Abstract
Background
Systemic lupus erythematosus (SLE) is characterised by dysregulation of autoreactive lymphocytes and antigen‐presenting cells. Signalling through Toll‐like receptor 9 (TLR9), a mediator of innate immune responses, has a role in activation of dendritic cells and autoreactive B cells.
Objective
To investigate whether TLR9 polymorphisms are associated with an increased risk of SLE.
Methods
DNA samples were obtained from 220 Japanese patients with SLE (with >4 American College of Rheumatology criteria for SLE) and 203 controls. The genetic variations of TLR9 were detected by PCR, followed by DNA sequencing. The promoter and enhancer activities of TLR9 were measured by luciferase reporter gene assay. The titres of anti‐dsDNA antibodies in sera from control or TLR9‐deficient mice were analysed by ELISA.
Results
The G allele at position +1174 (located in intron 1 of TLR9) is closely associated with an increased risk of SLE (p = 0.029). Furthermore, patients with SLE tend to have C allele at position −1486 (p = 0.11). Both alleles down regulate TLR9 expression by reporter gene assay. TLR9‐deficient mice under a C57BL/6 background possess higher titres of anti‐dsDNA serum antibodies than control C57BL/6 mice.
Conclusions
These results indicate that the presence of the G allele at position +1174 of TLR9 predisposes humans to an increased risk of SLE. It is speculated that TLR9 normally prevents the development of human SLE.
Systemic lupus erythematosus (SLE) is an autoimmune disease with a multifactorial aetiology characterised by impaired T cell responses and dysregulation of B cell activation, leading to production of autoantibodies.1,2 The role of genetics in the aetiology of SLE is firmly established, and many genes that are important for immune functions have been identified as candidates, including major histocompatibility antigens, PD‐1 and low‐affinity IgG receptors.2,3,4 Additionally, our group and others demonstrated that defective clearance of nucleosomal antigens and apoptotic cells is also involved in the development of SLE.5,6,7,8 The marked accumulation of nucleosomal antigens would allow low numbers of lymphocytes specific for nucleosomal antigens to expand vigorously. In agreement with this, patients with DNase1‐defective SLE have much higher titres of anti‐dsDNA antibodies than patients with SLE with normal DNase1 activity.6
Toll‐like receptor 9 (TLR9) recognises hypomethylated CpG DNA and generally acts as a sensor for bacterial infection.9,10 In addition, signalling through both TLR9 and IgM in B cells is involved in increased production of autoantibodies.11 Subsequent studies demonstrated that TLR9 contributes to the production of autoantibodies in mouse SLE‐like syndrome.12 However, Wu and Peng13 reported that TLR9 protects against mouse SLE‐like syndrome, as supported by the finding that TLR9‐deficient mice have defective suppressive activity of CD4CD25 regulatory T cells. Therefore, it remains to be clarified whether TLR9 is a causative or a protective molecule for human SLE. In addition, TLR9 signalling is involved in the production of interferon‐γ (IFNγ) from plasmacytoid dendritic cells.10 Although there is accumulating evidence that IFNγ contributes to progression of SLE,14,15 it is unclear whether TLR9‐mediated IFNγ production is responsible for the progression of SLE.
Three studies conducted in the UK, Korea and Hong Kong have investigated possible associations of genetic variations of TLR9 with SLE.16,17,18 Those studies did not detect an association of TLR9 gene variations with SLE susceptibility. As for the relationship of TLR9 genetic variations with immune‐associated diseases, Lazarus et al19 found an increased risk for asthma among European Americans with a C allele at position −1237, although the function of this region was not investigated.
In the present study, we investigated whether genetic variations of TLR9 are involved in the susceptibility of a Japanese population to SLE. We found that the presence of a G allele at position +1174 located in intron 1 of TLR9 is associated with an increased risk of SLE. The C allele at position −1486 was also closely correlated with the risk of SLE. The combination of both alleles down regulated the transcription of TLR9, as determined by reporter gene assay. Furthermore, we found that TLR9‐deficient mice have higher anti‐dsDNA antibody titres than control mice. These studies indicate that genetic variations of TLR9 that down regulate TLR9 expression are associated with an increased risk of SLE in a Japanese population.
