997 patients randomised into part I, 838 entered part II and 463 entered the 86‐week extension studies (fig 1). The baseline characteristics were similar among patients receiving placebo, etoricoxib, and naproxen; baseline patient characteristics from part I are representative of the patients in the extension, and remained similar among the etoricoxib and naproxen groups (table 1). The most common reasons for discontinuations in part I were clinical AEs and a lack of efficacy. In part II, patients discontinued owing to clinical AEs, lack of efficacy, and withdrawal of consent. Most common reasons for discontinuation during the 86‐week extension were clinical AEs, withdrawal of consent, and lack of efficacy (fig 1).
Figure 1 Patient accounting and study design displaying part I (weeks 0–12), part II (weeks 12–52), and the extension (weeks 52–138).
Table 1 Baseline patient characteristics.
| Characteristics | Baseline patient characteristics for all enrolled patients | Baseline patient characteristics for patients who entered the extension study | |||
|---|---|---|---|---|---|
| Placebo (n = 112) | Etoricoxib 60 mg (n = 446) | Naproxen (n = 439) | Etoricoxib (n = 246) | Naproxen (n = 217) | |
| Gender, No (%) | |||||
| Female | 82 (73.2) | 322 (72.2) | 314 (71.5) | 191 (77.6) | 156 (71.9) |
| Male | 30 (26.8) | 124 (27.8) | 125 (28.5) | 55 (22.4) | 61 (28.1) |
| Race, No (%) | |||||
| Asian | 1 (0.9) | 2 (0.4) | 3 (0.7) | 1 (0.4) | 1 (0.5) |
| Black | 6 (5.4) | 18 (4.0) | 19 (4.3) | 10 (4.1) | 5 (2.3) |
| Multiracial | 6 (5.4) | 24 (5.4) | 20 (4.6) | 26 (10.6) | 19 (8.8) |
| Other | 10 (8.9) | 42 (9.4) | 35 (8.0) | 31 (12.6) | 22 (10.1) |
| White | 89 (79.5) | 360 (80.7) | 362 (82.5) | 178 (72.4) | 170 (78.3) |
| Age (years) | |||||
| Mean (SD) | 63.8 (10.2) | 62.59 (9.8) | 62.7 (9.7) | 62.19 (9.0) | 61.51 (9.4) |
| Range | 40 to 87 | 35 to 92 | 40 to 87 | 40 to 84 | 40 to 87 |
| Body weight (kg) | |||||
| Mean (SD) | 86.4 (18.5) | 84.28 (18.9) | 85.09 (18.9) | 83.90 (18.67) | 86.03 (18.44) |
| Range | 51.3 to 138.0 | 44.8 to 176.9 | 48.00 to 158.8 | 47.6 to 176.9 | 48.0 to 142.9 |
| Primary OA joint, No (%) | |||||
| Hip | 20 (17.9) | 100 (22.4) | 99 (22.6) | 40 (16.3) | 38 (17.5) |
| Knee | 92 (82.1) | 346 (77.6) | 340 (77.4) | 206 (83.7) | 179 (82.5) |
| ARA function class, No (%) | |||||
| I | 24 (21.4) | 99 (22.2) | 90 (20.5) | 48 (19.5) | 48 (22.1) |
| II | 69 (61.6) | 246 (55.2) | 269 (61.3) | 144 (58.5) | 126 (58.1) |
| III | 19 (17.0) | 101 (22.6) | 80 (18.2) | 54 (22.0) | 43 (19.8) |
ARA, American Rheumatism Association.
