Abstract
Objective
To compare epicutaneous ketoprofen in Transfersome (ultra‐deformable vesicles, IDEA‐033) versus oral celecoxib and placebo for relief of signs and symptoms in knee osteoarthritis.
Methods
This was a multicentre, randomised, double‐blind, controlled trial; 397 patients with knee osteoarthritis participated and 324 completed the trial. They were randomly assigned 110 mg epicutaneous ketoprofen in 4.8 g Transfersome plus oral placebo (n = 138), 100 mg oral celecoxib plus placebo gel (n = 132), or both placebo formulations (n = 127) twice daily for 6 weeks. Primary efficacy outcome measures were the changes from baseline to end of the study on the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index pain subscale, physical function subscale and patient global assessment (PGA) of response.
Results
The mean WOMAC pain subscale scores in the intent to treat population were reduced by 18.2 (95% confidence interval −22.1 to −14.3), 20.3 (−24.3 to −16.2) and 9.9 (−13.9 to −5.8) in the IDEA‐033, celecoxib and placebo groups, respectively, and the physical function subscale score by 14.6 (−18.1 to −11.0), 16.6 (−20.2 to −13.0) and 10.2 (−13.8 to −6.6), respectively. The mean PGA of response scores were 1.8 (1.6 to 2.1), 1.7 (1.5 to 1.9) and 1.3 (1.1 to 1.5), respectively. The differences in change between IDEA‐033 and placebo were statistically significant for pain subscale (p<0.01) and PGA of response (p<0.01). Gastrointestinal adverse events for IDEA‐033 were similar to placebo.
Conclusion
IDEA‐033 is superior to placebo and comparable with celecoxib in relieving pain associated with an acute flare of knee osteoarthritis.
Keywords: Osteoarthritis, ketoprofen, celecoxib, ultra‐deformable vesicles
Osteoarthritis is the most prevalent form of arthritis, and is often associated with significant pain, disability and impaired quality of life due to cartilage degeneration and synovial inflammation.1 Current treatment recommendations for osteoarthritis focus on relieving pain and stiffness and on maintaining physical function. Nonsteroidal anti‐inflammatory drugs (NSAIDs) are one of the cornerstones of these guidelines, but face increasing concerns related to long‐term use due to their safety profiles.2,3,4,5 Nonspecific cyclooxygenase (COX) inhibitors have the potential to cause gastrointestinal bleeding in a dose‐related manner, and recent studies have shown that Cox‐II inhibitors may increase the risk of cardiovascular events such as myocardial infarctions.6
IDEA‐033 is a formulation containing ketoprofen, a well‐established NSAID,7 in Transfersomes. Transfersomes are ultra‐deformable carriers loaded with an active substance and applied epicutaneously in an aqueous suspension. Once the Transfersomes are on the skin, water starts to evaporate and deprive carriers of their suspending medium. Carriers reaching their solubility limit are attracted by the higher water content in the skin, resulting in spontaneous migration of IDEA‐033 through the skin barrier.8 The cutaneous microcirculation cannot clear these carriers because of their large size. The maximum depth of ketoprofen delivery from IDEA‐033 in soft tissue is controlled by the applied dose per skin area. We compared this innovative delivery form of ketoprofen with the first approved specific COX‐2 inhibitor, celecoxib,9 and with placebo for relief of signs and symptoms in knee osteoarthritis. We selected celecoxib at its approved standard dose for use in osteoarthritis of 100 mg twice daily.10
Methods
The study took place at 30 outpatient units in Germany between July 2003 and January 2004. The ethics committees or institutional review boards of each centre approved the protocol.
Participants
We considered for inclusion patients with a minimum of 6 months' history of osteoarthritis of the knee who met two of the following three clinical criteria: (1) morning stiffness of <30 minutes' duration, crepitus on motion and age ⩾40 years; (2) rating their pain in the index knee as ⩾3 on a five‐point Likert scale; and (3) taking oral NSAIDs at least 3 days per week for the past 3 months or for >25 of the past 30 days. Moreover, patients had to meet three osteoarthritis flare criteria: (1) pain in the index knee on walking ⩾40 mm on a visual analogue scale (VAS); (2) increased by ⩾15 mm compared with pain on prestudy treatment (screening); and (3) patient global assessment (PGA) score for osteoarthritis of 3–5 and at least one grade increase from screening.