Patients and methods
Subjects
We enrolled 423 Japanese individuals, including 220 patients with SLE and 203 unrelated healthy individuals, through the Kyushu University Hospital, Fukuoka, Japan, for genetic studies. All affected individuals fulfilled the American College of Rheumatology's Revised Criteria for the Classification of SLE.20,21 We obtained approval for the study from the ethics committee at Kyushu University, Fukuoka, Japan and Tokushima University, Tokushima, Japan, before patient enrolment. All individuals studied gave informed consent.
Mice
C57BL/6 mice were purchased from Japan CLEA Inc (Hamamatsu, Japan). The TLR9‐deficient mice were established as reported previously,22 and maintained at Kyushu University. All animal studies were approved by the animal research committees of The University of Tokushima and Kyushu University.
Sequencing and genotyping
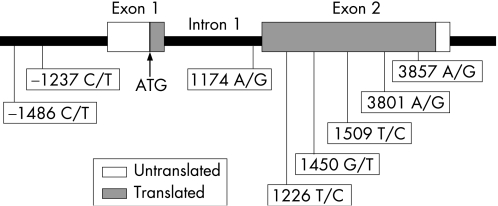
Genomic DNA samples from 220 Japanese patients with SLE and 203 normal controls were extracted from peripheral blood mononuclear cells. We searched the database of the National Institutes of Health (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db = snp&cmd = search&term = ) for the SNPs of TLR9 (GeneBank accession number NM‐017442). We first selected five non‐synonymous single‐nucleotide polymorphisms (SNPs) within exon 2. We also added one SNP within intron 1 and two SNPs in the 5′ region of TLR9.19 Figure 1 illustrates the location of these SNPs. The region containing each SNP was amplified by PCR. PCR was performed in a 10 μl volume using rTaq DNA polymerase kit (Toyobo, Osaka, Japan) with 50 ng of genomic DNA and 5 pmol of each primer. The cycling conditions were 2 min at 94°C followed by 40 cycles consisting of 30 s at 96°C, 30 s at 68°C and 10 min at 72°C. The PCR products were sequenced using a Big Dye Teminator V.3.1 Cycle Sequence Kit (Perkin‐Elmer Applied Biosystems, Foster City, California, USA), and the reactions were analysed on an ABI PRISM 3100 Genetic Analyser (Perkin‐Elmer Applied Biosystems).
Figure 1 Single‐nucleotide polymorphisms (SNPs) in Toll‐like receptor 9 (TLR9). Position of SNPs in the human TLR9 gene. Open or filled squares indicate the untranslated or translated regions of the exon, respectively. SNPs of offsets were calculated by taking the A of the TLR9 ATG start codon as position 1, based on GenBank Accession No NM_017442.
Luciferase reporter assay
The haplotype C at −1486 and G at +1174 were representative of patients with SLE, whereas the haplotype T at −1486 and A at +1174 were representative of normal individuals. Three types of plasmids were prepared. The pGL‐3 basic vector had an insert of the 5′ region of TLR9 containing haplotype T or C at −1486 (termed “promoter”). The pGL‐3 promoter vector encoding the SV40 promoter had an insert of intron 1 of TLR9 containing haplotype A or G at 1174 (termed “intron”). The pGL‐3 basic vector had an insert of both of these regions (termed “promoter+intron”). The 5′ region of the TLR9 gene containing haplotype C or T at −1486 was amplified with forward primer 5′‐ACTTGTGCTTGGCCCTGAGAG‐3′ and reverse primer 5′‐TCAAAGCCACAGTCCACAGATG‐3′ under the previously described PCR conditions. The region containing the translation initiation codon within exon 1 and haplotype A or G at +1174 within intron 1 was amplified with forward primer 5′‐ATGGTAGGACAACAGCTCTCAG‐3′ and reverse primer 5′‐GGAGCTCACAGGGTAGGAAG‐3′. These PCR products were blunted using T4 polynucleotide kinase (New England Biolabs, Schwalbach, Germany). The amplification product was ligated into pGL3 luciferase reporter vector using Ligation kit V.2.1 (Takara, Kyoto, Japan).