Efficacy results
Efficacy over 52 weeks
Etoricoxib 60 mg and naproxen 1000 mg demonstrated significantly greater improvements than placebo over the 12‐week treatment period for all efficacy end points. For the three co‐primary efficacy end points, etoricoxib was comparable to naproxen 1000 mg, as shown by the 95% CIs for the between‐group mean differences, which were contained within the ±10 mm equivalence bound. The placebo group had a significantly higher discontinuation rate due to lack of efficacy than both the etoricoxib (p<0.001) and naproxen 1000 mg groups (p<0.001) (fig 1). Efficacy responses in the etoricoxib and naproxen groups were not significantly different. Furthermore, differences between the active treatments and placebo were seen at the earliest time point of measurement (2 weeks after the start of the study drug) and persisted at about the same magnitude for the 12 weeks. Onset of treatment effect, as assessed by the WOMAC pain walking on flat surface and patient global assessment of response to treatment recorded at 4 hours after the dose, was seen as early as day 1. Duration of treatment effect after treatment with etoricoxib or naproxen, as assessed by the WOMAC pain walking on flat surface and patient global assessment of response to treatment recorded at 24 hours after the dose, was significantly different relative to placebo from day 2 onwards.
For patients who continued to receive the same treatment (etoricoxib or naproxen) during parts I and II of the studies, treatment effects, as measured by the time‐weighted average change from baseline over the entire 52 weeks of the studies, were similar between etoricoxib and naproxen 1000 mg. Efficacy was maintained at a consistent level over the 52 weeks of the studies for both the etoricoxib and naproxen 1000 mg treatment groups.
Efficacy over 138 weeks
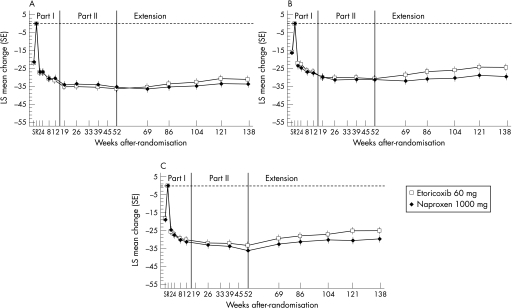
Graphical examination of the adjusted mean changes from baseline for the three primary end points (WOMAC pain subscale, WOMAC physical function subscale, and patient global assessment of disease) demonstrated a relatively constant treatment effect over the entire 138‐week extension period; results were similar for the etoricoxib and naproxen groups (fig 2). Clinically important treatment effects from etoricoxib and naproxen were seen from the first treatment period at week 2; these treatment effects were significantly better than those of placebo during part I (table 2; fig 2).
Figure 2 Mean (SE) change from baseline over time in patients continuing to receive the same treatment from baseline to week 121 for the primary end points: (A) WOMAC pain subscale (100 mm visual analogue scale (VAS)); (B) WOMAC physical function subscale (100 mm VAS); (C) patient and investigator global assessments of disease status (100 mm VAS).
Table 2 LS mean changes (95% CI) from baseline in the 52‐week base studies (analysis of time‐weighted average response to treatment).
| Treatment group | WOMAC pain subscale (VAS*) | WOMAC physical function subscale (VAS*) | Patient global assessment of disease status (VAS*) |
|---|---|---|---|
| Part I (12‐week treatment period) | |||
| Placebo | −15.31 (−19.25 to −11.37) | −10.27 (−14.19 to −6.35) | −13.38 (−17.51 to −9.26) |
| Etoricoxib 60 mg | −27.94 (−30.03 to −25.85) | −22.81 (−24.89 to −20.74) | −26.39 (−28.57 to −24.21) |
| Naproxen 1000 mg | −28.57 (−30.68 to −26.47) | −23.70 (−25.78 to −21.61) | −26.46 (−28.66 to −24.26) |
| Parts I and II (52‐week treatment period; in patients receiving the same treatment for 52 weeks) | |||
| Etoricoxib 60 mg | −31.03 (−33.19 to −28.86) | −25.96 (−28.24 to −23.69) | −27.58 (−29.83 to −25.32) |
| Naproxen | −30.60 (−32.82 to −28.39) | −26.06 (−28.39 to −23.73) | −27.82 (−30.14 to −25.51) |
WOMAC, Western Ontario and McMaster Universities OA Index; VAS, visual analogue scale.
*0–100 mm scale.
Safety results
All treatments were generally well tolerated in all periods of the studies. The percentage of patients with any AEs and serious AEs was similar among all treatment groups. In each study period, the naproxen group had a numerically greater percentage of patients discontinuing owing to an AE as well as the greatest percentage of patients with drug‐related AEs. Regardless of treatment group, the most common AEs in the three study periods overall were upper respiratory infection and hypertension (table 3).