Exclusion criteria were (1) grade 1 or grade 4 severity of the index knee based on the Kellgren and Lawrence radiographic criteria;11 (2) intra‐articular injections or arthroscopy of the index knee within the 3 months before screening; (3) signs of any clinically important inflammation of the index knee; crystalline‐induced synovitis in the index knee; and (4) a history, physical examination, or radiography results suggestive of acute inflammatory arthritis, rheumatoid arthritis, psoriatic arthritis, septic arthritis, gout, pseudogout, fibromyalgia, lupus erythematosus, or other types of inflammatory arthritis of the index knee.
Study design
Patients with pain in the non‐index knee during the 2 weeks before baseline received epicutaneous treatment of both knees (group 1). Patients with no pain in the non‐index knee received epicutaneous treatment of the index knee only (group 2). We used a computer‐generated centralised randomisation list to produce blocks of six patients, balanced per study centre and within groups 1 and 2, respectively. Randomisation to group 2 was restricted to 3 of 12 subjects, whereas randomisation to group 1 was unrestricted. Randomisation was performed by a centralised telephone procedure.
Patients returned for study visits after 2, 4 and 6 weeks. At each visit, the VAS version of the WOMAC, Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index,12,13 PGA of response, PGA of osteoarthritis, and safety assessments were performed.
Interventions
Patients received either 110 mg epicutaneous ketoprofen in 4.8 g Transfersome (a semi‐solid formulation, IDEA‐033) and oral placebo, 100 mg oral celecoxib and placebo gel, or both placebo formulations every 12 hours for 6 weeks. IDEA‐033 was accurately dosed using a commercially available applicator. The dose of 110 mg ketoprofen in 4.8 g Transfersome had demonstrated an acceptable safety profile in previous studies and was the maximum feasible dose, taking <30 minutes to dry on the skin. Patients could take up to 2000 mg paracetamol per day as rescue medication for knee pain for 3 days in any week, apart from the 48 hours preceding a study visit.
Outcome measures
All efficacy outcome measures were assessed in the index knee.
Primary outcome measures
We selected the following primary efficacy outcome measures: changes from baseline to end of study on the WOMAC Osteoarthritis Index VA3.1 pain subscale, physical function subscale and PGA of response (5‐point Likert scale: 0, none; 4, excellent) in the intent to treat (ITT) population.
Secondary outcome measures
Secondary efficacy outcome measures were the changes from baseline to end of study in PGA of osteoarthritis (5‐point classification),14 WOMAC stiffness subscale, use of rescue medication, discontinuation due to lack of efficacy, and primary outcome measures after 2 and 4 weeks of treatment.
All WOMAC subscale scores were normalised to a scale of 0 to 100 by dividing the sum subscale score by the number of questions of each subscale score.
In a post hoc analysis, calculation of responder rates was modified in line with the recently updated Outcome Measures in Rheumatology initiative/Osteoarthritis Research Society International (OMERACT‐OARSI) criteria.15
Safety assessments consisted of physical examination, standard haematology and biochemistry tests, questioning about adverse events, plasma concentrations of ketoprofen, and scoring of erythema and oedema of the skin area exposed to Transfersome.
Sample size
We calculated the sample size for this study based on the criteria recommended by the OARSI Standing Committee for Clinical Trial Response.16 By these criteria, a patient is classified as a responder if they demonstrate the following response in at least two of the following three domains: a 10‐mm improvement on pain, a 15‐mm improvement on physical function and a 35% improvement on PGA. This was the first phase II study of IDEA‐033, thus we used the placebo group's mean PGA score of 1.33 from a rofecoxib trial17 as an expected placebo response. A difference in mean PGA of 0.47 and a standard deviation (SD) estimate of 1.24 were used for the PGA score. Differences of 10 and 15 were used for the WOMAC pain and physical function scores, respectively, as well as a common SD of 27.18 A total of 120 patients per treatment group would provide ⩾80% power to detect differences in all three symptomatic domains, and 132 patients per group would account for a 10% dropout rate.
