Abstract
Background
Non‐vertebral (NV) fractures are responsible for a great amount of morbidity, mortality and cost attributable to osteoporosis.
Objectives
To identify risk factors for NV fractures in postmenopausal women with osteoporosis, and to design an assessment tool for prediction of these fractures.
Methods
2546 postmenopausal women with osteoporosis included in the placebo groups of three risedronate controlled trials were included (mean age 72 years, mean femoral T‐score −2.5; 60% and 53% of patients with prevalent vertebral and NV fractures, respectively). Over 3 years, 222 NV fractures were observed. Baseline data on 14 risk factors were included in a logistic regression analysis.
Results
6 risk factors were associated with NV fracture risk: prevalent NV fracture (p = 0.004), number of prevalent vertebral fractures (p<0.001), femoral T‐score (p = 0.031), serum level of 25‐hydroxyvitamin D (p<0.001), age (p = 0.012) and height (p = 0.037). An NV risk index was developed by converting the multivariate logistic equation into an additive score. In the group of women with a score ⩾2.1, the incidence of NV fracture was 13.2% (95% CI 11.1 to 15.3), 1.5 times higher than that of the general population.
Conclusions
The NV risk index is a convenient tool for selection of patients with osteoporosis with a high risk for NV fractures, and may help to choose from available treatments those with a proven efficacy for reduction of NV fracture risk.
Non‐vertebral (NV) fractures are common, affecting 1% of the population/year,1 and are responsible for a large proportion of the morbidity, mortality and cost attributable to osteoporosis. Studies have shown an increase in mortality associated with hip fractures,2 which account for 10% of all fractures and 40% of the total fractures after the age of 80. However, other NV fractures are frequent; wrist fractures are as common as hip fractures, and the lifetime risk of either fracture in postmenopausal women is about 16%.3 Some NV fractures, such as fractures of the proximal humerus, pelvis and ribs, show an increase in incidence in older women.1,4 Proximal humerus fractures are the third most frequent fracture after hip and Colles' fracture in older women, and are responsible for impairment of functional capacity for an average of 3 months.5 There is a slight but significant increase in mortality in the year after a shoulder fracture.2 A short‐term increase in morbidity has been shown in some patients after limb fracture, related to algodystrophy, which may be responsible for persistent disability.6 The incidence of hip and limb fractures is increasing in Western populations. Moreover, a previous fracture is a strong predictor of a future one,7 and the risk of vertebral and hip fractures increases fivefold in patients with Colles' fractures.8,9,10 Focusing on hip fracture underestimates the consequences of osteoporosis and the burden of all non‐vertebral osteoporotic fractures.
Reductions in the risk of vertebral fracture have been very consistent in various trials with different agents11,12,13,14,15,16 decreasing significantly the burden of osteoporosis related to these fractures. By contrast, the reported effects of these treatments on NV fractures have varied significantly.17,18,19,20 It remains unclear to what extent the differences in the magnitude of effect reflect differences in study populations and trial design rather than differences in antifracture efficacy. There are no means to compare the anti‐fracture effect of the different treatments, as no head‐to‐head studies have been conducted with such an end point. However, the different effect on NV fracture risk may be one of the determinants in the choice of the treatment for a postmenopausal woman with osteoporosis.
The assessment of NV fracture risk in postmenopausal women is difficult and the relationship between risk factors and fractures differs by type of fracture.21,22 NV fractures usually occur in conjunction with falls: preventive actions against falls are recommended,23 but there is no evidence that they have efficacy for reduction of fracture risk.24 By contrast, the other factor driving NV fracture risk is skeletal fragility, and the number and severity of vertebral fractures significantly predict NV fracture risk.25,26,27 Moreover, a small number of hip fractures occur spontaneously, without any trauma,28,29 indicating that bone fragility can be responsible for such a fracture before a fall. Thus, the risk of NV fracture may be assessed at least in part using bone parameters.
The objectives of this study were to identify risk factors for NV fractures in postmenopausal women with osteoporosis, and to design an assessment tool for prediction of these fractures.
