Renal insufficiency is a common comorbidity in patients with inflammatory rheumatic diseases such as rheumatoid arthritis and psoriatic arthritis. Decreased renal function is a limiting factor for the use of non‐steroidal anti‐inflammatory drugs and disease‐modifying antirheumatic drugs. Therefore, the availability of therapeutic alternatives would improve our treatment options for these patients. For tumour necrosis factor α (TNFα) inhibitors, no data about their use in patients with impaired renal function are available, because it has been an exclusion criterion in all major clinical trials.
We therefore retrospectively analysed all patients from our institution who had increased serum creatinine levels before or during treatment with TNFα antagonists. All patients with a serum creatinine ⩾1.1 mg/dl and treatment with infliximab, etanercept or adalimumab were included. We were able to identify nine patients with rheumatoid arthritis, one patient with psoriatic arthritis and one patient with juvenile rheumatoid arthritis who had end‐stage renal disease (ESRD) and was receiving haemodialysis (excluded from the analysis because of ESRD). Table 1 shows demographic data, reasons for decreased renal function and comorbidities.
Table 1 Characteristics of patients with renal insufficiency and anti‐tumour necrosis factor α therapy.
| Patient | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Age (years) | 30 | 64 | 65 | 65 | 67 | 71 | 72 | 63 | 76 | 50 | 34 |
| Sex | M | M | F | F | F | F | F | F | M | M | F |
| Diagnosis | RA | RA | RA | RA | RA | RA | RA | RA | RA | PsA | JRA |
| Use of anti‐TNFα therapy | ETC | ETC | ETC | ETC | ETC | ADM, INX, ETC | ADM, INX, ETC | INX | INX | INX | INX |
| Concomitant diseases: | |||||||||||
| Hypertension | + | − | + | + | + | + | + | + | + | + | + |
| Diabetes | + | − | − | − | − | + | − | + | + | − | − |
| Non‐steroidal anti‐inflammatory drug nephropathy | − | − | − | − | − | − | + | + | − | − | − |
| Sarcoidosis | − | − | − | − | − | − | − | − | − | − | + |
| EULAR response | Good | Good | None | Good | None | Mod | None | None | None | Rem | Good |
| Creatinine (mg/dl) | |||||||||||
| Month 0 | 1.34 | 4.32 | 1.13 | 1.5 | 1.99 | 1.5 | 1.09 | 1.34 | 1.57 | 1.12 | ESRD |
| Month 3 | 1.41 | 4.33 | 1.19 | 1.54 | 1.81 | 1.6 | 1.23 | 1.34 | 1.21 | 1.13 | |
| Month 6 | 1.38 | 4.85 | 1.07 | 1.59 | 1.96 | 1.9 | 1.12 | 1.46 | 1.05 | 1.04 | |
| End of study interval (months) | 1.71 (17) | 5.46 (22) | 1.26 (24) | 2.2 (19) | 2.47 (13) | 2.1 (15) | 1.13 (10) | 1.45 (22) | 2.14 (21) | 1.2 (20) | |
| Maximum | 1.71 | 5.46 | 1.26 | 2.2 | 2.47 | 2.1 | 1.31 | 1.68 | 2.14 | 1.24 | |
ADM, adalimumab; ETC, etanercept; ESRD, end stage renal disease; EULAR, European League Against Rheumatism; F, female; INX, infliximab; JRA, juvenile rheumatoid arthritis; M, male; Mod, moderate response; NSAID, non‐steroidal anti‐inflammatory drug; PsA, psoriatic arthritis; RA, rheumatoid arthritis; Rem, clinical remission; TNFα, tumour necrosis factor α.
Two patients were treated with all biological therapy (ETC, ADM and INX); the others were treated with only one biological therapy. The end of the study interval indicates the duration of our observation period with any of the biological treatments. Patients responding to this treatment continued to receive anti‐TNFα therapy. Values in parentheses denote the period of the study time of the study interval for each patient in months.
The mean age of patients was 62.3 years (range 30–76 years) and the mean creatinine level at the start of the study was 1.6 mg/dl. Risk factors for kidney disease were hypertension (n = 10), diabetes (n = 4) and non‐steroidal anti‐inflammatory drug induced nephropathy (n = 2).