The luciferase activity was assayed according to the Dual‐Luciferase Reporter Assay System (Promega, Madison, Wisconsin, USA). Transient transfections of Jurkat cells were performed using DMRIE‐C lipofection reagent (Invitrogen, Carlsbad, California, USA) according to the manufacturer's protocol. At 48 h after transfection, luciferase luminescence of each sample was read on the MiniLumat LB 9506 Luminometer (Perkin‐Elmer, Bad Wildbad, Germany).
Cell culture
We verified that Jurkat cells expressed TLR9, using reverse transcriptase‐polymerase chain reaction (data not shown). Jurkat cells were maintained in RPMI 1640 media supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin and 0.1% of 2‐mercaptoethanol at 37°C with 5% CO2.
ELISA
Sera from TLR9‐deficient or control mice were serially diluted, and each was plated in 96‐well plates coated with dsDNA. After incubation with sera, plates were washed, and alkaline phosphatase‐conjugated anti‐mouse IgG, IgG1 and IgG2a (Southern Biotech, Birmingham, Alabama, USA) were added to the plates. After washing and addition of p‐nitrophenyl phosphate tablets (Sigma, St Louis, Missouri, USA), chemiluminescence was measured.
Statistical analysis
Statistical analysis was performed using χ2 test for the allele frequencies of TLR‐9 and Student's t test for the comparison of luciferase activity and titre of anti‐dsDNA antibody. A value of p<0.05 was considered significant.
Results
Genotype frequency of TLR9 gene in patients with SLE
Genetic variations in the TLR9 gene are reported in the National Institutes of Health database (http://www.ncbi.nlm.nih.gov/SNP) and are shown in fig 1. In this database, five SNPs are located in exon 2, one in intron 1 and two in the 5′ region of TLR9 (fig 1). We analysed patients with SLE and normal controls for those eight SNPs (−1486, −1237, +1174, +1226, +1450, +1509, +3801 and +3857) of the TLR9 gene by PCR amplification of target sites followed by DNA sequencing. Table 1 shows the TLR9 genotype frequencies of patients with SLE and controls. In all, 84 patients with SLE and 84 controls had the same genotypes at +1226, +1450, +1509 SNPs, 86 patients with SLE and 88 controls had the same genotypes at, +3801, +3857 SNPs, and 183 patients with SLE and 198 controls had the same genotype at −1237 SNP. Thus, no polymorphisms at these regions were apparent in this Japanese population. By contrast, genetic variations were observed at positions +1174 and −1486 (table 1). The G/G genotype at +1174 within intron 1 was more frequent in patients with SLE (controls: 18% vs patients with SLE: 25%) and the A/A genotype at +1174 was more frequent in controls (controls: 30% vs patients with SLE: 22%). Although patients with SLE tended to have G/G genotypes at +1174 compared with healthy controls, there was no statistical significance. We also did not find any statistical difference in genotypes at position −1486.
Table 1 Genotype frequencies in patients with systemic lupus erythematosus and controls.
| Offset | ref SNP ID | Genotype | Controls n (%) | SLE n (%) | p Value |
|---|---|---|---|---|---|
| −1486 | rs187084 | C/T | 108/198 (54.5) | 95/183 (51.9) | 0.225 |
| T/T | 51/198 (25.8) | 49/183 (26.8) | |||
| C/C | 39/198 (19.7) | 39/183 (21.3) | |||
| −1237 | rs5743836 | T/T | 198/198 (100) | 183/183 (100) | |
| 1174 | rs352139 | A/G | 105/203 (51.7) | 115/220 (52.3) | |
| A/A | 61/203 (30.0) | 49/220 (22.3) | 0.083 | ||
| G/G | 37/203 (18.2) | 56/220 (25.5) | |||
| 1226 | rs5743842 | C/C | 84/84 (100) | 84/84 (100) | |
| 1450 | rs5743843 | T/T | 84/84 (100) | 84/84 (100) | |
| 1509 | rs5743844 | C/C | 84/84 (100) | 84/84 (100) | |
| 3801 | rs5743845 | G/G | 88/88 (100) | 86/86 (100) | |
| 3857 | rs5743846 | G/G | 88/88 (100) | 86/86 (100) |
SLE, systemic lupus erythematosus; SNP, single‐nucleotide polymorphism.