Patients and methods
Postmenopausal women included in the placebo groups of three risedronate prospective controlled trials were included in this study; these trials have been described in detail elsewhere.11,12,30 These multicentre studies were randomised, double‐blind, placebo‐controlled, parallel‐group trials conducted in postmenopausal women with osteoporosis. In the North American Vertebral Efficacy with Risedronate Therapy (VERT‐NA)11 and Multinational Vertebral Efficacy with Risedronate Therapy (VERT‐MN) studies,12 subjects were enrolled if they had at least two prevalent vertebral fractures. In the North American Vertebral Efficacy with Risedronate Therapy study,11 subjects could also be enrolled if they had one prevalent vertebral fracture and a lumbar spine bone mineral density (BMD) T‐score ⩽2 SD below the normal mean for young adults. In both trials, women were ambulatory, <85 years old, and at least 5 years postmenopausal. In the Hip Intervention Programme trial,30 women were aged 70–79 years, with a low femoral neck BMD—that is, T‐score >4 SD below the mean peak value in young adults, or <–3SD, together with a non‐skeletal risk factor for hip fracture. All patients received 1000 mg of calcium daily and up to 500 IU daily of vitamin D if baseline serum hydroxyvitamin D level was <16 ng/ml (40 nmol/l). The primary end point of these trials was the incidence of fractures, which were classified using the same procedures.
On the basis of known risk factors and items available in the studies, a total of 14 parameters were assessed (table 1) using information collected at baseline. Six NV fractures were considered for this analysis: hip, leg, pelvis, wrist, humerus and clavicle.31 Information on these fractures was collected prospectively during follow‐up and confirmed by reviewing either the x ray or the radiology report. Fractures due to pathological causes or resulting from high‐energy trauma were excluded from the analysis.
Table 1 Characteristics of the 2546 women in the study to determine the risk of non‐vertebral fractures.
| Parameter | ITT population (n = 3043) | All risk factor data (n = 2546) |
|---|---|---|
| Trials (number of patients) | ||
| VERT‐MN | 407 | 381 |
| VERT‐NA | 815 | 777 |
| HIP group 1 | 1821 | 1388 |
| Age (years) | ||
| Mean (SD) | 72 (5.7) | 72 (5.9) |
| Years since menopause | ||
| Mean (SD) | 26 (8.6) | 26 (8.8) |
| Height (cm) | ||
| Mean (SD) | 157 (7.0) | 157 (7.0) |
| Weight (cm) | ||
| Mean (SD) | 63 (11.9) | 63 (11.9) |
| BMI (kg/m2) | ||
| Mean (SD) | 25.5 (4.5) | 25.6 (4.5) |
| Prevalent vertebral fracture | ||
| Mean (SD) | 2 (2.4) | 2 (2.4) |
| Yes/total | 1618/2670 (60%) | 1531/2546 (60%) |
| Prevalent NV fracture status | ||
| Yes/total | 1612/3043 (53%) | 1342/2546 (53%) |
| FN T‐score (NHANES III) | ||
| Mean (SD) | −2.57 (0.698) | −2.53 (0.717) |
| Vitamin D serum 25 (OH) D3 (ng/ml) | ||
| Mean (SD) | 60.9 (30.2) | 62.1 (30.5) |
| Alcohol status | ||
| Current | 1300 (43%) | 1104 (43%) |
| Never | 1469 (49%) | 1223 (48%) |
| Previous | 247 (8%) | 219 (9%) |
| Smoking status | ||
| Current | 474 (16%) | 390 (15%) |
| Never | 1687 (55%) | 1417 (56%) |
| Previous | 882 (29%) | 739 (29%) |
| Breast cancer | ||
| Yes/total | 14/3043 (0.5%) | 10/2546 (0.4%) |
| History of rheumatoid arthritis | ||
| Yes/total | 103/3043 (3.4%) | 83/2546 (3.3%) |
| Use of thiazide diuretics during trial | ||
| Yes/total | 165/3043 (5.4%) | 144/2546 (5.7%) |
BMI, body mass index; FN, femoral neck; HIP, Hip Intervention Programme; ITT, Intent‐to‐Treat; NHANES III, Third National Health and Nutrition Examination Survey; NV, non‐vertebral; VERT‐MN, Multinational Vertebral Efficacy with Risedronate Therapy; VERT‐NA, North American Vertebral Efficacy with Risedronate Therapy.
