Abstract
Background
Fatigue is an important symptom in psoriatic arthritis (PsA).
Aim
To determine the reliability and validity of the Functional Assessment of Chronic Illness Therapy‐Fatigue (FACIT fatigue) Scale in PsA.
Methods
Consecutive patients attending the PsA clinic were assessed with the FACIT fatigue Scale twice, 1 week apart. Patients were assessed clinically according to a standardised PsA clinic protocol. Internal consistency of the 13 items on the FACIT fatigue questionnaire was measured using Cronbach's α; test–retest reliability by the intraclass correlation coefficient (ICC), and validity by the correlation of the FACIT fatigue results with other fatigue measures and disease characteristics.
Results
135 patients (80 men and 55 women, mean (SD) age 52 (13) years, mean (SD) disease duration 17 (10) years) participated. The mean FACIT fatigue score was 35.8 (12.4). Cronbach's α was 0.96. Repeat questionnaires were returned by 54% of patients. No difference in disease characteristics was observed between those who did and did not return the questionnaires. The ICC for first and repeat FACIT fatigue scores was 0.95. The correlation between the FACIT fatigue and modified Fatigue Severity Score was −0.79 (95% CI −0.85 to −0.72). FACIT fatigue scores were lower in patients with overwhelming fatigue and fibromyalgia than in those without (p<0.001). The FACIT fatigue was correlated with the actively inflamed joint count (−0.43, 95% CI −0.56 to −0.28, p<0.001), but not with the clinically damaged joint count (−0.06, 95% CI −0.23 to 0.11, p = 0.51).
Conclusion
The FACIT fatigue results were reproducible, and correlated with other fatigue measures as well as with disease activity in patients with PsA. Therefore, the FACIT fatigue is a reliable and valid instrument to measure fatigue in PsA.
Fatigue is an important symptom in patients with chronic diseases,1 such as multiple sclerosis,2 systemic lupus erythematosus,3 chronic liver disease,4 rheumatoid arthritis (RA)5 and ankylosing spondylitis.6 It is an important symptom contributing to decreased quality of life in patients with inflammatory arthritis, especially RA.7,8 It is defined as an overwhelming, sustained sense of exhaustion and decreased capacity for physical and mental work.9 It is measured using questionnaires that attempt to measure the subject's perception and severity of fatigue. The scales developed have to be validated for the study population.
A number of self‐reported scales are used to measure fatigue in patients with arthritis. These include the 16‐item Multidimensional Assessment of Fatigue (MAF) Scale,10 the vitality scale from the Medical Outcomes Study Short‐Form 36 (SF‐36),11 the Brief Fatigue Inventory,12 visual analogue scale (VAS) fatigue scale,5 Fatigue Severity Scale (FSS),13 Fatigue Impact Scale14 and Functional Assessment of Chronic Illness Therapy‐Fatigue (FACIT fatigue).15 The FACIT fatigue Scale has been used more recently to demonstrate decrease in fatigue in drug trials, which has led to a greater interest in fatigue in patients with rheumatological disorders.16,17,18,19,20
There are only a few studies comparing the various fatigue questionnaires. The single‐item VAS was compared with the longer MAF, vitality scale from the SF‐36 and the Brief Fatigue Inventory in subjects with RA.21 The VAS scale performed as well as or better than the longer scales with respect to correlation with clinical variables and sensitivity to change. However, in a more recent study, the FSS performed better than the VAS and the Fatigue Impact Scale in patients with postpoliomyelitis syndrome.22 Therefore, different fatigue measurement tools might perform differently with different diseases.
