Abstract
Background
A Mediterranean‐type diet rich in fish, fruit and vegetables and low in saturated fats has been associated with health benefits, including improved cardiovascular profile and benefit in RA.
Objective
To overcome obstacles to healthy eating by a community‐based intervention promoting a Mediterranean‐type diet in patients with RA living in socially deprived areas of Glasgow.
Methods
130 female patients with RA aged 30–70 years (median 55), disease duration 8 years were recruited from three hospital sites. The intervention group (n = 75) attended weekly 2‐hour sessions for 6 weeks in the local community, including hands‐on cooking classes backed up with written information. The control group (n = 55) were given dietary written information only. Both groups completed food frequency questionnaires (FFQs), and clinical and laboratory measures were assessed at baseline, 3 and 6 months.
Results
Significant benefit was shown in the intervention group compared with controls for patient global assessment at 6 months (p = 0.002), pain score at 3 and 6 months (p = 0.011 and 0.049), early morning stiffness at 6 months (p = 0.041) and Health Assessment Questionnaire score at 3 months (p = 0.03). Analysis of the FFQs showed significant increases in weekly total fruit, vegetable and legume consumption and improvement in the ratio of monounsaturated:saturated fat intake and systolic BP in the intervention group only. The cooking classes were positively received by patients and tutors; cost/patient for the 6 week course was £84 (€124).
Conclusions
Results demonstrate that a 6 week intervention can improve consumption of healthier foods. If implemented more widely it may prove a popular, inexpensive and useful adjunct to other RA treatment.
Keywords: rheumatoid arthritis, diet, cardiovascular risk
In the 1950s, the cook and writer Elizabeth David introduced A Book of Mediterranean Food1 to a postwar Britain still under food rationing and so started our enthusiasm for this delicious cuisine. More recently, the health benefits of the Mediterranean diet have emerged. Characteristically this type of diet includes a high intake of fruit, vegetables, legumes, a moderate to high intake of fish, a low intake of dairy products and red meat and a high intake of unsaturated fats (especially olive oil) complemented by a modest amount of alcohol (mainly in the form of wine).
A Mediterranean diet has been associated with increased survival in older people in a large, prospective cohort study involving nine European countries2 and has proved an effective intervention in both the primary3 and secondary4 prevention of coronary heart disease. An improved cardiovascular risk profile is probably mediated through a number of factors, including modification of hyperlipidaemia, hypertension and obesity as well as reduction in C reactive protein.5 This last effect is potentially important in arthritis. A prospective, nested, case–control study6 identified a high level of red meat consumption as a dietary risk factor for the development of inflammatory polyarthritis, while a similar study7 noted that patients with a low intake of fruit and vitamin C (exogenous antioxidants) were more likely to develop arthropathy than matched controls. The precise mechanism of this effect is uncertain; these factors may be acting as markers in a group of people at increased risk from other, possibly lifestyle‐related, factors. Indeed a recent cross‐sectional study has shown that wine buyers purchase more healthy food items than people who buy beer.8
A 12 week randomised trial of Mediterranean diet intervention in 51 patients with RA demonstrated positive benefits, with a reduction in disease activity (measured by the 28 joint count Disease Activity Score (DAS28)), an improvement in physical function (Health Assessment Questionnaire (HAQ)) and increased vitality,9 effects likely to be multifactorial. Further analysis showed an increase in reported consumption of antioxidant‐rich foods during the Mediterranean diet intervention.10 Intriguingly, the discovery of ibuprofen‐like activity in extra‐virgin olive oil may help to explain its effect.11
It is not clear, however, whether a Mediterranean‐type diet could achieve similar results in patients with RA in a true‐to‐life setting, particularly in a population with high levels of social deprivation such as Glasgow, the largest city in Scotland. Any intervention requiring a change in lifestyle or behaviour, especially those which may be life long and culturally driven, is difficult to achieve and sustain. However, behavioural counselling to increase consumption of fruit and vegetables in lower income adults in the general population has led to sustained increases in intake.12
The gain from a Mediterranean‐type diet intervention in patients with RA is potentially twofold. Firstly, improvement in disease activity and secondly, reduction in cardiovascular risk—people with RA are known to be at increased cardiovascular risk13,14,15 and have increased cardiovascular disease mortality.16 Social deprivation has an additional negative impact on both RA17 and cardiovascular risk.18
In this study we wished to explore the feasibility of introducing a Mediterranean‐type diet to our female patients with RA living in areas of social deprivation and to assess change, if any, in lifestyle, disease activity and cardiovascular risk.