Lateral and thoracolumbar radiographs were obtained at baseline and annually throughout the studies and assessed for fractures using standard methods.32
Statistical analysis
A logistic regression statistical model using the stepwise variable selection method was used to identify potential risk factors associated with incident NV osteoporosis‐related fracture status within the population. For the stepwise variable selection method, explanatory variables were included/omitted using an alpha level of 10%. The final model consisted of explanatory variables, which were significantly associated with incident NV fracture status using an alpha level of 5%. To evaluate the effect of using a variable selection method on the analysis, backward and forward variable selection methods were also applied, giving the same result. The goodness of fit of the final statistical model was examined by (a) calculating the Hosmer and Lemshow goodness‐of‐fit test and (b) producing a receiver operating characteristic (ROC) curve. Because some patients had missing data for the exploratory variables, the final logistic regression model was anticipated not to include all of the intent‐to‐treat population. Therefore, descriptive statistics of important baseline and post‐baseline variables were summarised for the intent‐to‐treat population, and the patients were included in the final regression model. Then, we developed an index using a methodology similar to that previously proposed by Black et al,33 by converting the multivariate logistic equation into an additive score. The range of the predictor variables was divided into intervals. For each variable, the product of its regression coefficient with the midpoint of the intervals determined the index contribution. The index values due to each variable were adjusted so as to make the minimum possible index contribution 0.
Results
A total of 3043 patients, included in the placebo groups of the three trials, and 2546 women, for whom data on the 14 risk factors were available, were the basis of this study. Table 1 shows the characteristics of these women. During the 3 years of follow‐up, 222 NV fractures were observed in 206 patients, including hip (n = 50), wrist (n = 71), humerus (n = 36), pelvis (n = 22), leg (n = 41) and clavicle (n = 2). An additional seven NV fractures were observed during follow‐up visits. Evaluable paired spinal radiographs were available for 2038 patients. The proportion of women with incident NV fractures was 8.6% (95% CI 7.6% to 9.6%), and the incidence of vertebral fracture was 14.8% (13.3% to 16.3%).
The following baseline parameters were identified by logistic regression analysis as independent predictors of NV fracture risk: prevalent NV fracture (OR 1.24; 95% CI 1.13% to 2.08%; p = 0.006), number of prevalent vertebral fractures (OR 1.12; 95% CI 1.06% to 1.18%; p<0.001), femoral neck T‐score (OR 1.29; 95% CI 1.02% to 1.63% per 1 SD decrease; p = 0.034), age (OR 1.04; 95% CI 1.02 to 1.07; p = 0.019), height (OR 1.02; 95% CI 1.00 to 1.05; p = 0.037) and serum level of 25‐hydroxyvitamin D3 (25OHD3; OR 1.01; 95% CI 1.0 to 1.02 per 1 nmol/l decrease; p<0.001). In the 48 patients who had a baseline serum hydroxyvitamin D <16 ng/ml (as defined by inclusion criteria), the incidence of NV fractures was 14.58% (95% CI 4.60 to 24.57). In the 2498 patients with baseline 25OHD3 ⩾16 ng/ml, the incidence was 8.25% (95% CI 7.17 to 9.33).
The predicted NV fracture risk (probability) is given by the equation: risk = exp(x)/[1+exp(x)], where x = −9.1119+0.0371×age+0.0236×height−0.2549×femoral neck T‐score−0.0106×serum 25OHD3+0.1073×number of prevalent vertebral fractures −0.2207×prevalent NV fracture. Prevalent NV fracture was assigned the value 1 if the woman had no previous NV fractures, and −1 otherwise. When the analysis was conducted using prevalent vertebral fracture as a binary parameter (yes/no), instead of number of fractures, the findings were similar.