Table 3 Incidence of clinical adverse experiences (AEs), by study period.
| 12‐Week part I of base studies | 40‐Week part II of base studies | 86‐Week extension period | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 112) | Etoricoxib (n = 446) | Naproxen (n = 439) | Etoricoxib (n = 434) | Naproxen (n = 404) | Etoricoxib (n = 246) | Naproxen (n = 217) | ||
| Any AE | 57 (50.9) | 262 (58.7) | 279 (63.6) | 301 (69.4) | 276 (68.3) | 179 (72.8) | 181 (83.4) | |
| Serious AEs | 1 (0.9) | 6 (1.3) | 9 (2.1) | 32 (7.4) | 30 (7.4) | 26 (10.6) | 33 (15.2) | |
| Discontinuations due to AEs | 9 (8.0) | 24 (5.4) | 44 (10.0) | 37 (8.5) | 46 (11.4) | 24 (9.8) | 28 (12.9) | |
| Drug related AEs | 19 (17.0) | 96 (21.5) | 128 (29.2) | 76 (17.5) | 91 (22.5) | 42 (17.1) | 58 (26.7) | |
| Most common AEs (⩾5.0% in any treatment group) | ||||||||
| Abdominal pain | 2 (1.8) | 7 (1.6) | 22 (5.0) | 8 (1.8) | 10 (2.5) | 2 (0.8) | 11 (5.1) | |
| Influenza‐like disease | 2 (1.8) | 13 (2.9) | 13 (3.0) | 27 (6.2) | 13 (3.2) | 5 (2.0) | 11 (5.1) | |
| Upper respiratory infection | 6 (5.4) | 34 (7.6) | 35 (8.0) | 47 (10.8) | 43 (10.6) | 33 (13.4) | 18 (8.3) | |
| Hypertension | 7 (6.3) | 23 (5.2) | 13 (3.0) | 32 (7.4) | 17 (4.2) | 27 (11.0) | 23 (10.6) | |
| Dyspepsia | 2 (1.8) | 9 (2.0) | 22 (5.0) | 11 (2.5) | 11 (2.7) | 6 (2.4) | 5 (2.3) | |
| Epigastric discomfort | 3 (2.7) | 13 (2.9) | 24 (5.5) | 13 (3.0) | 17 (4.2) | 6 (2.4) | 9 (4.1) | |
| Heartburn | 4 (3.6) | 12 (2.7) | 23 (5.2) | 10 (2.3) | 10 (2.5) | 4 (1.6) | 4 (1.8) | |
| Nausea | 4 (3.6) | 14 (3.1) | 23 (5.2) | 8 (1.8) | 4 (1.0) | 3 (1.2) | 4 (1.8) | |
| Sinusitis | 2 (1.8) | 9 (2.0) | 7 (1.6) | 8 (1.8) | 15 (3.7) | 13 (5.3) | 12 (5.5) | |
| Back pain | 6 (5.4) | 3 (0.7) | 6 (1.4) | 21 (4.8) | 12 (3.0) | 15 (6.1) | 13 (6.0) | |
| Bronchitis | 1 (0.9) | 9 (2.0) | 6 (1.4) | 14 (3.2) | 12 (3.0) | 11 (4.5) | 12 (5.5) | |
| Urinary tract infection | 0 (0.0) | 14 (3.1) | 11 (2.5) | 21 (4.8) | 20 (5.0) | 13 (5.3) | 18 (8.3) | |
Results are shown as No (%).
Hypertension occurred in 6.3% (placebo), 5.2% (etoricoxib), and 3.0% (naproxen) of patients during the placebo‐controlled part I; 7.4% (etoricoxib) and 4.2% (naproxen) during part II of the base period; and 11% (etoricoxib) and 10.6% (naproxen) during the 86‐week extension period. The observed increase in incidence over time in all treatment groups is not unexpected as these results represent a cumulative incidence of AEs over time. Other renovascular AEs such as lower extremity oedema and congestive heart failure occurred at a lower incidence than that found with hypertension AEs and with similar incidence among the treatment groups in all three treatment periods. Discontinuations owing to renovascular AEs were rare in all treatment groups (table 4).