We next examined the association of individual genotypes at +1174 and −1486 (table 2). Within the SLE patient group, 97.8% or 95.1% of individuals with G/G or A/A genotypes at position +1174 had C/C or T/T genotypes, respectively, at position −1486 (table 2). This result suggests that the G and A alleles at position +1174 are closely linked with C or T alleles, respectively, at position −1486, as reported previously.19
Table 2 Genotype of single nucleotide polymorphism in the promoter region.
| TLR‐9 SNP | +1174 | −1486 (%) |
|---|---|---|
| Genotype | A/A | T/T (95.1%) |
| C/T (2.4%) | ||
| C/C (2.4%) | ||
| G/G | C/C (97.8%) | |
| C/T (2.2%) | ||
| T/T (0%) | ||
| A/G | C/T (96.9%) | |
| T/T (0%) | ||
| C/C (3.1%) |
SNP, single‐nucleotide polymorphism; TLR9, Toll‐like receptor 9.
Allele frequencies at +1174 and −1486 regions of TLR9 in patients with SLE
To further examine the contribution of genetic variations in +1174 and −1486 of TLR9 to the risk of SLE, we compared the allele frequencies at both regions of TLR9 (table 3). The G allele at position +1174 was associated with a significantly increased risk of SLE (p = 0.029). The C allele at −1486 tended to be more frequent in patients with SLE than in healthy controls, but significance was not achieved (p = 0.112).
Table 3 Allele frequencies in patients with systemic lupus erythematosus and controls.
| Locus | Haplotype | Controls n (%) | SLE n (%) | p Value |
|---|---|---|---|---|
| −1486 | T | 210 (53.0) | 173 (47.3) | 0.112 |
| C | 186 (47.0) | 193 (52.7) | ||
| +1174 | A | 227 (56.0) | 213 (48.4) | 0.029 |
| G | 179 (44.0) | 227 (51.6) |
SLE, systemic lupus erythematosus.
Downregulation of TLR9 expression by the two SNPs frequently observed in patients with SLE
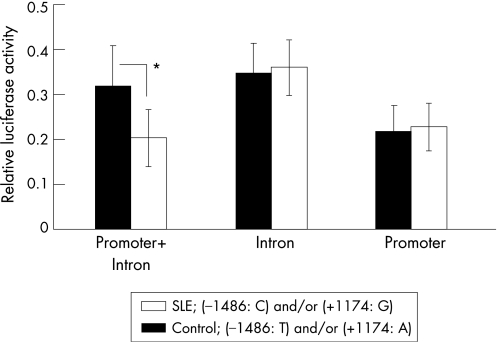
We next examined whether two regions (−1486 in the 5′ region of the TLR9 gene and +1174 within intron 1) regulated the transcription of the TLR9 gene, as neither is located in a coding region. The C allele at −1486 and G allele at +1174, or the T allele at −1486 and A allele at +1174, were used as representatives of patients with SLE or controls, respectively. We constructed three plasmids. The first plasmid (promoter) had the 5′ region of TLR9 containing an SNP located in −1486. The second plasmid (intron) encoding SV40 promoter had intron 1 of TLR9 containing an SNP located in +1174. The third plasmid (promoter+intron) had both regions without any promoter sequence. Each reporter plasmid was transfected into Jurkat cells, and luciferase activity was compared between SLE and healthy controls. Transfection of the promoter plasmid of control type induced luciferase activity in Jurkat cells, which indicates that this region encodes the TLR9 promoter (fig 2). However, we did not observe any difference in luciferase activity between SLE and control types when the promoter plasmid was transfected (fig 2). Transfection of intron plasmids of SLE and control types also showed similar enhancer activities for SV40 promoter (fig 2). In contrast, the transfection of promoter+intron plasmid showed lower TLR9 promoter activity in SLE than did control type (fig 2). Thus, the combination of C at −1486 and G at +1174, which is frequently observed in SLE, has lower regulatory activity for TLR9 transcription compared with that of T at −1486 and A at +1174.