The area under the ROC curve, depicting the level of sensitivity and specificity associated with predicting the NV fracture risk on the basis of the six predictors in the final model, was 0.66. The ROC results were used to define a subgroup of women with high risk of NV fracture. A cut‐off value for the predicted probability of NV fracture was selected to minimise the absolute difference between sensitivity and specificity. The value was 0.086 (ie, 8.6% NV fracture risk at baseline), and it corresponded to a sensitivity of 62.4% and a specificity of 62.3%.
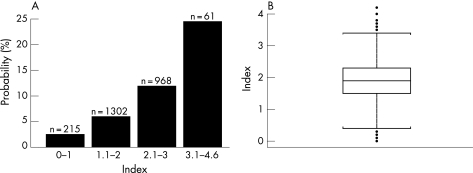
Table 2 reports the NV fracture risk index. The result can be a value in the range from 0 (no risk) to 4.6 (highest risk). Patients with an NV index ⩾2.1 belonged to the high‐risk subgroup—that is, with a 3‐year probability of fracture of at least 8.6%.
Table 2 Simplified non‐vertebral fracture risk index.
| Variable | Group | Index value |
|---|---|---|
| Age (years) | <65 | 0 |
| 65–70 | 0.2 | |
| 70–75 | 0.4 | |
| 75–80 | 0.6 | |
| ⩾80 | 0.8 | |
| Height (cm) | <147 | 0 |
| 147–153 | 0.2 | |
| 153–161 | 0.4 | |
| 161–167 | 0.5 | |
| ⩾167 | 0.7 | |
| FN T‐score | <−3.8 | 0.8 |
| ⩾−3.8 and <−3.0 | 0.6 | |
| ⩾−3.0 and <−2.2 | 0.4 | |
| ⩾−2.2 and −1.4 | 0.2 | |
| ⩾−1.4 | 0 | |
| Vitamin D (ng/ml) | <30 | 0.8 |
| 30–50 | 0.6 | |
| 50–70 | 0.4 | |
| 70–90 | 0.2 | |
| ⩾90 | 0 | |
| Prevalent vertebral fracture* | 0 | 0 |
| 1 | 0.1 | |
| 2–6 | 0.4 | |
| ⩾7 | 1.1 | |
| Prevalent NV fracture | Yes | 0.4 |
NV, non‐vertebral.
In the subgroup of women (n = 998) with an index of NV fracture risk ⩾2.1, the incidence of NV fractures was 13.2% (11.1–15.3%)—that is, 1.5 times higher than the average of the population (fig 1).
Figure 1 (A) Probability of non‐vertebral fracture in 3 years in postmenopausal women with osteoporosis, according to baseline index risk; (B) distribution of the index values, including outliers.
Discussion
This study shows that, in postmenopausal women with osteoporosis already selected as being at high risk of fracture, six factors easily assessable in clinical practice, such as age, height, prior NV fracture, number of prevalent vertebral fractures, femoral neck T‐score and serum 25‐hydroxyvitamin D, are important predictors of the risk of NV fractures.
Risk factors have been described to be different for different NV fractures. No single factor is responsible for the occurrence of a fracture, and the combination of factors may differ between individuals. Some studies have focused on hip fracture or Colles' fracture.21,22 For example, previous wrist fracture, low body weight, low calcium intake and non‐use of hormone replacement therapy have been reported to be risk factors specific to Colles' fracture. But the direction of the fall also makes a difference: a forward fall is a risk factor for Colles' fracture, and a sideways fall, is a risk for hip fracture. We did not focus our analysis on a particular NV fracture; we were interested in the clinical use of prediction of such fractures as a group in clinical management of postmenopausal women with osteoporosis.
From a biomechanical point of view, it is logical that increasing height is a risk factor for NV fracture, as it increases the energy released during trauma.34 Low body weight is a strong risk factor for hip fracture 34,35,36; however, thinner women have lower BMD for several reasons, including low transmitted gravitational forces and lower levels of endogenous oestrogens. Thus, femoral neck T‐score was included in our multivariate analysis. BMD is a strong risk factor for NV fractures.37,38,39 A low hip BMD is a stronger predictor of hip fracture than BMD at other sites.37 In the Study of Osteoporotic Fracture, the incidence of most fracture types was found to be significantly increased in older women with low BMD (measured at the radius or the os calcis).34,40 Prevalent vertebral fractures are an expected risk factor for incident NV fractures—for either their severity25 or their number. This reflects the systemic bone fragility.