Methods
One hundred and thirty female patients with RA aged 30–70 years were recruited over 9 months from three hospital sites—we aimed at recruiting residents from within any of the Social Inclusion Partnership areas in Glasgow, which are areas of social deprivation.
Intervention group
Patients in the intervention group (n = 75) attended a 6 week cookery course (with emphasis on a Mediterranean‐type diet) organised by Greater Glasgow Health Board's Health Promotion Department (GGHBHPD) and delivered by nutritionists and teaching staff from local colleges. Occupational therapy staff advised about provision of aids for food preparation. The patients attended a weekly 2 hour cookery class, with a maximum of 10 participants in each session. Participants received a folder with written information on a Mediterranean‐type diet, healthy eating and recipes which promoted the increased consumption of fruits, vegetables and legumes, along with the substitution of saturated fat with monounsaturated fat in the form of olive oil or spreads containing olive oil. In addition to “hands‐on” food preparation, cooking and tasting, the participants received information about food hygiene, nutrition and local accessibility of affordable ingredients.
Control group
Control patients (n = 55) received readily available written information on healthy eating only.
Allocation
We originally intended to allocate patients randomly to intervention and control groups. However, a limiting factor proved to be the availability of a cookery course in a venue close to the patient's home at a time suitable to them. A more pragmatic approach was necessary, resulting in those able to attend on certain dates being allocated to the intervention group and those unavailable on dates of programmed courses becoming the control group.
Patient assessment
Patients in both groups were assessed at baseline, 3 and 6 months.
Clinical features
Tender and swollen joint count, patient global pain score, duration of early morning stiffness (EMS), DAS28, HAQ score, erythrocyte sedimentation rate, C reactive protein, and interleukin 6 (IL6) were measured. IL6 is a proinflammatory cytokine and acts as a mediator in the acute phase response (higher levels of IL6 are present in more active disease).
Cardiovascular risk
Assessment included documentation of smoking habits, systolic and diastolic blood pressure, total and high‐density lipoprotein cholesterol, glutathione and body mass index. Glutathione has important roles in preventing oxidative stress, metabolising nutrients and regulating cellular events. A deficiency of glutathione contributes to oxidative stress and can be implicated in the pathogenesis of heart disease.
Dietary assessment
Dietary data were collected using a previously validated food frequency questionnaire (FFQ),19 which was completed by participants at the clinical assessment visits. The Mediterranean diet is rich in fruits, vegetables and legumes, which are good sources of the antioxidant vitamins A, C and E. If the intervention were successful in promoting dietary change, we would expect to see increases in intake of these food groups as well as the associated nutrients. A composite score of the weekly total number of servings of the three food groups was calculated. Additional questions about fruit intake were included in the FFQ as the DietQ FFQ collects only limited data on fruit consumption. These questions were analysed separately using the diet 5 computer package, and the nutrient data added to the data estimated by DietQ to calculate the daily intake of vitamins A, C and E.
Deprivation
The Carstairs grouping for each patient was noted20 (derived from postcode, based on male employment, overcrowding, car ownership and social class).
Statistical analysis
A Wilcoxon matched‐pairs signed‐ranks test was used for within‐group analyses and a Mann–Whitney U test for comparison between intervention and control groups.
Ethics
Local ethics committee approval was given before staring this study.
Results
Table 1 shows that age, disease duration and body mass index were similar in both intervention and control groups.