Figure 2 Regulation of Toll‐like receptor 9 (TLR9) transcription. Jurkat cells were transfected with reporter plasmids, and luciferase activity was examined. The C allele at −1486 and G allele at +1174, or the T allele at −1486 and A allele at +1174, were used as representatives of systemic lupus erythematosus (SLE; open) or controls (closed), respectively. The plasmid (promoter) has the 5′ region of TLR9 containing a single‐nucleotide polymorphism (SNP) located at −1486. The plasmid (intron) encoding the SV40 promoter has an intron 1 of TLR9 containing an SNP located at +1174. The plasmid (promoter+intron) has both regions without any promoter sequence. Relative luciferase activity was expressed by Renilla reniformis/Photinus pyralis luciferase activity. Results are expressed as the mean (SD) of three samples and p value was calculated. *p<0.05.
Increased titre of anti‐dsDNA antibody in sera from TLR9‐deficient mice
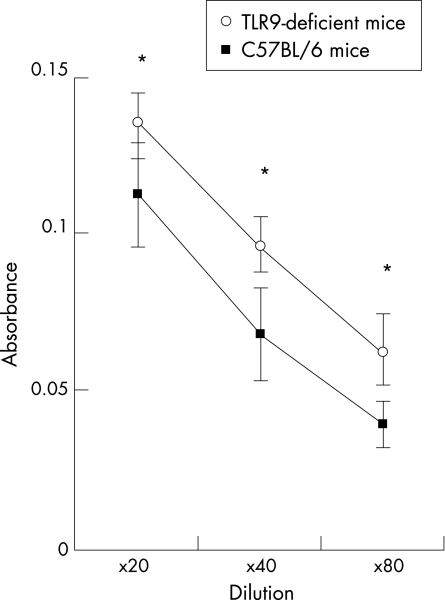
To determine whether there is a functional link between low expression of TLR9 and increased risk for SLE, we examined anti‐dsDNA levels in serially diluted sera from TLR9‐deficient and control C57BL/6 mice at the age of 10 weeks. The anti‐dsDNA antibody levels were higher in TLR9‐deficient mice than in C57BL/6 mice (fig 3). These results indicate that diminished TLR9 expression contributes to increased production of anti‐dsDNA antibody in mice even under low autoimmune background genes.
Figure 3 Anti‐dsDNA levels in sera from Toll‐like receptor 9 (TLR9)‐deficient mice. Sera from TLR9‐deficient (open circle) and C57BL/6 (closed square) mice were serially diluted and the levels of anti‐dsDNA were evaluated using ELISA. Results were expressed as mean (SD) of triplicate samples and p value calculated. *p<0.05.
Discussion
TLR9, which is activated by hypomethylated CpG DNA, triggers innate immune responses.9,10 Although TLR9 signalling has a crucial role in controlling infections, recent studies using autoimmune‐prone mouse models have suggested that TLR9 is associated with progression of autoimmune diseases.11,23,24 Thus, we investigated whether genetic variations of TLR9 are involved in the increased risk of SLE in a Japanese population. We found that a G allele at a position +1174 located in intron 1 of TLR9 is associated with an increased risk of SLE. The combination of the G allele at position +1174 with a C allele at position −1486 has the ability to down regulate TLR9 expression. Furthermore, TLR9‐deficient mice at 10 weeks of age had higher titres of anti‐dsDNA antibody than control C57BL/6 mice. These results strongly suggest a significant association between low TLR9 expression and increased risk of SLE.