Vitamin D deficiency and secondary hyperparathyroidism are common in older women and play a major role in the pathogenesis of hip fracture.41,42 The role of this mechanism in the occurrence of other NV fractures is unknown. However, vitamin D deficiency has a role not only in bone fragility but also in muscle function. Falls are mainly related to muscle weakness, postural instability and incoordination. In the Mediterranean Osteoporosis study,36 there was a continuous increase in hip fracture incidence with decreasing sunlight exposure, without an apparent threshold. The decline in renal function with age, including impaired 1−α hydroxylase activity, may be an additional factor. In the prospective Occupational Physicians Reporting Activity study,43 older women with low levels of 25‐hydroxyvitamin D had both an inferior physical activity and postural stability and an increased risk of fractures. In both active and inactive women aged ⩾60 years, the lower the 25‐hydroxyvitamin D concentration, the lower the musculoskeletal function in the lower extremities.44 Studies have shown that vitamin D may reduce the risk of fractures45 and also the risk of falls.43 Vitamin D supplementation does not have a direct effect on muscle strength, but may improve neuromuscular or neuroprotective function.46 In our prospective study, although patients received supplements of calcium and vitamin D, the low baseline level of 25‐hydroxyvitamin D was still a significant risk factor for fracture, which may be related to a long‐term effect of sarcopenia caused by vitamin D deficiency.47 Moreover, the dose of vitamin D which would be beneficial is unknown, and trials using <800 IU/day of vitamin D did not find a consistent effect on fracture risk.46 We could not account for changes in vitamin D status over time, as no repeated measurements were made. By contrast, we observed similar results regardless of when baseline vitamin D measurement was taken, indicating that the effect was not season‐dependent (data not shown).
The main limitation of our study is that information on falls is not available. The low value of our ROC curve area may be related to this ignored parameter, which is of crucial importance in explaining the fractures.48,49,50 In the Mediterranean Osteoporosis study,36 the best combination of risk factors led to a sensitivity of 55% and a specificity of 65% (excluding age). However, as in our study, this combination has a high attributable risk. A retrospective study of postmenopausal women showed an increased risk for fractures during the preceding year in women who reported a fall during that period and had low BMD, but not in women with a history of falling and normal BMD, nor in women who reported no falls irrespective of their BMD.51 These results suggest that the risk for clinical, mainly NV, fractures is increased only in women with a combination of low BMD and incident falls. Studies of fall prevention have shown varying results, but none have shown a decrease in the number of fractures.52 This apparent lack of anti‐fracture efficacy might reflect the fact that these studies were not performed in patients selected on the basis of low BMD. Anti‐osteoporotic treatments have shown their efficacy only in women who have low BMD or prevalent vertebral fractures. It remains to be clarified whether measures to prevent falls—in combination with drug therapy—might further reduce the risk of fracture.
Our study suggests that, in women with osteoporosis, a proportion of patients with a high risk of NV fractures can be selected, and that an index is a convenient tool for this selection in clinical practice. First‐line treatment for these patients must be chosen from those available with a proven efficacy for reduction of NV fracture risk.53
Abbreviations
BMD - bone mineral density
NV - non‐vertebral
ROC - receiver operating characteristic
Footnotes
Competing interests: None declared.