Table 4 AEs of special interest.
| Special interest AEs | 12‐Week part I of base studies | 40‐Week part II of base studies | 86‐Week extension period | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 112) | Etoricoxib (n = 446) | Naproxen (n = 439) | Etoricoxib (n = 434) | Naproxen (n = 404) | Etoricoxib (n = 246) | Naproxen (n = 217) | ||
| GI nuisance symptoms | ||||||||
| GI nuisance AEs | 14 (12.5) | 50 (11.2) | 102 (23.2) | 54 (12.4) | 51 (12.6) | 19 (7.7) | 31 (14.3) | |
| Discontinuations | 2 (1.8) | 5 (1.1) | 18 (4.1) | 6 (1.4) | 8 (2.0) | 0 (0.0) | 3 (1.4) | |
| Renovascular AEs | ||||||||
| Hypertension | 7 (6.3) | 23 (5.2) | 13 (3.0) | 32 (7.4) | 17 (4.2) | 27 (11.0) | 23 (10.6) | |
| Discontinuations | 0 (0.0) | 1 (0.2) | 1 (0.2) | 1 (0.2) | 0 (0.0) | 1 (0.4) | 0 (0.0) | |
| Lower extremity oedema | 2 (1.8) | 10 (2.2) | 9 (2.1) | 10 (2.3) | 13 (3.2) | 8 (3.3) | 7 (3.2) | |
| Discontinuations | 1 (0.9) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.4) | 0 (0.0) | |
| Congestive heart failure | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 2 (0.5) | 0 (0.0) | 2 (0.9) | |
| Discontinuations | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | |
| Confirmed serious thrombotic CV AEs and upper GI bleeding AEs | ||||||||
| Confirmed serious thrombotic CV AEs | 0 (0.0) | 1 (0.2) | 0 (0.0) | 10 (2.3) | 2 (0.5) | 2 (0.8) | 4 (1.8) | |
| Confirmed upper GI bleeding AEs | 0 (0.0) | 0 (0.0) | 7 (1.6) | 5 (1.2) | 11 (2.7) | 2 (0.8) | 13 (5.9) | |
Results are shown as No (%).
AEs, adverse experiences; GI, gastrointestinal; CV, cardiovascular.
In part I, the incidence of GI nuisance AEs (ie, abdominal pain, acid reflux, dyspepsia, epigastric discomfort, heartburn, nausea and vomiting) was similar for etoricoxib and placebo; GI nuisance AEs were more common in the naproxen group. In part II, the etoricoxib and naproxen groups had a similar proportion of patients with GI nuisance AEs, whereas they occurred with greater frequency in the naproxen group compared with the etoricoxib group during the extension period (table 4).
Upper GI PUB events (confirmed by an external adjudication committee) did not occur in patients receiving etoricoxib during part I of the base period, while 7 (1.6%) patients receiving naproxen experienced a GI PUB. In part II of the base period, 5 (1.2%) patients receiving etoricoxib and 11 (2.7%) patients receiving naproxen experienced a GI PUB event. GI PUB events occurred in 2 (0.8%) patients in the etoricoxib group and 13 (5.9%) patients in the naproxen group during the 86‐week extension period. The following specific PUB events occurred in the etoricoxib group: duodenal ulcer and upper GI haemorrhage. In the naproxen group, duodenal ulcers, gastric ulcers, and upper GI haemorrhages occurred.
During part I, 1 (0.2%) patient in the etoricoxib group had a confirmed serious CV AE compared with none in the naproxen group. In part II, 10 (2.3%) etoricoxib patients had thrombotic CV events compared with 2 (0.5%) naproxen patients. Confirmed thrombotic CV events occurred in 2 (0.8%) etoricoxib patients and 4 (1.8%) naproxen patients in the 86‐week extension period. These thrombotic CV events included acute myocardial infarction and ischaemic stroke in the etoricoxib group, and, in the naproxen group, acute myocardial infarction, transient ischaemic attack, and a pulmonary embolism. All patients with confirmed thrombotic CV events recovered.