Table 1 Group demographics at baseline.
| Demographics | Intervention (n = 75) | Control (n = 55) | ||||
|---|---|---|---|---|---|---|
| Median | Mean | Interquartile range | Median | Mean | Interquartile range | |
| Age (years) | 58 | 55 | 47–64 | 52 | 53 | 45–61 |
| Disease duration (years) | 7 | 9.3 | 3.5–14 | 7 | 9.6 | 4–12 |
| BMI (kg/m2) | 25.86 | 26.75 | 22–33 | 27.65 | 27.95 | 24–31 |
BMI, body mass index.
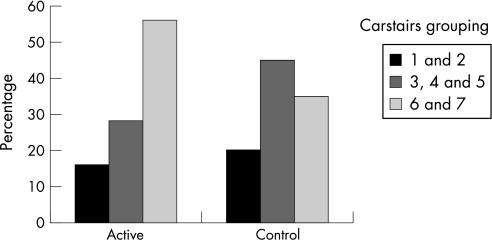
As expected by the design of the study, the patients in the intervention group were more likely to be in the most deprived social classes 6 and 7, living in a Social Inclusion Partnership area (fig 1). Baseline cardiovascular risk based on blood pressure, age and smoking status was calculated for all patients using readily available and validated graphs21; none of the recruited patients had diabetes mellitus. Sixty per cent had a calculated cardiovascular disease risk of <10% over the next 10 years, 30% a risk of 10–20% and 10% a >20% risk.
Figure 1 Influence of deprivation.
Consumption of fruit, vegetables and legumes was below the recommended minimum of five portions a day, in both groups at baseline. By 3 months this had improved significantly in the intervention group who were attending cooking classes (table 2).
Table 2 Food frequency diaries at baseline and 3 months in the two groups.
| Intervention (n = 75) | Control (n = 55) | |||||
|---|---|---|---|---|---|---|
| 0 | 3 Months | p Value | 0 | 3 Months | p Value | |
| Fruit, vegetables and legumes (portions/week) | 23.5 | 26 | 0.016 | 21.5 | 23 | 0.84 |
| Monounsaturated fats:saturated fats | 0.86 | 0.92 | 0.022 | 0.82 | 0.83 | 0.726 |
| Vitamin A (µg/day) | 1108 | 1246 | 0.101 | 922 | 974 | 0.403 |
| Vitamin C (mg/day) | 94 | 104 | 0.081 | 94 | 94 | 0.929 |
| Vitamin E (mg/day) | 7.0 | 6.8 | 0.626 | 5.8 | 5.5 | 0.448 |
Results are shown as medians.
At the same time, this group also had a significant improvement in the ratio of monounsaturated:saturated fats consumed. Alcohol consumption was low in both groups with a mean consumption of 1.5 units/week in the intervention group and 1.9 units/week in the control group. We reviewed disease modifying antirheumatic drug (DMARD) treatment, examining any escalation of dose or addition of extra DMARD over the study period. Within the 6 months, 21.3% of the intervention group and 23.6% of the control group had such a change in their treatment.
Clinical assessments showed a significant benefit in the intervention group compared with the control group for patient global assessment at 6 months (p = 0.002), pain score at 3 and 6 months (p = 0.011 and 0.049), EMS at 6 months (p = 0.041) and HAQ at 3 months (p = 0.03)—Mann–Whitney calculations (table 3).
Table 3 Baseline Disease Activity Scores (DAS) and clinical outcomes at baseline, 3 and 6 months.