Statistical analysis
Primary efficacy analysis
We performed the primary efficacy analysis on all randomised patients who used at least one dose of study medication, the ITT population. The per protocol (PP) population included patients who did not have significant protocol deviations such as use of rescue medication >3 days in any week or within the 48 hours preceding visits. All efficacy outcome measures were also analysed in the PP population.
For the primary analysis, we used a closed step‐down approach:19 first we compared celecoxib with placebo, then if p was ⩽0.05, we compared IDEA‐033 with placebo. Thus no adjustment for type I error was necessary. We considered p<0.05 as statistically significant.
Secondary efficacy analysis
For WOMAC pain and function subscales, we calculated changes from baseline at each visit for each subject, based on available data at that visit. We did not impute missing data except for carrying the last observation forward for patients who had only baseline data.
We analysed the changes from baseline on the WOMAC pain and function subscales using analysis of covariance models with treatment and investigator as fixed effects and the corresponding baseline value as a covariate. We included the treatment by investigator interaction in the final model if p was <0.10. We analysed PGA using an analysis of covariance model with treatment and investigator as fixed effects and the WOMAC pain score at baseline as a covariate. We set missing PGA scores at zero, the worst possible outcome. We used least squares means for statistical comparisons between the two active treatments and placebo.
Results
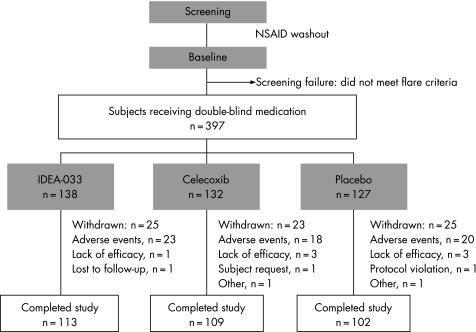
Between July and December 2003, 499 patients were screened and 397 randomised (figure 1). All patients received the allocated treatment, and 324 patients completed the study. The number of patients withdrawn due to adverse events was similar in all three groups (figure 1) as were baseline characteristics (table 1).
Figure 1 Flow of patients through trial. All results presented are based on the analysis of the intent‐to‐treat population, i.e. 138, 132 and 127 patients of the three treatment groups, applying the last observation carried forward technique.
Table 1 Baseline characteristics of patients randomised to IDEA‐033, celecoxib, or placebo.
| Characteristic | IDEA‐033 (n = 138) | Celecoxib (n = 132) | Placebo (n = 127) | |
|---|---|---|---|---|
| Mean (SD) age (years) | 63.3 (10.1) | 62.4 (9.6) | 62.8 (9.8) | |
| Men | 63 (45.7) | 50 (37.9) | 47 (37.0) | |
| Women | 75 (54.3) | 82 (62.1) | 80 (63.0) | |
| WOMAC Index | ||||
| Mean (SD) pain score† | 55.1 (18.4) | 56.1 (18.6) | 59.9 (17.3) | |
| Mean (SD) stiffness score† | 49.4 (21.1) | 50.6 (22.2) | 53.1 (21.1) | |
| Mean (SD) physical function score† | 53.8 (20.4)* | 54.6 (21.0) | 58.9 (19.6) | |
| Mean (SD) PGA of osteoarthritis | 3.9 (0.5) | 3.9 (0.6) | 4.0 (0.5) |
PGA, physician's global assessment; WOMAC, Western Ontario and McMaster Universities.
*One patient did not provide a baseline physical function score (n = 137).
†All WOMAC subscale scores were normalised to a scale of 0 to 100 by dividing the sum subscale score by the number of questions of each subscale score.
Values are numbers (percentages) unless stated otherwise.