References
- 1.Eastell R, Reid D M, Compston J, Cooper C, Fogelman I, Francis R M.et al Secondary prevention of osteoporosis: when should a non‐vertebral fracture be a trigger for action? Q J Med 200194575–597. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis J A, Odèn A, Sernbo I, Redlund‐Johnell I, Petterson C.et al Mortality after osteoporotic fracture. Osteoporos Int 20041538–42. [DOI] [PubMed] [Google Scholar]
- 3.Melton LJ I I I, Chrischilles E A, Cooper C, lane A W, Riggs B L. How many women have osteoporosis? J Bone Miner Res 199271005–1010. [DOI] [PubMed] [Google Scholar]
- 4.Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M, Vuori I. Increasing number and incidence of osteoporotic fractures of the proximal humerus in elderly people. BMJ 19933131051–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S H, Dargent‐Molina P, Bréart G, for the EPIDOS Group Risk factors for fractures of the proximal humerus: results from the EPIDOS prospective study. J Bone Miner Res 200217817–825. [DOI] [PubMed] [Google Scholar]
- 6.Bickerstaff D R, Kanis J A. Algodystrophy: an under‐recognized complication of minor trauma. Br J Rheumatol 199433240–248. [DOI] [PubMed] [Google Scholar]
- 7.Klotzbuecher C M, Ross P D, Landsman P B, Abbott III T A, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 200015721–739. [DOI] [PubMed] [Google Scholar]
- 8.Cuddihy M T, Gabriel S E, Crowson C S, O'Fallon V M, Melton LJ I I I. Forearm fractures as predictors of subsequent osteoporotic fractures. Osteoporos Int 19999469–475. [DOI] [PubMed] [Google Scholar]
- 9.Mallmin H, Ljunghall S. Distal radius fracture is an early sign of general osteoporosis: bone mass measurements in a population‐based study. Osteoporos Int 19944357–361. [DOI] [PubMed] [Google Scholar]
- 10.Peel N F, Barrington N A, Smith T W, Eastell R. Distal forearm fracture as risk factor for vertebral osteoporosis. BMJ 19943081543–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris S T, Watts N B, Genant H K, McKeever C D, Hangartner T, Keller M.et al Effects of risedronate treatment on‐vertebral and non‐vertebral fractures in women with postmenopausal osteoporosis. A randomized controlled trial. JAMA 19992821344–1352. [DOI] [PubMed] [Google Scholar]
- 12.Reginster J Y, Minne H W, Sorensen O H, Hooper M, Roux C, Brandi M L.et al Randomized trial of the effects of risedronate on‐vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 20001183–91. [DOI] [PubMed] [Google Scholar]
- 13.Black D M, Cummings S R, Karpf D B, Cauley J A, Thompson D E, Nevitt M C.et al Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 19963481535–1541. [DOI] [PubMed] [Google Scholar]
- 14.Ettinger B, Black D M, Mitlak B H, Knickerbocher R K, Nickelsen T, Genant H K.et al Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3‐year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999282637–645. [DOI] [PubMed] [Google Scholar]
- 15.Neer R M, Arnaud C D, Zanchetta J R, Prince R, Gaich G A, Reginster J Y.et al Effect of parathyroid hormone (1‐34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 20013441434–1441. [DOI] [PubMed] [Google Scholar]
- 16.Meunier P J, Roux C, Seeman E, Ortolani S, Badurski J E, Spector T.et al The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 2004350459–468. [DOI] [PubMed] [Google Scholar]
- 17.Reginster J Y, Seeman E, de Vernejoul M C, Adami S, Compston J, Phenekos C.et al Strontium ranelate reduces the risk of non‐vertebral fractures in post menopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab 2005902816–2822. [DOI] [PubMed] [Google Scholar]
- 18.Karpf D B, Shapiro D R, Seeman E, Ensrud K E, Johnston C C, Adami S, for the Alendronate Osteoporosis Treatment Study Groups et al Prevention of non‐vertebral fractures by alendronate: a meta‐analysis. JAMA 19972771159–1164. [PubMed] [Google Scholar]