In this report, we demonstrate that a G allele at position +1174 (located in intron 1 of TLR9) is associated with an increased risk of SLE in Japanese population. The G allele seems to down regulate TLR9 expression at the transcriptional level. Previous studies have not demonstrated any genetic variations of TLR9 associated with an increased risk of SLE in UK, Korean and Hong Kong Chinese populations.16,17,18 Korean and Chinese populations do not differ in allele frequencies at position +1174 between patients with SLE and healthy controls; the variation of this region was not evaluated in the UK population.16,17,18 The genetic variation at position −1237 is associated with an increased risk of asthma in European American populations.19 However, we did not detect any SNP at position −1237 in our study (data not shown), and Korean and Chinese populations also have very small variations at position −1237.17,18 Taken together, those results suggest that the genetic variations of TLR9 observed at position +1174 might be specific for Japanese SLE populations. In fact, ethnic differences in the genetic polymorphisms have been reported in genes such as PDCD1 and PTPN22, which are known to be related to autoimmune diseases.25 Nevertheless, it should be determined whether genetic variation within other molecules related to TLR9 signalling pathways and/or regulatory molecules of TLR9 expression are involved in the susceptibility of different ethnic groups to SLE. In addition, we examined whether genetic variations of TLR9 have any relationship with age of onset or Systemic Lupus International Collaborating Clinics damage index,26 but we could not detect any relationship (supplementary fig 1 is available online at http://ard.bmj.com/supplemental). We believe that it will be important to examine whether TLR9 gene variations affect the disease activity, including anti‐dsDNA antibody or susceptibility to drug treatment, in future studies.
Based on studies of autoimmune‐prone mice, there is accumulating evidence that TLR9 is involved in the progression of autoimmune diseases. Leadbetter et al11 first reported that the recognition of immune complexes (DNA and immunoglobulin) by both IgM and TLR9 was required for the production of anti‐immunoglobulin antibody. Furthermore, Ehlers et al27 reported that TLR9 is an MyD88‐dependent inducer of IgG2a and 2b autoantibodies, which are implicated in the progression of lupus‐prone mice. Christensen et al12 have demonstrated that lpr mice crossed with TLR9‐deficient mice produce low amounts of anti‐dsDNA antibody, although no effect on the development of clinical autoimmune disease was observed. Those results have suggested that TLR9 signalling is associated with progression of autoimmune diseases including SLE. In contrast, Wu and Peng13 reported that genetic ablation of TLR9 has a protective role in the onset of SLE‐like syndrome in MRL/lpr mice because TLR9‐deficient animals have low suppressive activity of CD4+CD25+ regulatory T cells (Treg). One difference between Wu's and Christensen's studies is the genetic background of the mice. Wu and Peng backcrossed TLR9 mice to the MRL background more than six times, but Christensen et al backcrossed one or two times.12,13 While it is unclear why different groups reach distinctly different conclusions, previous studies have investigated the roles of TLR9 in the production of autoantibodies under autoimmune‐prone backgrounds, including the lpr mutation and the MRL strain.12,13 Therefore, TLR9 signal‐mediated cellular responses may change depending on the level of autoimmune‐prone background. To avoid the effect of other autoimmune‐related genes as much as possible, we used TLR9‐deficient mice under C57BL/6 background in this study. We demonstrated that such TLR9‐deficient mice possess a higher titre of anti‐dsDNA antibody than control C57BL/6 mice, which supports Wu and Peng's13 studies, although those mice did not develop autoimmune pathology by the age of 10 weeks (data not shown). Thus, diminished expression of TLR9 could be involved in enhanced production of autoantibodies. It will be important to evaluate whether aged TLR9‐deficient mice lacking other autoimmune‐related genes develop autoimmune diseases.
Regarding the mechanism by which low TLR9 expression is involved in SLE, one previous paper has suggested that Treg are defective in TLR9‐deficient mice under MRL background.13 We also have data showing that stimulation of dendritic cells through TLR9 during parasite infection strengthens the suppressive function of Treg, but that CpG oligodeoxynucleotide stimulation of dendritic cells cannot induce such effects (studied by Hisaeda et al in 2007, unpublished observations). These results suggest that unknown ligands for TLR9 activate dendritic cells, which increases the suppressive function of Treg. As for the contribution of Treg to human SLE, there are several papers indicating that the function and number of Treg are decreased in patients with SLE.28,29 Thus, TLR9‐mediated activation of Treg may be involved in protecting against SLE.