Efficacy
Primary efficacy analysis
The changes from baseline to end of study in mean WOMAC pain score were similar in the IDEA‐033 and celecoxib groups and superior to placebo (table 2). Changes in mean WOMAC physical function score were less pronounced on IDEA‐033 compared with celecoxib (ITT population) (table 2). Mean PGA of response at end of study were assessed as fair (2) for both active treatments and poor (1) for placebo (table 2). Thus, superiority of IDEA‐033 compared with placebo was shown for two of three primary efficacy outcomes for the ITT population.
Table 2 Primary efficacy outcomes observed in patients with osteoarthritis (intent to treat and per protocol populations).
| Outcomes | IDEA‐033 | Celecoxib | Placebo |
|---|---|---|---|
| Intent‐to treat population (n) | 138 | 132 | 127 |
| WOMAC Index | |||
| Mean (SD) change in pain score | −19.4 (21.2) | −20.7 (22.7) | −12.4 (20.8) |
| LS mean (SE) change | −18.2 (2.0)* | −20.3 (2.1)* | −9.9 (2.1) |
| 95% CI | −22.1 to −14.3 | −24.3 to −16.2 | −13.9 to −5.8 |
| p Value, comparison with placebo | 0.0041 | 0.0004 | |
| Mean (SD) change in physical function score | −16.0 (20.3) | −18.1 (22.5) | −12.3 (19.2) |
| LS mean (SE) change | −14.6 (1.8) | −16.6 (1.8)* | −10.2 (1.8) |
| 95% confidence interval | −18.1 to −11.0 | −20.2 to −13.0 | −13.8 to −6.6 |
| p Value, comparison with placebo | 0.08 | 0.01 | |
| PGA of response at end of study | |||
| None (0), n (%) | 35 (25.4) | 34 (25.8) | 51 (40.2) |
| Poor (1), n (%) | 16 (11.6) | 23 (17.4) | 20 (15.7) |
| Fair (2), n (%) | 23 (16.7) | 24 (18.2) | 21 (16.5) |
| Good (3), n (%) | 48 (34.8) | 37 (28.0) | 30 (23.6) |
| Excellent (4), n (%) | 16 (11.6) | 14 (10.6) | 5 (3.9) |
| Mean (SD) score | 2.2 (1.3) | 1.9 (1.3) | 1.5 (1.3) |
| LS mean (SE) score | 1.8 (0.1)* | 1.7 (0.1)* | 1.3 (0.1) |
| 95% CI | 1.6 to 2.1 | 1.5 to 1.9 | 1.1 to 1.5 |
| p Value, comparison with placebo | <0.01 | 0.02 |
LS, least squares; PGA, patient global assessment; WOMAC, Western Ontario and McMaster Universities;
*Statistically significant difference (p<0.05) compared with placebo.
Results are given as changes from baseline to end of study unless stated otherwise.
Secondary efficacy analysis
Planned secondary efficacy outcomes revealed similar results for both active treatments (table 3). Both active treatments were significantly superior to placebo. The post hoc analysis of responder rates according to the recently updated OMERACT‐OARSI criteria15 essentially confirmed the results in planned efficacy measures (table 3). IDEA‐033 had a significantly superior responder rate compared with placebo, in contrast to celecoxib.
Table 3 Secondary efficacy outcomes observed in patients with osteoarthritis (intent‐to‐treat population).