The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system23 is a comprehensive compilation of questions that measure health‐related quality of life in patients with cancer and other chronic diseases (http://www.facit.org). The core of the FACIT system is the Functional Assessment of Cancer Therapy‐General,24 a 27‐item general version of the questionnaire, which serves as a foundation to the questions added to address specific problems related to a particular disease site, treatment or symptom. The FACIT fatigue questionnaire was developed to assess fatigue associated with anaemia.25 Thirteen fatigue‐related questions (FACIT fatigue) were added to the Functional Assessment of Cancer Therapy‐General to make the FACIT‐F. The responses to the 13 items on the FACIT fatigue questionnaire are each measured on a 4‐point Likert scale. Thus, the total score ranges from 0 to 52. High scores represent less fatigue. The FACIT fatigue Scale has been validated in the general population,15 in patients with cancer25 and in patients with RA.26 In patients with cancer, the FACIT fatigue Scale showed excellent internal consistency and reliability, and differentiated patients by haemoglobin level and patient‐rated performance status.25 In RA too, FACIT fatigue showed good internal consistency, strong association with the vitality scales of SF‐36 and MAF, and the ability to differentiate patients according to clinical change using the American College of Rheumatology response criteria.26
Psoriatic arthritis (PsA) is an inflammatory arthritis associated with psoriasis, usually seronegative for rheumatoid factor.27,28 Fatigue is an important symptom of PsA. Fatigue was assessed using FSS as modified by Gladman et al.29,30 Among patients with PsA, 45% reported fatigue on clinical assessment, and their fatigue score on the FSS was significantly higher than patients who did not report fatigue.31 Changes in fatigue were shown to reflect changes in clinical disease activity in PsA.32 The FACIT fatigue was shown to improve with treatment with adalimumab in patients with PsA.33 In patients with psoriasis treated with etanercept, improvement in fatigue (using the FACIT fatigue) was shown to correlate with decreasing joint pain.34 However, the FACIT fatigue has not been validated in patients with PsA. Therefore, the purpose of this study was to determine the internal consistency, test–retest reliability, criterion and construct validity of the FACIT fatigue in patients with PsA.
Patients and methods
Consecutive patients attending the University of Toronto Psoriatic Arthritis Clinic, Toronto, Ontario, Canada, were enrolled into the study over a 3‐month period. They were given two sets of questionnaires to be completed twice—once in clinic and again 1 week later:
the 13‐point FACIT fatigue questionnaire; and
the modified Fatigue Severity Scale (mFSS), a 9‐point questionnaire that assesses the effect of fatigue on daily activities. Each item is scored on a scale from 0 to 10, as modified from the original 1–7 scale,29,30 with an average overall score (0–10) being computed. A higher score denotes more severe fatigue. The mFSS has been previously validated in PsA in our clinic.30,31,32
Patients were assessed clinically according to a standardised PsA clinic protocol, which includes a complete history, physical examination and laboratory evaluation. The clinical measures of actively inflamed joints (joint line tenderness, stress pain or swelling) and clinically damaged joints (deformity, ankylosed or flail joints) have been shown to be reliable.35 The protocol includes a question on the presence or absence of overwhelming fatigue. The presence or absence of fibromyalgia is also noted on the basis of fatigue, chronic widespread musculoskeletal pain not restricted to joints, and ⩾11 fibromyalgia tender points, which are counted at each assessment. Laboratory testing includes haemoglobin and erythrocyte sedimentation rate (ESR). Anaemia was defined as a haemoglobin <130 g/dl for men and <120 g/dl for women. Increased ESR was defined as >15 mm/h for men and >20 mm/h for women.
Statistical analysis
The internal consistency of the 13 items on the FACIT fatigue questionnaire was measured using Cronbach's α. The test–retest reliability was tested by the intraclass correlation coefficient. Construct validity was tested by correlation with the mFSS, presence or absence of overwhelming fatigue, the presence or absence of fibromyalgia, and disease characteristics (actively inflamed joint count, swollen joint count and clinically damaged joint count). Pearson's correlation coefficient, Student's t test and Wilcoxon's rank sum test were used as appropriate using SAS V.8.02.
This study was approved by the University Health Network Research Ethics Board.
Results
A total of 135 patients were recruited for the study. Table 1 describes the demographic and disease characteristics, as well as the scores of FACIT fatigue and mFSS fatigue scales of those enrolled.
Table 1 Patients' demographics, disease characteristics and fatigue scale scores.
| Total number of patients | 135 |
|---|---|
| Men/women | 80/55 |
| Mean (SD) age (years) | 52 (13) |
| Mean (SD) disease duration (years) | 17 (10) |
| Mean (SD) actively inflamed joint count (ACR 68/66)* | 4.5 (7) |
| Mean (SD) swollen joint count (ACR 68/66)† | 1.3 (2) |
| Mean (SD) clinically damaged joint count (ACR 68) | 8.7 (12) |
| Mean (SD) Psoriasis Area Severity Index score | 3.5 (5) |
| Patients with fibromyalgia, n (%) | 16 (12) |
| Patients with anaemia, n (%) | 27 (20) |
| Patients with raised ESR, n (%) | 63 (47) |
| Patients with overwhelming fatigue, n (%) | 26 (19) |
| Mean (SD) FACIT fatigue score | 35.8 (12.4) |
| Mean (SD) mFSS score | 4.9 (2.7) |
ACR, American College of Rheumatology; ESR, erythrocyte sedimentation rate; FACIT fatigue, Functional Assessment of Chronic Illness Therapy‐Fatigue; mFSS, modified Fatigue Severity Scale.