- 19.Black D M, Thompson D E, Bauer D C, Ensrud K, Musliner T, Hochberg M C.et al Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. J Clin Endocrinol Metab 2000854115–4124. [DOI] [PubMed] [Google Scholar]
- 20.Chesnut CH I I I, Skag A, Christiansen C, Recker R, Stakkestad J A, Hoiseth A.et al Effects of oral ibandronate administrated daily or intermittently on fracture risk in post menopausal osteoporosis. J Bone Miner Res 2004191241–1249. [DOI] [PubMed] [Google Scholar]
- 21.Graafmans W C, Ooms M E, Bezemer P D, Bouter L M, Lips P. Different risk profiles for hip fractures and distal forearm fractures: a prospective study. Osteoporos Int 19966427–431. [DOI] [PubMed] [Google Scholar]
- 22.Honkanen R, Tuppurainen M, Kröger H, Alhava E, Saarikoski S. Relationship between risk factors and fractures differ by type of fracture: a population‐based study of 12192 perimenopausal women. Osteoporos Int 1998825–31. [DOI] [PubMed] [Google Scholar]
- 23.Dargent‐Molina P, Favier F, Grandjean H, Baudoin C, Schott A M, Hausherr E, for the EPIDOS Group et al Fall‐related factors and risk of hip fracture: the EPIDOS prospective study. Lancet 1996348145–149. [DOI] [PubMed] [Google Scholar]
- 24.Tinetti M E, Baker K I, McAvay G, Claus E B, Garrett P, Gottschalk M.et al A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med 1994331821–827. [DOI] [PubMed] [Google Scholar]
- 25.Delmas P D, Genant H K, Crans G G, Stock J L, Wong M, Siris E.et al Severity of prevalent vertebral fractures and the risk of subsequent vertebral and non‐vertebral fractures: results from the MORE trial. Bone 200333522–532. [DOI] [PubMed] [Google Scholar]
- 26.Black D M, Arden N K, Palermo L, Pearson J, Cummings S R. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res 199914821–828. [DOI] [PubMed] [Google Scholar]
- 27.Melton L J, Atkinson E J, Cooper C, O'Fallon W M, Riggs B L. Vertebral fractures predict subsequent fractures. Osteoporos Int 199910214–221. [DOI] [PubMed] [Google Scholar]
- 28.Tountas A A. Insufficiency stress fractures of the femoral neck in elderly women. Clin Orthop 1993292202–209. [PubMed] [Google Scholar]
- 29.Parker M J, Twemlow T R. Spontaneous hip fractures. Acta Orthopaed Scand 199768325–326. [DOI] [PubMed] [Google Scholar]
- 30.McClung M R, Geusens P, Miller P D, Zippel H, Bensen W G, Roux C, the Hip Intervention Program Study Group et al Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 2001344333–340. [DOI] [PubMed] [Google Scholar]
- 31.Seeley D G, Browner W S, Nevitt M C, Genant H K, Scott J C, Cummings S R, the Study of Osteoporotic Fractures Research Group Which fractures are associated with low appendicular bone mass in elderly women? Ann Intern Med 1991115837–842. [DOI] [PubMed] [Google Scholar]
- 32.Genant H K, Wu C Y, Van Kuyk C, Nevitt M C. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 199381137–1148. [DOI] [PubMed] [Google Scholar]
- 33.Black D M, Steinbuch M, Palermo L, Dargent‐Molina P, Lindsay R, Hoseyni M S.et al An assessment tool for predicting fracture risk in post‐menopausal women. Osteoporos Int 200112519–528. [DOI] [PubMed] [Google Scholar]
- 34.Ensrud K E, Lipschutz R C, Cauley J A, Seeley D, Nevitt M C, Scott J, for the Study Osteoporotic Fractures Research Group et al Body size and hip fracture risk in older women: a prospective study. Am J Med 1997103274–280. [DOI] [PubMed] [Google Scholar]
- 35.Cummings S R, Nevitt M C, Browner W S, Stone K, Fox K M, Ensrud K E, the Study of Osteoporotic Fractures Research Group et al Risk factors for hip fracture in white women. N Engl J Med 1995332767–773. [DOI] [PubMed] [Google Scholar]
- 36.Johnell O, Gullberg B O, Kanis J A, Allander E, Elffors L, Dequeker J.et al Risk factors for hip fractures in European women: the MEDOS study. J Bone Miner Res 1995101802–1815. [DOI] [PubMed] [Google Scholar]