| Outcomes | IDEA‐033 (n = 138) | Celecoxib (n = 132) | Placebo (n = 127) | |
|---|---|---|---|---|
| PGA of osteoarthritis at end of study | ||||
| Very good (1), n (%) | 12 (8.7%) | 13 (9.8%) | 4 (3.1%) | |
| Good (2), n (%) | 62 (44.9%) | 48 (36.4%) | 41 (32.3%) | |
| Fair (3), n (%) | 47 (34.1%) | 50 (37.9%) | 46 (36.2%) | |
| Poor (4), n (%) | 14 (10.1%) | 16 (12.1%) | 29 (22.8%) | |
| Very poor (5), n (%) | 3 (2.2%) | 5 (3.8%) | 7 (5.5%) | |
| Mean (SD) | 2.5 (0.9) | 2.6 (1.0) | 3.0 (1.0) | |
| LS mean (SE) | 2.5 (0.1)* | 2.6 (0.1)* | 2.9 (0.1) | |
| 95% CI | 2.3 to 2.7 | 2.4 to 2.8 | 2.7 to 3.1 | |
| p Value, comparison with placebo | 0.0009 | 0.0141 | ||
| WOMAC Index stiffness score | ||||
| Mean (SD) chang | −14.7 (22.8) | −16.7 (26.4) | −10.0 (21.3) | |
| LS mean (SE) change | −14.3 (1.9)* | −15.8 (2.0)* | −8.2 (2.0) | |
| 95% CI | −18.1 to −10.5 | −19.7 to −12.0 | −12.1 to −4.3 | |
| p Value, comparison with placebo | 0.0215 | 0.0044 | ||
| Use of rescue medication† | ||||
| Mean (SD) | 0.24 (0.43) | 0.16 (0.34) | 0.37 (0.60) | |
| LS mean (SE) | 0.26 (0.04)* | 0.17 (0.04)* | 0.38 (0.04) | |
| 95% CI | 0.2 to 0.3 | 0.1 to 0.2 | 0.3 to 0.5 | |
| p Value, comparison with placebo | 0.0291 | 0.0002 | ||
| OMERACT‐OARSI responder‡ | ||||
| n (%) at end of study | 95 (68.8)* | 84 (63.6) | 70 (55.1) | |
| 95% CI | 61.1 to 76.6 | 55.4 to 71.8 | 46.5 to 63.8 | |
| p Value, comparison with placebo | 0.0247 | 0.1551 | — | |
| Number needed to treat (vs placebo) | 8.0 | 12.0 | — | |
| 95% CI | 2.1 to 25.3 | −3.4 to 20.4 | — | |
LS, least squares; OMERACT‐OARSI, Outcome Measures in Rheumatology initiative/Osteoarthritis Research Society International.
*Statistically significant difference (p<0.05) compared with placebo.
†Total number of rescue medication capsules taken/total number of days in study.
‡Post hoc analysis of responder rates according to the recently updated OMERACT‐OARSI criteria.15
Results are given as changes from baseline to end of study unless stated otherwise.
Safety
Adverse events
All randomised patients were evaluated for safety. No gastrointestinal (GI) bleeding occurred. Non‐serious GI adverse events for IDEA‐033 (9.4%) were similar to placebo and numerically lower than for celecoxib (13.6%). One patient treated with celecoxib had a myocardial infarction, one patient treated with placebo had angina and no serious cardiovascular adverse event occurred in patients treated with IDEA‐033. Most adverse events were evenly spread throughout all three treatment groups (table 4). There was a tendency for more patients reporting erythema in the IDEA‐033 group. IDEA‐033 seemed to cause more skin irritation than the matching placebo gel, with the popliteal fossa being the predominant skin area concerned.
Table 4 Most commonly reported adverse events.
| MedDRA SOC preferred term | IDEA‐033 (n = 138) | Celecoxib (n = 132) | Placebo (n = 127) | |
|---|---|---|---|---|
| Any SOC, any adverse events | 74 (53.6) | 66 (50.0) | 62 (48.8) | |
| Gastrointestinal disorders, any adverse events | 13 (9.4) | 18 (13.6) | 12 (9.4) | |
| Abdominal pain, upper | 2 (1.4) | 4 (3.0) | 3 (2.4) | |
| Constipation | 3 (2.2) | 0 (0.0) | 1 (0.8) | |
| Diarrhoea NOS | 1 (0.7) | 2 (1.5) | 0 (0.0) | |
| Dyspepsia | 1 (0.7) | 4 (3.0) | 1 (0.8) | |
| Flatulence | 0 (0.0) | 2 (1.5) | 0 (0.0) | |
| Gastritis NOS | 3 (2.2) | 0 (0.0) | 3 (2.4) | |
| Nausea | 2 (1.4) | 3 (2.3) | 2 (1.6) | |
| Toothache | 0 (0.0) | 3 (2.3) | 1 (0.8) | |
| Musculoskeletal and connective tissue disorders, any adverse events | 12 (8.7) | 19 (14.4) | 20 (15.7) | |
| Arthralgia | 3 (2.2) | 3 (2.3) | 4 (3.1) | |
| Back pain | 6 (4.3) | 6 (4.5) | 4 (3.1) | |
| Joint effusion | 2 (1.4) | 2 (1.5) | 1 (0.8) | |
| Sciatica | 0 (0.0) | 4 (3.0) | 1 (0.8) | |
