A review of 341 adult‐onset Still's disease (AOSD) patients noted that 69% of all reported cases1 and 84% (69/82) of our series2 displayed sore throat early in the disease course. Despite the presence of severe sore throat, physical examinations showed normal findings or only mild pharyngeal infection, and imaging studies (including computed tomography (CT) scans) of the neck were negative.1,2,3,4 The lesions responsible for sore throat in active AOSD patients have not yet been explored.
We performed magnetic resonance imaging (MRI) of the larynx5 in 6 active AOSD patients (3 females and 3 males; mean age 33.5 years; table 1) presenting with sore throat and fulfilling the Yamaguchi criteria.6 Our aim was to identify the lesions responsible for sore throat in AOSD patients. Throat swabs for bacterial cultures were negative and serological tests for viruses were non‐diagnostic in all AOSD patients. Serum levels of C‐reactive protein (CRP) were elevated in all of our active AOSD patients. Three AOSD patients were available for MRI examination both at the active phase when presenting with sore throat, and at the remission phase (defined as the absence of systemic manifestation and sore throat within 6 months of effective therapy). The Ethics Committee of Clinical Research, Taichung Veterans General Hospital, approved this study protocol.
Table 1 Summary of clinical and MRI findings of 6 patients with adult‐onset Still's disease during sore throat.
| Case | Age/Sex | Clinical features of sore throat | Findings of indirect laryngoscopic examination | CRP levels (mg/dl) | MRI findings | |
|---|---|---|---|---|---|---|
| T2‐weighted | Post‐contrast T1 | |||||
| 1 | 28/F | Left‐sided sore throat | Normal | 4.2 | Increased signal intensity at left‐sided crico‐thyroid cartilage | Marked enhancement |
| 2 | 34/F | Sore throat | Normal | 1.6 | Increased signal intensity at pre‐epiglottic area | Mild enhancement |
| 3 | 27/M | Sore throat | Normal | 5.7 | Increased signal intensity along bilateral thyroid cartilages | Marked enhancement |
| 4 | 35/M | Sore throat | Normal | 6.6 | Increased signal intensity near cricoid cartilage | Mild enhancement |
| 5 | 25/F | Severe sore throat with odynophagia | Redness of pharyngeal mucosa | 10.7 | Increased signal intensity at crico‐thyroid cartilages and pharynx | Marked enhancement |
| 6 | 52/M | Severe sore throat | Redness of laryngeal mucosa | 11.3 | Increased thickness with high signal intensity at the tissue surrounding vocal cord | Marked enhancement |
M, male; F, female; CRP, C‐reactive protein; MRI, magnetic resonance imaging.
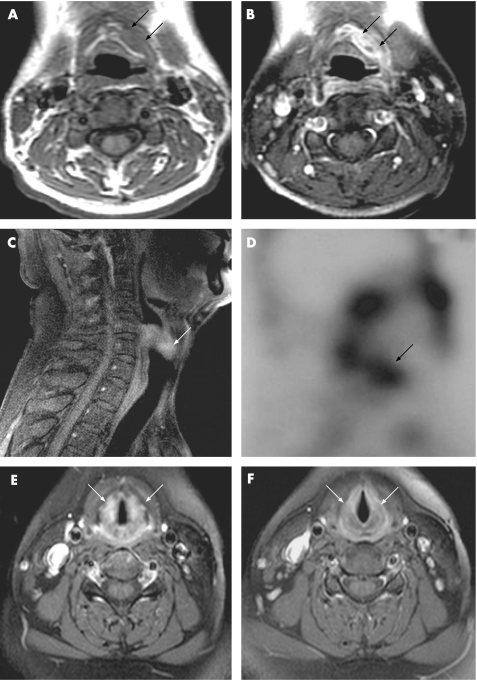
A brief summary of clinical and MRI findings of our 6 AOSD patients during sore throat was shown in table 1. The T1‐weighted images showed increased thickness of soft tissue near the crico‐thyroid cartilage (case 1 and fig 1A), and the post‐contrast T1‐weighted images demonstrated marked enhancement at the perichondral tissue (fig 1B). In an AOSD patient presenting with odynophagia, the post‐contrast T1‐weighted image showed marked enhancement at the soft tissue near cricoid‐thyroid cartilages and the pharynx (case 5 and fig 1C). Gallium‐67 scintography showed an increased uptake intensity at the corresponding region (fig 1D). In a patient who had redness of the laryngeal mucosa shown by indirect laryngoscope, a T2‐weighted image illustrated increased signal intensity at the soft tissue surrounding the vocal cord (case 6). During a longitudinal follow‐up, the inflammatory signs shown by MRI markedly subsided (fig 1E, 1F), paralleling clinical remission and the decrease in CRP (mean ± SD, 6.5 ± 4.8 mg/dl vs 0.1 ± 0.0 mg/dl).