Discussion
The present report provides data from a combined analysis of two, long‐term studies of identical design comparing the efficacy and safety of etoricoxib 60 mg and naproxen 1000 mg in patients with OA. The 12‐week, placebo‐controlled period of the international study from the present analysis was previously reported. The efficacy of etoricoxib was better than that of placebo and similar to that of naproxen; both etoricoxib and naproxen were also generally well tolerated.14 The base period of the American study demonstrated similar results.15 In the present, combined analysis of both studies, the efficacy of etoricoxib and naproxen was comparable; clinical improvements, as assessed by the primary efficacy end points, were observed by the first treatment visit, 2 weeks after randomisation, and maintained for up to 138 weeks. Both treatments were generally well tolerated. Although a similar proportion of patients in each treatment group experienced an AE over the entire course of these studies, the specific types of AE that occurred in each treatment group differed to some degree.
Hyptertension is a common comorbidity in patients with OA and is also a condition that may be associated with the use of both selective and traditional NSAIDs owing to their effects on renal prostaglandins.16,17 In a pooled analysis of studies in the etoricoxib development programme, etoricoxib demonstrated a shallow dose response with a generally similar incidence of hypertension to that of traditional NSAIDs. In comparisons with ibuprofen, the incidence was slightly lower with etoricoxib, whereas in comparisons with naproxen the incidence was slightly higher with etoricoxib; none of these differences were interpreted as clinically meaningful.12 In a large, randomised, controlled trial in patients with OA comparing etoricoxib 90 mg (1.5 times the recommended OA dose) versus the traditional NSAID, diclofenac 150 mg, etoricoxib 90 mg demonstrated a significantly higher incidence of hypertension.18 In the current studies, hypertension was among the most common AE to occur for both etoricoxib and naproxen. The incidence of hypertension was numerically greater with etoricoxib than with naproxen, which is consistent with the results of previous analyses.12 The medical significance of these observations is probably limited, however, as discontinuations from hypertension were infrequent and generally similar among both groups. Furthermore, the occurrence of other renovascular AEs, such as lower extremity oedema and congestive heart failure, were generally similar among patients who received etoricoxib and naproxen throughout the 138‐week treatment period. These data demonstrate the importance of monitoring the blood pressure of all patients who are treated with any NSAID, including etoricoxib.
Previous studies have suggested that etoricoxib is associated with a lower frequency of gastrointestinal AEs than patients receiving chronic treatment with traditional, non‐selective NSAIDs.19,20 The present analysis supports the outcomes from these previous studies; patients receiving etoricoxib experienced GI AEs with a reduced incidence in comparison with patients receiving naproxen during part I of the study. Owing to the self‐selected nature of the study group beyond part I of the study, the lack of a demonstrable difference in GI tolerability among the treatment groups was not unexpected. However, there was an observable difference in GI tolerability during the extension period in favour of etoricoxib, although the extension data should be viewed with caution because they also are not representative of a randomised patient group. Additionally, the proportion of patients with GI perforations, ulcers, or bleeding was smaller in the etoricoxib group than in the naproxen group.
Although these studies were not powered specifically to examine CV risk, this report presents the available data obtained from them. In the current studies, the incidence of thrombotic CV events was low in each treatment group, with a greater proportion of patients experiencing a thrombotic CV event in the etoricoxib group than in the naproxen group. These results are consistent with a previous analysis of CV data from the etoricoxib development programme, in which confirmed thrombotic CV events occurred at a similar rate among patients treated with etoricoxib and traditional NSAIDs, with the exception of naproxen. In comparisons with naproxen, the rate of confirmed thrombotic CV events was higher for etoricoxib.21 These results are also consistent with CV safety data observed in randomised trials of other COX‐2 selective inhibitors, in which a lower incidence of thrombotic CV events was observed with naproxen.7,22,23
Conclusions
In summary, etoricoxib 60 mg and naproxen 1000 mg had similar efficacy for the treatment of OA, which was maintained over 138 weeks. Both agents were generally well tolerated. Although these studies were not powered to evaluate the relative risk of GI or CV events, the safety data from these studies suggest that etoricoxib has a more favourable GI safety and tolerability profile than naproxen, whereas naproxen is associated with a numerically lower incidence of thrombotic CV events.