*Joints with tenderness and/or swelling.
†Joints with swelling only.
Internal consistency
The internal consistency of the 13 items on the FACIT fatigue questionnaire as measured by Cronbach's α was 0.96.
Test–retest reliability of FACIT fatigue score
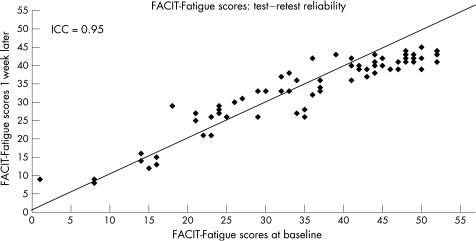
Repeat questionnaires were returned by 73 (54%) patients. No difference was seen in the disease characteristics between those who did and did not return the questionnaires. However, when compared with patients who did not return the repeat questionnaires, patients who returned the questionnaires were less likely to be working, and more likely to have fibromyalgia. Indeed, when patients with fibromyalgia were excluded, there was no longer a difference in employment status. There was no difference in the FACIT fatigue scores between those who responded to the repeat questionnaires and those who did not (35.9 (12.7) vs 35.7 (12.2), p = 0.94). There was also no difference in the mFSS scores between the responders and non‐responders (5.0 (2.9) vs 4.8 (2.6), p = 0.7). The intraclass correlation coefficient between the first and repeat questionnaires was 0.95 (fig 1).
Figure 1 Intraclass correlation (ICC) between Functional Assessment of Chronic Illness Therapy (FACIT) Fatigue‐scores at the first and repeat assessments.
Criterion validity
The FACIT fatigue scores were lower in patients reporting overwhelming fatigue than in those not reporting the same (24.8 (13.9) and 38.5 (10.4), respectively, p<0.001). The FACIT fatigue scores were also lower in patients with fibromyalgia than in those without fibromyalgia (19.6 (9.0) and 38.1 (11.3), respectively, p<0.001). The FACIT fatigue scores were compared with the mFSS scores. There was a good correlation between the FACIT fatigue and mFSS scores (r = −0.79, 95% CI −0.85 to −0.72). The negative sign reflects that higher scores on the FACIT fatigue scale indicate less fatigue whereas higher scores on the mFSS scale indicate more fatigue.
Construct validity
On comparing with mean (SD) FACIT fatigue scores obtained from the general population15 (43.6 (9.4), n = 1010), the scores obtained for this cohort of patients was lower (35.8 (12.4)), indicating that patients with PsA have more fatigue than the the general population. The FACIT fatigue scores correlated with actively inflamed joint count (−0.43, 95% CI −0.56 to −0.28, p<0.001) and less with swollen joint count (−0.27, 95% CI −0.42 to −0.01, p = 0.002), but not with the clinically damaged joint count (0.06, 95% CI −0.23 to 0.11, p = 0.51). There was no difference in the scores between patients with and without increased ESR (34.0 (13.7) vs 37.0 (11.3), p = 0.31) and anaemia (36.0 (12.3) vs 35.7(13.5), p = 0.93), respectively. Men and women also scored similarly (males 37.2 (11.0), females 33.6 (14.2), p = 0.12). Table 2 depicts the correlations between demographic and disease characteristics and FACIT fatigue scores.
Table 2 Correlations with demographic and disease characteristics.
| Variable | FACIT fatigue score | mFSS score | ||
|---|---|---|---|---|
| Correlation coefficient (95% CI) | p Value | Correlation coefficient (95% CI) | p Value | |
| Age | 0.01 (−0.16 to 0.18) | 0.88 | 0.05 (−0.12 to 0.22) | 0.55 |
| Disease duration of psoriasis | −0.01 (−0.18 to 0.16) | 0.93 | 0.03 (−0.14 to 0.20) | 0.72 |
| Disease duration of PsA | −0.03 (−0.20 to 0.14) | 0.78 | 0.02 (−0.16 to 0.19) | 0.79 |
| Actively inflamed joints | −0.43 (−0.56 to −0.28) | <0.001 | 0.37 (0.21 to 0.51) | <0.001 |
| Swollen joints | −0.27 (−0.42 to −0.10) | 0.002 | 0.15 (−0.02 to 0.31) | 0.09 |
| Damaged joints | −0.06 (−0.23 to 0.11) | 0.51 | 0.16 (−0.01 to 0.32) | 0.07 |
FACIT fatigue, Functional Assessment of Chronic Illness Therapy‐Fatigue; mFSS, modified Fatigue Severity Scale; PsA, psoriatic arthritis.