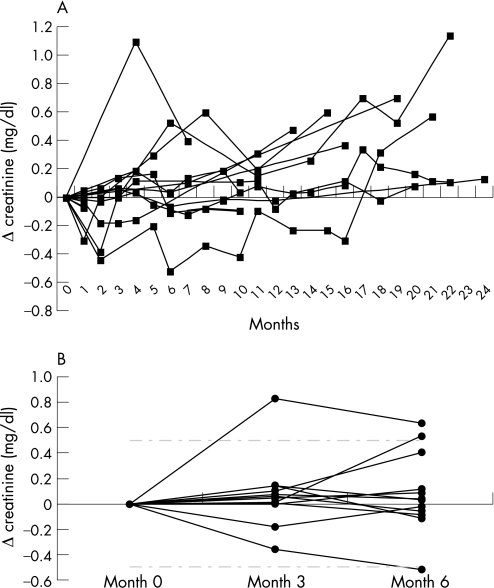
Figure 1A shows the course of serum creatinine concentrations during anti‐TNFα therapy. The follow‐up period was up to 24 months. In a statistical analysis of creatinine levels after 3 and 6 months using Friedman's test, no significant increases from baseline could be detected (p>0.05, fig 1B). Additionally, no differences among the TNFα antagonists could be observed. Adalimumab, however, was given to only two of the 10 outpatients for a relatively short period. The mean change in creatinine was 0.07 mg/dl after 3 months and 0.09 mg/dl after 6 months. However, in some patients, there was a trend towards an increase in creatinine at later time points, presumably because of a progression of the underlying kidney disease. No severe infections or other serious adverse events were observed in our patients during anti‐TNFα therapy. According to the European League Against Rheumatism response criteria, we observed four patients with good response, one with moderate response and five with no response in the first 3 months.
Figure 1 Course of creatinine in renal insufficiency with concomitant anti‐tumour necrosis factor alpha (TNFα) therapy. (A) Time course of change (Δ) in serum creatinine concentrations in 10 patients during anti‐TNFα therapy. (B) Creatinine changes (Δ) within the first 6 months of anti‐TNFα therapy. Dashed‐dotted line depicts change in creatinine of 0.5 mg/dl. No significant increases, as analysed by Friedman's test.
Reports on the occurrence of renal side effects during anti‐TNFα therapy are scarce. These have included immune complex vasculitis, lupus nephritis and nephritic syndrome.1,2,3 In these cases, renal symptoms were reversible after withdrawal of TNFα antagonists. One case report demonstrated safe application in a patient with ESRD receiving haemodialysis, which is confirmed by one case reported here.4 In addition, anti‐TNFα treatment was well tolerated and safe in the 15 patients with renal involvement of amyloid A amyloidosis.5 No side effects on renal function have been reported so far from randomised clinical trials; however, patients with impaired renal function have been excluded from these trials.6,7,8 In conclusion, our data indicate that the use of TNFα inhibitors has no negative effect on renal function in patients with kidney disease.
Acknowledgements
We thank the Committee “Pharmakotherapie” of the German Society of Rheumatology for support, and Dr Uppenkamp (Klinikum Ludwigshafen, Germany) and Dr Schmitter (Munich, Germany) for providing patient data; we also thank Dr F. Schiller for the statistical analysis.
Abbreviations
ESRD - end‐stage renal disease
TNFα - tumour necrosis factor α
Footnotes
Funding: AJH received funding for lectures from Abbott. AT has nothing to disclose. BM received honoraria for lectures and participation in advisory boards from Abbott, Wyeth and Schering‐Plough, and GS received honoraria for lectures and participation in advisory boards from Abbott, Amgen, Essex, Bristol‐Myers Squibb and Schering‐Plough.
Competing interests: None.
References
- 1.Stokes M B, Foster K, Markowitz G S, Ebrahimi F, Hines W, Kaufman D.et al Development of glomerulonephritis during anti‐TNF‐alpha therapy for rheumatoid arthritis. Nephrol Dial Transplant 2005201400–1406. [DOI] [PubMed] [Google Scholar]
- 2.Chin G, Luxton G, Harvey J M. Infliximab and nephrotic syndrome. Nephrol Dial Transplant 2005202824–2826. [DOI] [PubMed] [Google Scholar]
- 3.den Broeder A A, Assmann K J, van Riel P L, Wetzels J F. Nephrotic syndrome as a complication of anti‐TNFalpha in a patient with rheumatoid arthritis. Neth J Med 200361137–141. [PubMed] [Google Scholar]
- 4.Singh R, Cuchacovich R, Huang W, Espinoza L R. Infliximab treatment in a patient with rheumatoid arthritis on hemodialysis. J Rheumatol 200229636–637. [PubMed] [Google Scholar]
- 5.Gottenberg J E, Merle‐Vincent F, Bentaberry F, Allanore Y, Berenbaum F, Fautrel B.et al Anti‐tumor necrosis factor alpha therapy in fifteen patients with AA amyloidosis secondary to inflammatory arthritides: a followup report of tolerability and efficacy. Arthritis Rheum 2003482019–2024. [DOI] [PubMed] [Google Scholar]
- 6.Lee S J, Kavanaugh A. Adverse reactions to biologic agents: focus on autoimmune disease therapies. J Allergy Clin Immunol 2005116900–905. [DOI] [PubMed] [Google Scholar]
- 7.Roberts L, McColl G J. Tumour necrosis factor inhibitors: risks and benefits in patients with rheumatoid arthritis. Intern Med J 200434687–693. [DOI] [PubMed] [Google Scholar]
- 8.Voulgari P V, Alamanos Y, Nikas S N, Bougias D V, Temekonidis T I, Drosos A A. Infliximab therapy in established rheumatoid arthritis: an observational study. Am J Med 2005118515–520. [DOI] [PubMed] [Google Scholar]