Impaired clearance of apoptotic cells or self‐DNA has been implicated in the pathogenesis of human SLE.1 Indeed, the C1q defect or decreased activity of DNase I is observed in some patients with SLE.1,30 By contrast, the present studies demonstrated that TLR9 genotypes down regulating TLR9 expression are associated with increased SLE susceptibility, which might inhibit SLE pathology caused by impaired clearance of apoptotic cells or nucleosomes. Therefore, genetic variations of TLR9 and impaired clearance of apoptotic cells or nucleosomes would independently affect the susceptibility of SLE. In addition, a recent paper demonstrated that there is a TLR9‐independent sensing mechanism to activate the innate immune responses against the endogenous DNA,31 which also supports the independent contribution of TLR9 and clearance of antigens on SLE susceptibility.
In summary, these data strongly suggest that TLR9 genetic variations associated with low TLR9 levels have a crucial role in establishing an autoimmune background in human SLE. Although the precise mechanisms are unclear, those results suggest that a TLR9 agonist might be useful for the treatment of SLE.
Supplementary table 1 is available online at http://ard.bmj.com/supplemental.
Supplementary Material
Acknowledgements
We thank Mrs Suga and Yamakawa for their technical and editorial assistance. This work was supported by the Mitsubishi Foundation and by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations
IFNγ - interferon‐γ
SLE - systemic lupus erythematosus
SNP - single‐nucleotide polymorphism
TLR9 - Toll‐like receptor 9
Treg - regulatory T cells
Footnotes
Competing interests: None declared.
Supplementary table 1 is available online at http://ard.bmj.com/supplemental.
References
- 1.Yasutomo K. Pathological lymphocyte activation by defective clearance of self‐ligands in systemic lupus erythematosus. Rheumatology (Oxford) 200342214–222. [DOI] [PubMed] [Google Scholar]
- 2.Wakeland E K, Liu K, Graham R R, Behrens T W. Delineating the genetic basis of systemic lupus erythematosus. Immunity 200115397–408. [DOI] [PubMed] [Google Scholar]
- 3.Nath S K, Kilpatrick J, Harley J B. Genetics of human systemic lupus erythematosus: the emerging picture. Curr Opin Immunol 200416794–800. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan S, Chowdhury B, Tsokos G C. Autoimmunity in systemic lupus erythematosus: integrating genes and biology. Semin Immunol 200618230–243. [DOI] [PubMed] [Google Scholar]
- 5.Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz H G, Moroy T. Features of systemic lupus erythematosus in Dnase1‐deficient mice. Nat Genet 200025177–181. [DOI] [PubMed] [Google Scholar]
- 6.Yasutomo K, Horiuchi T, Kagami S, Tsukamoto H, Hashimura C, Urushihara M.et al Mutation of DNASE1 in people with systemic lupus erythematosus. Nat Genet 200128313–314. [DOI] [PubMed] [Google Scholar]
- 7.Munoz L E, Gaipl U S, Franz S, Sheriff A, Voll R E, Kalden J R.et al SLE—a disease of clearance deficiency? Rheumatology (Oxford) 2005441101–1107. [DOI] [PubMed] [Google Scholar]
- 8.White S, Rosen A. Apoptosis in systemic lupus erythematosus. Curr Opin Rheumatol 200315557–562. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Takeda K. Toll‐like receptor signalling. Nat Rev Immunol 20044499–511. [DOI] [PubMed] [Google Scholar]
- 10.Takeda K, Kaisho T, Akira S. Toll‐like receptors. Annu Rev Immunol 200321335–376. [DOI] [PubMed] [Google Scholar]
- 11.Leadbetter E A, Rifkin I R, Hohlbaum A M, Beaudette B C, Shlomchik M J, Marshak‐Rothstein A. Chromatin‐IgG complexes activate B cells by dual engagement of IgM and Toll‐like receptors. Nature 2002416603–607. [DOI] [PubMed] [Google Scholar]