| Psychiatric disorders | 0 (0.0) | 6 (4.5) | 1 (0.8) | |
| Depression | 0 (0.0) | 3 (2.3) | 0 (0.0) | |
| Respiratory, thoracic and mediastinal disorders, any adverse events | 17 (12.3) | 14 (10.6) | 10 (7.9) | |
| Nasopharyngitis | 10 (7.2) | 11 (8.3) | 6 (4.7) | |
| Skin and subcutaneous tissue disorders, any adverse events | 39 (28.3) | 27 (20.5) | 28 (22.0) | |
| Dermatitis allergic | 2 (1.4) | 1 (0.8) | 0 (0.0) | |
| Erythema | 29 (21.0) | 18 (13.6) | 21 (16.5) | |
| Exanthema | 3 (2.2) | 2 (1.5) | 1 (0.8) | |
| Pruritus | 0 (0.0) | 5 (3.8) | 4 (3.1) | |
| Skin irritation | 2 (1.4) | 0 (0.0) | 0 (0.0) | |
| Urticaria NOS | 2 (1.4) | 1 (0.8) | 1 (0.8) |
MedDRA, Medical Dictionary for Regulatory Activities; NOS, not otherwise specified; SOC, System Organ Class.
Data are n (%). Patients who reported an adverse event more often than once during the study were only counted once.
Plasma ketoprofen concentrations
In the IDEA‐033 group, geometric mean (range) ketoprofen plasma concentrations immediately before the next dose of IDEA‐033 were 81.2 (4.6–677.8) ng/ml and 76.7 (4.6–598.8) ng/ml after 2 and 6 weeks of treatment, respectively. There were no significant differences between patients who received treatment for both knees and patients who received treatment for the index knee only.
Discussion
Efficacy
Patients with an acute flare of osteoarthritis treated epicutaneously with IDEA‐033 for 6 weeks had relief of pain and treatment responses judged by the patients comparable with the prototype of specific COX‐2 inhibitors, celecoxib. We selected celecoxib at its approved standard dose for use in osteoarthritis, at 100 mg twice daily.10 In a recent publication comparing twice‐daily doses of 100 mg and 200 mg celecoxib with diclofenac 50 mg and naproxen 500 mg twice daily in 13 274 osteoarthritis patients, both doses of celecoxib were as effective as diclofenac and naproxen.20 Thus, the celecoxib dose used in this study is a fully effective dose.
IDEA‐033 was superior to placebo in all efficacy outcomes, apart from the WOMAC physical function score in the ITT population, whereas in the PP population this outcome was also superior to placebo.
Although widely used for the treatment of osteoarthritis, conventional topical NSAIDs have been criticised frequently. A particular concern was the absorption of the NSAID through the skin and systemic availability through the intense cutaneous microcirculation, which may explain their effects in osteoarthritis. Topical diclofenac applied to the index knee resulted in similar diclofenac concentrations in the synovial fluids of both the index and non‐index knees.21
Topical NSAIDs applied as a conventional gel or a patch pass the skin barrier, which is located in the stratum corneum.22 The driving force of passive diffusion is the concentration gradient between topical NSAID and skin. Once drug molecules have crossed the stratum corneum, they are subject to clearance through the cutaneous microvasculature.23 In this aspect, IDEA‐033 is very different to conventional topical NSAIDs. The transport of IDEA‐033 into tissues such as muscles and joints deep below the skin application site is driven by the transdermal moisture gradient. The cutaneous microcirculation cannot clear Transfersomes, owing to their size. In studies in pigs, IDEA‐033 showed substantially higher drug concentrations in the target muscle and index knee,24 in contrast to results for conventional topical NSAIDs.21
Safety
IDEA‐033 proved to be safe and well tolerated. Although IDEA‐033 seemed to cause more skin irritations than the matching placebo gel, the intensity of erythema was generally mild and reversible.25 All other adverse events were evenly spread throughout all three treatment groups. GI adverse events for IDEA‐033 were similar to placebo.