Acknowledgements
We thank the following investigators who participated in these studies: Lebovicz, Richard; Bays, Harold; Beaulieu, A; Bernstein, David; Box, Jane; Brabham, A; Britt, David; Caldwell, Jacques; Collins, Gregory; Corn, Lydia; Divittorio, Gino; Dolan, Geoffrey; Edwards, William; Elinoff, Victor; Ettinger, Mark; Fisher, CL; Gillie, Edward; Goldberg, Marc; Greth, Warren; Gutierrez, Maria; Henry, Daniel; Zizic, Thomas; Sandall, Paul; Kay, Jonathan; Davis, Jeffrey; McIlwain, Harris; Miller, S David; Nies, Kenneth; O'Barr, Jr, Thomas; Offenberg, Howard; Paster, R Zorba; Resnick, Harvey; Ripley, Peter; Ryan, Michael; Safdi, Alan; Schiff, Michael; Severance, Randall; Shaul, Stephen; Tesser, John; Weaver, Arthur; Wolfe, Sanford; Abbott, Richard; Weerasinghe, Mervyn; Saxe, Philippe; Bockow, Barry; Sheldon, Eric; Hassman, David; Emori, H Walter; Saaibi, Diego; Garza‐Elizondo, Mario; Morales, Jorge; Gallacher, Alberto; Zanchetta, Jose; Castro, Ricardo; Garcia, Abraham; BarretoGameiro Silva, Marilia; Hidalgo, Arquimedes; Brighton, Stan; VanDuuren, Elsa; Sarembock, Brian; Poor, Gyula; Tamasi, Laszlo; Gal, Janos; Bereczki, Janos; Maluje, Viviana; Taylor, Sue; Handel, Malcolm; Mathers, David; Bell, Mary; Cividino, Alfred; Menard, Henri; Verdejo, Alfonso; Bianchi, Gerolamo; Mader, Reuven; Bonafede, Peter
Abbreviations
AEs - adverse experiences
CI - confidence interval
COX - cyclo‐oxygenase
CV - cardiovascular
GI - gastrointestinal
NSAIDs - non‐steroidal anti‐inflammatory drugs
OA - osteoarthritis
PUBs - perforations, ulcers, or bleeding events
VAS - visual analogue scale
WOMAC - Western Ontario and McMaster Universities OA Index
References
- 1.Dieppe P A, Lohmander L S. Pathogenesis and management of pain in osteoarthritis. Lancet 2005365965–973. [DOI] [PubMed] [Google Scholar]
- 2.Solomon L. Clinical features of osteoarthritis. In: Ruddy S, Harris ED, Sledge CB, eds. Kelley's textbook of rheumatology. 6th ed. Philadelphia, London, New York, St. Louis, Sydney, Toronto: Saunders, 20011409–1418.