As can be seen, no correlations with age or disease duration were noted. There was a moderate negative correlation with the total number of actively inflamed joints and a lower but significant correlation with the number of swollen joints, but no correlation with the number of clinically damaged joints. The mFSS also correlated with the total number of actively inflamed joints but not with the number of swollen joints.
Discussion
Fatigue is an important symptom in patients with inflammatory arthritis. At the outcome measures in PsA workshop during Outcome Measures in Rheumatoid Arthritis Clinical Trial 7, it was proposed to include methods to evaluate fatigue in patients with PsA as one of the research agenda.36 A recent clinical trial in PsA included fatigue as a secondary outcome measure.33 However, the measure used—the FACIT fatigue—had not been validated in patients with PsA.
The results of this study demonstrate that the FACIT fatigue score is a reliable measure in patients with PsA. Patients with PsA had lower FACIT fatigue scores than the general population. There was excellent correlation between the first and repeat questionnaires, the second of which was completed within 1 week of the first, assuming no significant change in clinical status between the two assessments. Notably, there was no difference between responders and non‐responders in terms of demographics and responses to the mFSS and FACIT fatigue scores. Although the response rate for the repeat questionnaire was only 54%, there were no differences in disease characteristics or the fatigue measure in the first visit between those who completed the second questionnaire and those who did not. The main difference was that people who completed the second questionnaire were less likely to be working and may thus have had more time to complete the questionnaire.
The FACIT fatigue showed a good (negative) correlation with the mFSS, a measure previously shown to be reliable in patients with PsA.30,31,32 Patients reporting overwhelming fatigue had lower scores (meaning more fatigue) than those not reporting the same. Patients with fibromyalgia are known to have significant fatigue.37 The FACIT fatigue scores were also lower in those patients with PsA who had fibromyalgia. The score was also correlated with measures of PsA disease activity (actively inflamed joint count and swollen joint count), but not with clinical damage or disease duration. Thus, the FACIT fatigue Scale showed construct validity, as one expects patients reporting overwhelming fatigue, having fibromyalgia and active PsA to have more fatigue, and therefore lower scores on the FACIT fatigue Scale.
There are similarities and differences between FSS and FACIT fatigue Scales. The FSS items measure a homogeneous and unidimensional attribute—that is, problems due to fatigue.38 Item response theory analysis has previously shown that there is a good coverage of the domain over the centre of the fatigue domain and the distribution of the item difficulty levels cluster in the centre of the domain.39 Although the FACIT fatigue Scale is also considered to be a unidimensional measure of fatigue, its items cover a broader concept of fatigue. This instrument might therefore be more appropriate to measure the entire continuum of fatigue in patients with inflammatory arthritis.26
The FACIT fatigue scores were not found to be lower in patients with PsA with anaemia. This is in contrast with patients with cancer with anaemia, where fatigue as measured by the FACIT fatigue was found to be higher.15 This is probably because most patients with PsA with anaemia had haemoglobin values just below normal limits, whereas in the study on cancer, patients with haemoglobin levels ⩽11.0 g/dl were included. The FACIT fatigue scores did not correlate with increased ESR. This again could be explained by the fact that only few patients with PsA had increased ESR and the increase was only mild to moderate. Acute‐phase reactants are increased in only about 50% of patients with PsA.39
In summary, the results of this study demonstrate that the FACIT fatigue Scale is a reliable and valid measure of fatigue in patients with PsA. The scores correlate with measures of disease activity and with other measures of fatigue. Although not tested in this study, the FACIT fatigue has demonstrated responsiveness in a randomised controlled trial using adalimumab in PsA.33 It thus fulfils the requirements of the Outcome Measures in Rheumatoid Arthritis Clinical Trials filter. It may therefore be used as an outcome measure in clinical trials of PsA. However, formal studies are required to determine which fatigue assessment tool is most suitable to assess fatigue in patients with PsA in the clinic and in clinical trials.
Acknowledgements
This work was supported by a grant from the Krembil Foundation. VC was supported by The Ogryzlo Fellowship from the Arthritis Society and the Arthritis Centre of Excellence, University Health Network. SB was supported by a Canadian Rheumatology Association Student Scholarship.