| Intervention (n = 75) | Control (n = 55) | Mann–Whitney between groups | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 Months | 6 Months | 0 | 3 Months | 6 Months | ||
| Tender joint count (0–28) | 5 | 5 | 4 | 6 | 6 | 6 | – |
| Swollen joint count (0–28) | 6 | 5 | 4 | 6 | 5 | 5 | – |
| Patient global VAS (0–100 mm) | 50 | 50 | 45 | 54 | 55 | 63 | 6 Months 0.002 |
| Pain score VAS (0–100 mm) | 50 | 50 | 50 | 55 | 62 | 63 | 3 Months 0.011 |
| 6 Months 0.049 | |||||||
| EMS (min) | 30 | 30 | 15 | 60 | 30 | 30 | 6 Months 0.041 |
| HAQ score (0–3) | 1.75 | 1.625 | 1.625 | 1.75 | 1.875 | 1.875 | 3 Months 0.03 |
| DAS28 | 4.7 | 4.5 | 4.4 | 5.0 | 4.7 | 4.8 | – |
| ESR (mm/1st h) | 19 | 20 | 16 | 19 | 19 | 16 | – |
| CRP (mg/l) | 10 | 10 | 10 | 8.5 | 8 | 8 | – |
| IL6 (pg/ml) | 4.7 | 3.85 | 3.35 | 4.1 | 3.8 | 5.3 | NS |
Results are shown as medians.
VAS, visual analogue scale; EMS, early morning stiffness; HAQ, Health Assessment Questionnaire; DAS28, 28 joint count Disease Activity Score; ESR, erythrocyte sedimentation rate; CRP, C reactive protein; IL6 interleukin 6; NS, not significant.
Evaluation of cardiovascular risk factors showed a significant drop in systolic blood pressure by an average of 4 mm Hg in the intervention group (p = 0.016), while the control group showed no change. No significant change in cholesterol or glutathione levels was found with this intervention (table 4).
Table 4 Cardiovascular risk factors at baseline, 3 and 6 months.
| Risk factors | Intervention (n = 75) | Control (n = 55) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 Months | 6 Months | Wilcoxon | 0 | 3 Months | 6 Months | Wilcoxon | |
| Ever smoker (%) | 64 | 62 | ||||||
| Systolic BP (mm Hg) | 132 | 130 | 128 | 0.016 | 130 | 129 | 130 | NS |
| Diastolic BP (mm Hg) | 80 | 78 | 80 | NS | 80 | 80 | 78 | NS |
| Total cholesterol (mmol/l) | 5.55 | 5.3 | 5.4 | NS | 5.3 | 5.18 | 5.4 | NS |
| HDL (mmol/l) | 1.55 | 1.6 | 1.6 | NS | 1.5 | 1.46 | 1.5 | NS |
| TC:HDL ratio | 3.44 | 3.40 | 3.40 | NS | 3.50 | 3.52 | 3.23 | NS |
| Glutathione (nnmol/ml) | 3.23 | 3.26 | 2.72 | NS | 2.94 | 3.2 | 2.66 | NS |
| Weight (kg) | 66.0 | 64.1 | 65.1 | NS | 70 | 70 | 72.5 | NS |
| BMI (kg/m2) | 25.86 | 25 | 25.39 | NS | 27.65 | 27.65 | 28.22 | NS |
BP, blood pressure; TC, total cholesterol; HDL, high‐density lipoprotein; BMI, body mass index; NS, not significant.
The cost per patient for the 6 week cookery course was £84 (€124) (met by the GGHBHPD).
Discussion
In this study we sought to assess whether we could modify dietary lifestyle, disease activity and cardiovascular risk in female patients with RA living in areas of social deprivation by introducing them to a Mediterranean‐type diet. Cookery classes to provide “hands‐on” experience of a Mediterranean‐type diet were an essential element in increasing knowledge and confidence in the participants.
This study shows that this intervention was achievable and well received by patients. Intake of fruit, vegetables and legumes increased significantly over 3 months in the intervention group and the use of monounsaturated compared with saturated fats improved. The majority of the participants felt that the recipes were straightforward to make and affordable. Only three stated they were unable to purchase the necessary ingredients, either because they were too costly or were unavailable in their local shops. There were also wider social benefits in that most felt they had learnt new skills in food use and preparation. Some women also noted an improvement in confidence and self‐esteem as they were now able to contribute more to cooking for themselves and their families at home.