The ketoprofen plasma concentrations detected in the IDEA‐033 group (4.6–677 ng/ml) corresponded to 0.1–10% of the maximum plasma concentrations detected following a usual therapeutic oral dose of 200 mg ketoprofen per day.26 Thus, the systemic exposure to ketoprofen following IDEA‐033 was substantially lower than after oral administration.
Conclusions
An ultra‐deformable carrier loaded with ketoprofen for epicutaneous application (IDEA‐033) was superior to placebo and similar to oral celecoxib in relieving pain of knee osteoarthritis over a 6‐week treatment period. New drug delivery systems may pave the way for evidence‐based long‐term targeted NSAID use in osteoarthritis.
Acknowledgements
Other investigators who participated in the study: Drs Gunar Baumann, Juergen Beck, Philipp Bolze, Jaroslaw Boronczyk, Gunter Boy, Hanns‐Gerd Dammann, Thorsten Decker, Joachim Deutmarg, Heike Dorow, Patrick Finkbeiner, Maik Lomp, Klaus Hammerle, Klaus Heil, Steffen Hein, Axel Kaden, Reiner Lehmann, Wolfgang Menke, Othard August Moller, Ludwig Radinger, Ulrich Scherer, Wolfgang Schmidl, Hartmut Schneider, Frank‐Detlef Stanek, Wilfried Trultzsch, Dieter Veith, Ruediger Zahn, Joerg Zeeh.
Abbreviations
COX - cyclooxygenase
GI - gastrointestinal
ITT - intent to treat
NSAIDs - nonsteroidal anti‐inflammatory drugs
OMERACT‐OARSI - Outcome Measures in Rheumatology initiative/Osteoarthritis Research Society International
PGA - patient global assessment
PP - per protocol
WOMAC - Western Ontario and McMaster Universities
VAS - visual analogue scale
Footnotes
IDEA AG (Germany) and McNeill Consumer & Specialty Pharmaceuticals (USA) sponsored the study and carried out on‐site monitoring of all participants. The sponsors had an opportunity to comment on the manuscript before submission, but the final version was the sole responsibility of the authors.
Clinical Trials.gov Identifier: NCT00317733
References
- 1.Scott J C, Hochbert M C. Arthritic and other musculosceletal diseases. In: Brownson RC, Remington PL, Davis JR, eds. Chronic disease: epidemiology and control Washington: American Public Health Association, 1993
- 2. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000431905–1915. [DOI] [PubMed] [Google Scholar]
- 3.Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B, Bijlsma J W.et al EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 200059936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazieres B, Bannwarth B, Dougados M, Lequesne M. EULAR recommendations for the management of knee osteoarthritis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials. Joint Bone Spine 200168231–240. [DOI] [PubMed] [Google Scholar]
- 5.Roddy E, Doherty M. Guidelines for management of osteoarthritis published by the American College of Rheumatology and the European League Against Rheumatism: why are they so different? Rheum Dis Clin North Am 200329717–731. [DOI] [PubMed] [Google Scholar]
- 6.Solomon D H, Schneeweiss S, Glynn R J, Kiyota Y, Levin R, Mogun H.et al Relationship between selective cyclooxygenase‐2 inhibitors and acute myocardial infarction in older adults. Circulation 20041092068–2073. [DOI] [PubMed] [Google Scholar]
- 7.Veys E M. 20 years' experience with ketoprofen. Scand J Rheumatol Suppl 199190(Suppl)1–44. [PubMed] [Google Scholar]
- 8.Cevc G, Schatzlein A, Richardsen H. Ultradeformable lipid vesicles can penetrate the skin and other semi‐permeable barriers unfragmented. Evidence from double label CLSM experiments and direct size measurements. Biochim Biophys Acta 2002156421–30. [DOI] [PubMed] [Google Scholar]