Figure 1 MRI findings of the larynx in case 1 presented with left‐sided sore throat lasting for 2 weeks. (A) Axial, T1‐weighted image shows increased thickness of soft tissue near the left half of the thyroid cartilage (arrows). (B) Post‐contrast T1‐weighted image demonstrates marked enhancement at the same region (arrows). In case 5, presenting with odynophagia, the sagittal plane of the post‐contrast T1‐weighted image (C) shows an increase in thickness with marked enhancement at the soft tissue near the cricoid‐thyroid cartilage and the pharynx (arrow). In this patient, Gallium‐67 scintography (D) shows increased uptake intensity at the corresponding region (arrow). A change in MRI findings after effective therapy was observed in case 6. The axial plane of the post‐contrast T1‐weighted image shows increased thickness with marked enhancement at the soft tissue near the vocal cord in the active phase (E, arrows), and it resumed to normal appearance at the same area (F, arrows) after 5 months' therapy in this patient.
Discussion
There are no further image studies concerning the cause of sore throat in AOSD patients following negative findings of CT scans.1 We demonstrated high signal intensity with contrast enhancement over soft tissue surrounding crico‐thyroid cartilage and/or vocal cords in active AOSD patients presenting with sore throat. Perichondritis of crico‐thyroid cartilage and/or corditis may explain the sore throat, and the close proximity between the involved area and pharyngeal constrictors may lead to odynophagia. The evidence of inflammation was supported by an increase in the uptake intensity of Gallium‐67 citrate, which has a high affinity for inflammatory lesions.7 MRI changes of crico‐thyroid cartilage parallel clinical remission and the decrease in serum CRP levels in AOSD patients. Our results suggest that crico‐thyroid perichondritis, demonstrated by MRI, may precipitate the pathogenesis of sore throat in AOSD.
Abbreviations
AOSD - adult‐onset Still's disease
CRP - C‐reactive protein
CT - computed tomography
MRI - magnetic resonance imaging
Footnotes
Competing interests: None declared.
References
- 1.Nguyen K H Y, Weisman M H. Severe sore throat as a presenting symptom of adult onset Still's disease: a case series and review of the literature. J Rheumatol 199724592–597. [PubMed] [Google Scholar]
- 2.Chen D Y, Lan J L, Hsieh T Y, Chen Y H. Clinical manifestations, disease course, and complications in eighty‐two patients with adult‐onset Still's disease in Taiwan. J Formos Med Assoc 2004103844–852. [PubMed] [Google Scholar]
- 3.Bywaters E G L. Still's disease in the adults. Ann Rheum Dis 197130121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohta A, Yamaguchi M, Kaneoka H, Nagayoshi T, Hiida M. Adult Still's disease: Review of 228 cases from the literature. J Rheumatol 1987141139–1146. [PubMed] [Google Scholar]
- 5.Castelijns J A, Doornbos J, Verbeeten B, Vielvoye G J, Bloem J L. Magnetic resonance imaging of the normal larynx. J Comput Assist Tomogr 19859919–925. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H.et al Preliminary criteria for classification of adult Still's disease. J Rheumatol 199219424–430. [PubMed] [Google Scholar]
- 7.Tsan M F. Mechanism of gallium‐67 accumulation in inflammatory lesions. J Nucl Med 19852688–92. [PubMed] [Google Scholar]