We failed to see a significant improvement in the intake of the antioxidant vitamins A, C and E. Possibly, the FFQ was not sufficiently sensitive to detect changes in the actual nutrient intake. The FFQ was originally developed to assess the intake of total energy and macronutrients—protein, fat and carbohydrate—at a time when antioxidants were not the focus of interest.19 The number of fruits and vegetables represented in the FFQ is relatively limited and it is possible that participants increased their intake with items not listed on the FFQ. A more accurate assessment of nutrient intake might have been achieved by using 7‐day weighed or estimated food diaries. However, this method places a heavy burden on the participant, which we did not think was appropriate given the age and health of our subjects. In addition, they are costly and time consuming to analyse: we did not have the funds to employ the specialist skills required to code and analyse food diaries.
We, like previous investigators,9 have shown a modest improvement in a number of measures of disease activity. Pain score was significantly better in the Mediterranean diet group than in the controls at 3 and 6 months. Patient global assessment and reported EMS were significantly better at 6 months. Patient function, as assessed by the HAQ score, was also better in the intervention group at 3 months. Overall the DAS28 score remained unchanged in both groups, but despite this, patients in the intervention group clearly felt better. The reasons for this are likely to be multifactorial and may, in part, reflect increased confidence and self‐esteem as well as dietary intervention. As it is impossible to conduct this type of study in a double‐blind fashion, we cannot entirely exclude the possibility of a placebo response, but this seems less likely as the same trend was seen over a number of measurements and was sustained.
Patients with RA are at increased risk of cardiovascular events13,14 and we also aimed to assess if we could modify this tendency in our patients. The intervention group lost weight (median 0.9 kg over the 6 month period), whereas the control group showed a weight gain (median 3 kg). However, this difference was not statistically significant. Cholesterol levels (at baseline and 6 months) and smoking status did not differ between the two groups. We noted a small (mean 4 mm Hg) but significant reduction in systolic blood pressure in the intervention group. This was not attributable to the prescription of, or changes to, anti‐hypertensive treatment. However, the magnitude of the change noted is perhaps what we might achieve with the introduction of a mild anti‐hypertensive agent in routine practice. The benefit to patients is that this was achieved without an addition to their drugs.
This study has shown that female patients with RA following a Mediterranean‐type diet derive modest benefits across a range of areas, suggesting that this type of intervention may be a useful therapeutic adjunct to conventional DMARDs, feasible in routine clinical practice and popular with patients.
The initial objectives when designing this study were to assess if lifestyle, disease activity or cardiovascular risk might be altered by this type of intervention. The results show that this is indeed achievable at low cost and is acceptable to patients with RA.
To act on and implement these findings we have approached local and national (Scottish) public health authorities to inform them of the results and discuss the potential impact of assessment in a larger population.
Acknowledgements
We are grateful to the following for assistance and support: Sisters Fiona MacDonald, Liz McIvor and Audrey Rowan for additional metrology input, occupational therapists at the three hospital sites, Mrs Dorothy McKnight for a supplementary statistical support, students in the Human Nutrition Department (University of Glasgow) who helped analyse the FFQs, the Community Nutrition Tutors for delivering the cookery courses, Dr H Burns for facilitating the input from the GGHBHPD and the Scottish Society of Physicians for additional financial support. Our thanks also go to our patients who participated in the study.