Abbreviations
ESR - erythrocyte sedimentation rate
FACIT fatigue - Functional Assessment of Chronic Illness Therapy‐Fatigue
FSS - Fatigue Severity Scale
MAF - Multidimensional Assessment of Fatigue
PsA - psoriatic arthritis
RA - rheumatoid arthritis
SF‐36 - Short‐Form 36
VAS - visual analogue scale
Footnotes
Competing interests: None declared.
References
- 1.Swain M G. Fatigue in chronic disease. Clin Sci (Lond) 2000991–8. [PubMed] [Google Scholar]
- 2.Freal J E, Kraft G H, Coryell J K. Symptomatic fatigue in multiple sclerosis. Arch Phys Med Rehabil 198465135–138. [PubMed] [Google Scholar]
- 3.Krupp L B, LaRocca N G, Muir J.et al A study of fatigue in systemic lupus erythematosus. J Rheumatol 1990171450–1452. [PubMed] [Google Scholar]
- 4.Jones E A. Fatigue associated with chronic liver disease: a riddle wrapped in a mystery inside an enigma. Hepatology 1995221606–1608. [PubMed] [Google Scholar]
- 5.Wolfe F, Hawley D J, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol 1996231407–1417. [PubMed] [Google Scholar]
- 6.Jones S D, Koh W H, Steiner A, Garrett S L, Calin A. Fatigue in ankylosing spondylitis: its prevalence and relationship to disease activity, sleep, and other factors. J Rheumatol 199623487–490. [PubMed] [Google Scholar]
- 7.Suurmeijer T P, Waltz M, Moum T, Guillemin F, van Sonderen F L, Briancon S.et al Quality of life profiles in the first years of rheumatoid arthritis: results from the EURIDISS longitudinal study. Arthritis Rheum 200145111–121. [DOI] [PubMed] [Google Scholar]
- 8.Rupp I, Boshuizen H C, Jacobi C E, Dinant H J, van den Bos G A. Impact of fatigue on health‐related quality of life in rheumatoid arthritis. Arthritis Rheum 200451578–585. [DOI] [PubMed] [Google Scholar]
- 9.North American Nursing Diagnosis Association Nursing diagnoses: definition and classification, 1997–1998. Philadelphia, PA: McGraw‐Hill, 199658
- 10.Belza B L, Henke C J, Yelin E H, Epstein W V, Gilliss C L. Correlates of fatigue in older adults with rheumatoid arthritis. Nurs Res 19934293–99. [PubMed] [Google Scholar]
- 11.Ware J E, Jr, Sherbourne C D. The MOS 36‐Item Short‐Form Health Survey (SF‐36). I. Conceptual framework and item selection. Med Care 199230473–483. [PubMed] [Google Scholar]
- 12.Mendoza T R, Wang X S, Cleeland C S, Morrissey M, Johnson B A, Wendt J K.et al The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999851186–1196. [DOI] [PubMed] [Google Scholar]
- 13.Krupp L B, LaRocca N G, Muir‐Nash J, Steinberg A D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989461121–1123. [DOI] [PubMed] [Google Scholar]
- 14.Fisk J D, Pontefract A, Ritvo P G, Archibald C J, Murray T J. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci 1994219–14. [PubMed] [Google Scholar]
- 15.Cella D, Lai J S, Chang C H, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 200294528–538. [DOI] [PubMed] [Google Scholar]
- 16.Weinblatt M E, Keystone E C, Furst D E, Moreland L W, Weisman M H, Birbara C A.et al Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA Trial. Arthritis Rheum 20034835–45. [DOI] [PubMed] [Google Scholar]
- 17.Emery P, Fleischmann R, Filipowicz‐Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A.et al The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double‐blind, placebo‐controlled, dose‐ranging trial. Arthritis Rheum 2006541390–1400. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S B, Emery P, Greenwald M W, Dougados M, Furie R A, Genovese M C.et al Rituximab for rheumatoid arthritis refractory to anti‐tumor necrosis factor therapy: results of a multicenter, randomized, double‐blind, placebo‐controlled, phase III trial evaluating primary efficacy and safety at twenty‐four weeks. Arthritis Rheum 2006542793–2806. [DOI] [PubMed] [Google Scholar]
- 19.Weisman M H, Breedveld F C, Cifaldi M A, Sterz R, Dietz B M, Spencer‐Green G T. Improvements in quality of life measures from adalimumab (HUMIRA®) plus methotrexate (MTX) translate into improved physical function and less fatigue in patients with early rheumatoid arthritis (RA). Arthritis Rheum 200552(Suppl 9)S395 [Google Scholar]