- 12.Christensen S R, Kashgarian M, Alexopoulou L, Flavell R A, Akira S, Shlomchik M J. Toll‐like receptor 9 controls anti‐DNA autoantibody production in murine lupus. J Exp Med 2005202321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X, Peng S L. Toll‐like receptor 9 signaling protects against murine lupus. Arthritis Rheum 200654336–342. [DOI] [PubMed] [Google Scholar]
- 14.Pascual V, Banchereau J, Palucka A K. The central role of dendritic cells and interferon‐alpha in SLE. Curr Opin Rheumatol 200315548–556. [DOI] [PubMed] [Google Scholar]
- 15.Ronnblom L, Alm G V. An etiopathogenic role for the type I IFN system in SLE. Trends Immunol 200122427–431. [DOI] [PubMed] [Google Scholar]
- 16.De Jager P L, Richardson A, Vyse T J, Rioux J D. Genetic variation in toll‐like receptor 9 and susceptibility to systemic lupus erythematosus. Arthritis Rheum 2006541279–1282. [DOI] [PubMed] [Google Scholar]
- 17.Hur J W, Shin H D, Park B L, Kim L H, Kim S Y, Bae S C. Association study of Toll‐like receptor 9 gene polymorphism in Korean patients with systemic lupus erythematosus. Tissue Antigens 200565266–270. [DOI] [PubMed] [Google Scholar]
- 18.Ng M W, Lau C S, Chan T M, Wong W H, Lau Y L. Polymorphisms of the toll‐like receptor 9 (TLR9) gene with systemic lupus erythematosus in Chinese. Rheumatology (Oxford) 2005441456–1457. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus R, Klimecki W T, Raby B A, Vercelli D, Palmer L J, Kwiatkowski D J.et al Single‐nucleotide polymorphisms in the Toll‐like receptor 9 gene (TLR9): frequencies, pairwise linkage disequilibrium, and haplotypes in three U.S. ethnic groups and exploratory case‐control disease association studies. Genomics 20038185–91. [DOI] [PubMed] [Google Scholar]
- 20.Tan E M, Cohen A S, Fries J F, Masi A T, McShane D J, Rothfield N F.et al The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982251271–1277. [DOI] [PubMed] [Google Scholar]
- 21.Hochberg M C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997401725. [DOI] [PubMed] [Google Scholar]
- 22.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H.et al A Toll‐like receptor recognizes bacterial DNA. Nature 2000408740–745. [DOI] [PubMed] [Google Scholar]
- 23.Anders H J. A Toll for lupus. Lupus 200514417–422. [DOI] [PubMed] [Google Scholar]
- 24.Rifkin I R, Leadbetter E A, Busconi L, Viglianti G, Marshak‐Rothstein A. Toll‐like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev 200520427–42. [DOI] [PubMed] [Google Scholar]
- 25.Mori M, Yamada R, Kobayashi K, Kawaida R, Yamamoto K. Ethnic differences in allele frequency of autoimmune‐disease‐associated SNPs. J Hum Genet 200550264–266. [DOI] [PubMed] [Google Scholar]
- 26.Gladman D D, Urowitz M B, Goldsmith C H, Fortin P, Ginzler E, Gordon C.et al The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum 199740809–813. [DOI] [PubMed] [Google Scholar]
- 27.Ehlers M, Fukuyama H, McGaha T L, Aderem A, Ravetch J V. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med 2006203553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crispin J C, Martinez A, Alcocer‐Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun 200321273–276. [DOI] [PubMed] [Google Scholar]
- 29.Alvarado‐Sanchez B, Hernandez‐Castro B, Portales‐Perez D, Baranda L, Layseca‐Espinosa E, Abud‐Mendoza C.et al Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun 200627110–118. [DOI] [PubMed] [Google Scholar]
- 30.Gaipl U S, Beyer T D, Heyder P, Kuenkele S, Bottcher A, Voll R E.et al Cooperation between C1q and DNase I in the clearance of necrotic cell‐derived chromatin. Arthritis Rheum 200450640–649. [DOI] [PubMed] [Google Scholar]
- 31.Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll‐like receptor‐independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp Med 20052021333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.