- 37.Marshall D, Johnell O, Wedel H. Meta‐analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 19963121254–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nevitt M C, Johnell O, Black D M, Ensrud K, Genant H K, Cummings S R. Bone mineral density predicts non‐spine fractures in very elderly women. Study of Osteoporotic Fractures Research Group. Osteoporos Int 19944325–331. [DOI] [PubMed] [Google Scholar]
- 39.Seeley D G, Browner W S, Nevitt M C, Genant H K, Scott J C, Cummings S R, the Study of Osteoporotic Fractures Research Group Which fractures are associated with low appendicular bone mass in elderly women? Am Intern Med 1991115837–842. [DOI] [PubMed] [Google Scholar]
- 40.Cummings S R, Black D M, Nevitt M C, Browner W, Cauley J, Ensrud K, the Study of Osteoporotic Fractures Research Group et al Bone density at various sites for prediction of hip fractures. Lancet 199334172–75. [DOI] [PubMed] [Google Scholar]
- 41.Lips P, Netelenbos J C, Jongen M J, van Ginkel F C, Althuis A L, van Schaik C L.et al Histomorphometric profile and vitamin D status in patients with femoral neck fracture. Metab Bone Dis Relat Res 1982485–93. [DOI] [PubMed] [Google Scholar]
- 42.Dawson‐Hughes B, Harris S S, Krall E A, Dallal G E, Falconer G, Green C L. Rates of bone loss in postmenopausal women randomly assigned to one of two dosages of vitamine D. Am J Clin Nutr 1995611140–1145. [DOI] [PubMed] [Google Scholar]
- 43.Gerdhem P, Ringsberg K A, Obrant K J, Akesson K. Association between 25‐hydroxy vitamin D levels, physical activity, muscle strenght and fractures in the prospective population‐based OPRA Study of elderly women. Osteoporos Int 2005 [DOI] [PubMed]
- 44.Bischoff‐Ferrari H A, Dietrich T, Orav E J, Hu F B, Zhang Y, Karlsson E W.et al Higher 25‐hydroxyvitamin D concentrations are associated with better lower‐extremity function in both active and inactive persons aged 60 years and more. Am J Clin Nutr 200480752–758. [DOI] [PubMed] [Google Scholar]
- 45.Chapuy M C, Arlot M E, Duboeuf F, Brun J, Crouzet B, Arnaud S.et al Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 19923271637–1642. [DOI] [PubMed] [Google Scholar]
- 46.Bischoff‐Ferrari H A, Dawson‐Hughes B, Willett W C, Staehelin H B, Bazemore M G, Zee R Y.et al Effect of vitamin D on falls. A meta‐analysis. JAMA 20042911999–2006. [DOI] [PubMed] [Google Scholar]
- 47.Visser M, Deeg D J H, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab 2003885766–5772. [DOI] [PubMed] [Google Scholar]
- 48.Winner S J, Morgan C A, Grimley Evans J. Perimenopausal risk of falling and incidence of distal forearm fracture. BMJ 19892981486–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crilly R G, Delaquerriere‐Richardson L, Roth J H, Vandervoort A A, Hayes K C, Mackenzie R A. Postural stability and Colles' fracture. Age Ageing 198716133–138. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen T, Sambrook P, Kelly P, Jones G, Lord S, Freund J.et al Prediction of osteoporotic fracture by postural instability and bone density. BMJ 19933071111–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geusens P, Autier P, Boonen S, Vanhoof J, Declerck K, Raus J. The relationship among history of falls, osteoporosis, and fractures in postmenopausal women. Arch Phys Med Rehabil 200283903–906. [DOI] [PubMed] [Google Scholar]
- 52.Tinetti M E. Clinical practice: preventing falls in elderly persons. N Engl J Med 200334842–49. [DOI] [PubMed] [Google Scholar]
- 53.Boonen S, Laan R F, Barton I P, Watts N B. Effect of osteoporosis treatments on risk of non vertebral fractures: review and meta analysis of intention to treat studies. Osteporos Int 2005161291–1298. [DOI] [PubMed] [Google Scholar]