- 3.Petersson I F. Occurrence of osteoarthritis of the peripheral joints in European populations. Ann Rheum Dis 199655659–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence R C, Helmick C G, Arnett F C, Deyo R A, Felson D T, Giannini E H.et al Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 199841778–799. [DOI] [PubMed] [Google Scholar]
- 5.Fries J F. NSAID gastropathy: the second most deadly rheumatic disease? Epidemiology and risk appraisal. J Rheumatol Suppl 1991286–10. [PubMed] [Google Scholar]
- 6.Gabriel S E, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti‐inflammatory drugs. A meta‐analysis. Ann Intern Med 1991115787–796. [DOI] [PubMed] [Google Scholar]
- 7.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos‐Vargas R, Davis B.et al Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 20003431520–1528. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto A K, Cavanaugh P F., Jr Etoricoxib. Drugs Today (Barc) 200440395–414. [PubMed] [Google Scholar]
- 9.Solomon S D, McMurray J J, Pfeffer M A, Wittes J, Fowler R, Finn P.et al Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 20053521071–1080. [DOI] [PubMed] [Google Scholar]
- 10.Bresalier R S, Sandler R S, Quan H, Bolognese J A, Oxenius B, Horgan K.et al Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 20053521092–1102. [DOI] [PubMed] [Google Scholar]
- 11.Chan A T, Manson J E, Albert C M, Chae C U, Rexrode K M, Curhan G C.et al Nonsteroidal antiinflammatory drugs, acetaminophen, and the risk of cardiovascular events. Circulation 20061131578–1587. [DOI] [PubMed] [Google Scholar]
- 12.Curtis S P, Ng J, Yu Q, Shingo S, Bergman G, McCormick C L.et al Renal effects of etoricoxib and comparator nonsteroidal anti‐inflammatory drugs in controlled clinical trials. Clin Ther 20042670–83. [DOI] [PubMed] [Google Scholar]
- 13.Gottesdiener K, Schnitzer T, Fisher C, Bockow B, Markenson J, Ko A.et al Results of a randomized, dose‐ranging trial of etoricoxib in patients with osteoarthritis. Rheumatology (Oxford) 2002411052–1061. [DOI] [PubMed] [Google Scholar]
- 14.Leung A T, Malmstrom K, Gallacher A E, Sarembock B, Poor G, Beaulieu A.et al Efficacy and tolerability profile of etoricoxib in patients with osteoarthritis: a randomized, double‐blind, placebo and active‐comparator controlled 12‐week efficacy trial. Curr Med Res Opin 20021849–58. [DOI] [PubMed] [Google Scholar]
- 15.Fisher C A, Curtis S P, Resnick H, Ripley P, Leung A T, Ko A T.et al Treatment with etoricoxib, a COX‐2 selective inihibitor, resulted in clinical improvement in knee and hip osteoarthritis (OA) over 52 weeks [abstract]. Arthritis Rheum 200144(Suppl)S135 [Google Scholar]
- 16.Breedveld F C. Osteoarthritis—the impact of a serious disease. Rheumatology (Oxford) 200443(Suppl 1)i4–i8. [DOI] [PubMed] [Google Scholar]
- 17.Brater D C. Anti‐inflammatory agents and renal function. Semin Arthritis Rheum 20023233–42. [DOI] [PubMed] [Google Scholar]
- 18.Baraf H S B, Fuentealba C, Greenwald M.et al Tolerability and effectiveness of etoricoxib compared to diclofenac sodium in patients with osteoarthritis: a randomized controlled study (EDGE trial). Arthritis Rheum 200450(Suppl)346–347. [Google Scholar]
- 19.Ramey D, Watson D J, Yu C, Bolognese J A, Curtis S P, Reicin A S. The incidence of upper gastrointestinal adverse events in clinical trials of etoricoxib vs. non‐selective NSAIDs: an updated combined analysis analysis, Curr Med Res Opin 200521715–722. [DOI] [PubMed] [Google Scholar]
- 20.Hunt R H, Harper S, Watson D J, Yu C, Quan H, Lee M.et al The gastrointestinal safety of the COX‐2 selective inhibitor etoricoxib assessed by both endoscopy and analysis of upper gastrointestinal events. Am J Gastroenterol 2003981725–1733. [DOI] [PubMed] [Google Scholar]
- 21.Curtis S P, Ko A T, Bolognese J A, Cavanaugh P F, Jr, Reicin A S. Pooled analysis of thrombotic cardiovascular events in clinical trials of the COX‐2 selective inhibitor etoricoxib. Curr Med Res Opin 2006222365–2374. [DOI] [PubMed] [Google Scholar]
- 22.Farkouh M E, Kirshner H, Harrington R A, Ruland S, Verheugt F W, Schnitzer T J.et al Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet 2004364675–684. [DOI] [PubMed] [Google Scholar]
- 23.Kearney P M, Baigent C, Godwin J, Halls H, Emberson J R, Patrono C. Do selective cyclo‐oxygenase‐2 inhibitors and traditional non‐steroidal anti‐inflammatory drugs increase the risk of atherothrombosis? Meta‐analysis of randomised trials. BMJ 20063321302–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]