- 9.FitzGerald G A, Patrono C. The coxibs, selective inhibitors of cyclooxygenase‐2. N Engl J Med 2001345433–442. [DOI] [PubMed] [Google Scholar]
- 10.GD Searle LLC (a division of Pfizer Inc). CELEBREX (celecoxib capsules) prescribing information. New York: GD Searle LLC, 2005
- 11.Kellgren J H, Lawrence J S. Radiological assessment of osteo‐arthrosis. Ann Rheum Dis 195716494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellamy N, Buchanan W W, Goldsmith C H, Campbell J, Stitt L W. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988151833–1840. [PubMed] [Google Scholar]
- 13.Bellamy N. Pain assessment in osteoarthritis: experience with the WOMAC osteoarthritis index. Semin Arthritis Rheum 19891814–17. [DOI] [PubMed] [Google Scholar]
- 14.American Rheumatism Association Glossary Committee Dictionary of the rheumatic diseases: signs and symptoms. New York: Contact Associates International, 1988
- 15.Pham T, van der Heijde D, Altman RD anderson J J, Bellamy N, Hochberg M.et al OMERACT‐OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage 200412389–399. [DOI] [PubMed] [Google Scholar]
- 16.Dougados M, Leclaire P, van der Heijde D, Bloch D A, Bellamy N, Altman R D. Response criteria for clinical trials on osteoarthritis of the knee and hip: a report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthritis Cartilage 20008395–403. [DOI] [PubMed] [Google Scholar]
- 17.Ehrich E W, Schnitzer T J, McIlwain H, Levy R, Wolfe F, Weisman M.et al Effect of specific COX‐2 inhibition in osteoarthritis of the knee: a 6 week double blind, placebo controlled pilot study of rofecoxib. Rofecoxib Osteoarthritis Pilot Study Group. J Rheumatol 1999262438–2447. [PubMed] [Google Scholar]
- 18.Davies G M, Watson D J, Bellamy N. Comparison of the responsiveness and relative effect size of the western Ontario and McMaster Universities Osteoarthritis Index and the short‐form Medical Outcomes Study Survey in a randomized, clinical trial of osteoarthritis patients. Arthritis Care Res 199912172–179. [DOI] [PubMed] [Google Scholar]
- 19.Maurer W, Hothorn L A, Lehmacher W. Multiple comparisons in drug clinical trials and preclinical assays: a‐priori ordered hypotheses. In: Vollmar J, ed. Testing principles in clinical and preclinical trials. Stuttgart, Germany: Gustav Fischer Verlag, 19953–18.
- 20.Singh G, Fort J G, Goldstein J L, Levy R A, Hanrahan P S, Bello A E.et al Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS‐I Study. Am J Med 2006119255–266. [DOI] [PubMed] [Google Scholar]
- 21.Radermacher J, Jentsch D, Scholl M A, Lustinetz T, Frolich J C. Diclofenac concentrations in synovial fluid and plasma after cutaneous application in inflammatory and degenerative joint disease. Br J Clin Pharmacol 199131537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadgraft J, Walters K A, Guy R H. Epidermal lipids and topical drug delivery. Semin Dermatol 199211139–144. [PubMed] [Google Scholar]
- 23.Kretsos K, Kasting G B. Dermal capillary clearance: physiology and modeling. Skin Pharmacol Physiol 20051855–74. [DOI] [PubMed] [Google Scholar]
- 24.Mazgareanu S, Vierl U, Rother M. Transfersome carriers for transdermal targeted delivery of NSAID into muscle and knee joint – pre‐clinical results with IDEA‐033. Osteoarthritis Cartilage 200412S87 [Google Scholar]
- 25.Lin J, Zhang W, Jones A, Doherty M. Efficacy of topical non‐steroidal anti‐inflammatory drugs in the treatment of osteoarthritis: meta‐analysis of randomised controlled trials. BMJ 2004329324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamali F, Brocks D R. Clinical pharmacokinetics of ketoprofen and its enantiomers. Clin Pharmacokinet 199019197–217. [DOI] [PubMed] [Google Scholar]