- 20.Calin A, Dijkmans B A, Emery P, Hakala M, Kalden J, Leirisalo‐Repo M.et al Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004631594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfe F. Fatigue assessments in rheumatoid arthritis: comparative performance of visual analog scales and longer fatigue questionnaires in 7760 patients. J Rheumatol 2004311896–1902. [PubMed] [Google Scholar]
- 22.Vasconcelos O M, Jr, Prokhorenko O A, Kelley K F, Vo A H, Olsen C H, Dalakas M C.et al A comparison of fatigue scales in postpoliomyelitis syndrome. Arch Phys Med Rehabil 2006871213–1217. [DOI] [PubMed] [Google Scholar]
- 23.Cella D.Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) measurement system. Evanston, IL: Center on Outcomes, Research and Education (CORE), Evanston Northwestern Healthcare and Northwestern University, 1997
- 24.Cella D F, Tulsky D S, Gray G, Sarafian B, Linn E, Bonomi A.et al The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 199311570–579. [DOI] [PubMed] [Google Scholar]
- 25.Yellen S B, Cella D F, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia‐related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 19971363–74. [DOI] [PubMed] [Google Scholar]
- 26.Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol 200532811–819. [PubMed] [Google Scholar]
- 27.Wright V, Moll J M H.Seronegative polyarthritis. Amsterdam: North‐Holland, 1976
- 28.Gladman D D. Psoriatic arthritis. In: Harris ED, Budd RC, Genovese MC, Firestein GS, Sargent JS, Sledge CB, eds. Kelley's textbook of rheumatology. 7th edn. Philadelphia, PA: WB Saunders, 20051155–1164.
- 29.Gladman D D, Urowitz M B, Ong A, Gough J, MacKinnon A. A comparison of five health status instruments in patients with systemic lupus erythematosus. Lupus 19965190–195. [DOI] [PubMed] [Google Scholar]
- 30.Schentag C T, Cichon J, MacKinnon A, Gladman D D, Urowitz M B. Validation and normative data for the 0–10 point scale version of the Fatigue Severity Scale (FSS). Arthritis Rheum 200043(Suppl 9)S177 [Google Scholar]
- 31.Schentag C T, Beaton M, Rahman P, Husted J, Gladman D D. Fatigue in psoriatic arthritis (PsA). J Rheumatol 1999261627 [Google Scholar]
- 32.Schentag C, Gladman D D. Changes in fatigue in psoriatic arthritis: disease activity or fibromyalgia. Arthritis Rheum 200246(Suppl 9)S424 [Google Scholar]
- 33.Mease P J, Gladman D D, Ritchlin C T, Ruderman E M, Steinfeld S D, Choy E H.et al Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double‐blind, randomized, placebo‐controlled trial. Arthritis Rheum 2005523279–3289. [DOI] [PubMed] [Google Scholar]
- 34.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A.et al Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double‐blind placebo‐controlled randomised phase III trial. Lancet 200636729–35. [DOI] [PubMed] [Google Scholar]
- 35.Gladman D D, Farewell V, Buskila D, Goodman R, Hamilton L, Langevitz P.et al Reliability of measurements of active and damaged joints in psoriatic arthritis. J Rheumatol 19901762–64. [PubMed] [Google Scholar]
- 36.Gladman D D, Mease P J, Krueger G, van der Heijde D, Antoni C, Helliwell P S.et al Outcome measures in psoriatic arthritis. J Rheumatol 2005322262–2269. [PubMed] [Google Scholar]
- 37.Burkham J, Harris E D.Fibromyalgia: a chronic pain syndrome, In: Harris ED, Budd RC, Genovese MC, Firestein GS, Sargent JS, Sledge CB, eds. Kelley's textbook of rheumatology. 7th edn. Philadelphia, PA: WB Saunders 2005522–536.
- 38.Kleinman L, Zodet M W, Hakim Z, Aledort J, Barker C, Chan K.et al Psychometric evaluation of the Fatigue Severity Scale for use in chronic hepatitis C. Qual Life Res 20009499–508. [DOI] [PubMed] [Google Scholar]
- 39.Gladman D D, Shuckett R, Russell M L, Thorne J C, Schachter R K. Psoriatic arthritis (PSA)—an analysis of 220 patients. Q J Med 198762127–141. [PubMed] [Google Scholar]