Abbreviations
DAS28 - 28 joint count Disease Activity Score
DMARD - disease modifying antirheumatic drug
EMS - early morning stiffness
FFQ - food frequency questionnaire
GGHBHPD - Greater Glasgow Health Board's Health Promotion Department
HAQ - Health Assessment Questionnaire
IL6 - interleukin 6
References
- 1.David E.A book of Mediterranean food. London: Penguin, 1998
- 2.Trichopoulou A, for members of the EPIC‐Elderly Prospective Study Group Modified Mediterranean diet and survival: EPIC‐Elderly prospective cohort study. BMJ 2005330991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trichopolou A, Costacou T, Bamia C, Trichopolos D. Adherence to Mediterranean diet and survival in a Greek population. N Engl J Med 20033482599–2608. [DOI] [PubMed] [Google Scholar]
- 4.De Lorgeril M, Renaud S, Mamelle N, Salen P, Martin J L, Monjaud I.et al Mediterranean alpha‐linolenic acid‐rich diet in secondary prevention of coronary heart disease. Lancet 1994343454–459. [DOI] [PubMed] [Google Scholar]
- 5.Ridker P M, Hennekens C H, Buring J E, Rifai N. C‐reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000342836–843. [DOI] [PubMed] [Google Scholar]
- 6.Pattison D J, Symmons D P M, Lunt M, Welch A, Luben R, Bingham S A.et al Dietary risk factors for the development of inflammatory polyarthritis. Evidence for a role of high level of red meat consumption. Arthritis Rheum 2004503804–3812. [DOI] [PubMed] [Google Scholar]
- 7.Pattison D J, Silman A J, Goodson N J, Lunt M, Bunn D, Luben R.et al Vitamin C and the risk of developing inflammatory polyarthritis: prospective nested case‐control study. Ann Rheum Dis 200463843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen D, Friis K, Skovenborg E, Grønbaek M. Food buying habits of people who buy wine or beer: cross‐sectional study. BMJ 200633219–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sköldstam L, Hagfors L, Johansen G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann Rheum Dis 200362208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagfors L, Leanderson P, Sköldstam L, Andersson J, Johansen G. Antioxidant intake, plasma antioxidants and oxidative stress in a randomised, controlled, parallel, Mediterranean dietary intervention study on patients with rheumatoid arthritis. Nutr J. 2003;2: 1–11, Available at http://www.nutritionj.com/content/2/1/5 (accessed 28 March 2007) [DOI] [PMC free article] [PubMed]
- 11.Beauchamp G K, Keast R S J, Morel D, Lins J, Pikas J, Han Q.et al Ibuprofen‐like activity in extra‐virgin olive oil. Nature 200543745–46. [DOI] [PubMed] [Google Scholar]
- 12.Steptoe A, Perkins‐Porras L, McKay C, Rink E, Hilton S, Cappuccio F P. Behavioural counselling to increase consumption of fruit and vegetables in low income adults: randomised trial. BMJ 2003326855–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEntegart A, Capell H A, Creran D, Rumley A, Woodward M, Lowe G. Cardiovascular risk factors, including thrombotic variables, in a population with rheumatoid arthritis. Rheumatology (Oxford) 200140640–644. [DOI] [PubMed] [Google Scholar]
- 14.Alkaabi J K, Ho M, Levison R, Pullar T, Belch J. Rheumatoid arthritis and macrovascular disease. Rheumatology (Oxford) 200342292–297. [DOI] [PubMed] [Google Scholar]
- 15.Hall F C, Dalbeth N. Disease modification and cardiovascular risk reduction: two sides of the same coin? Rheumatology (Oxford) 2005441473–1482. [DOI] [PubMed] [Google Scholar]
- 16.Goodson N, Marks J, Lunt M, Symmons D. Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Ann Rheum Dis 2005641595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McEntegart A, Morrison E, Capell H A, Duncan M R, Porter D, Madhok R.et al Effect of social deprivation on disease severity and outcome in patients with rheumatoid arthritis. Ann Rheum Dis 199756410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tunstall‐Pedoe H, Woodward M, for the SIGN group on risk estimation By neglecting deprivation, cardiovascular risk scoring will exacerbate social gradients in disease. Heart 200592307–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarnell J W G, Fehily A M, Milbank J E, Sweetname P M, Walker C L. A short dietary questionnaire for use in an epidemiological survey: comparison with weighed dietary records. Hum Nutr Appl Nutr 198337A103–112. [PubMed] [Google Scholar]
- 20.Carstairs V, Morris R.Deprivation and health in Scotland. Aberdeen: Aberdeen University Press, 1991
- 21.Anonymous British National Formulary. 51st ed. London: Pharmaceutical Press, 2006