Abstract
Background
In the context of preclinical development, we studied the potential of intra‐articular gene delivery using a recombinant adeno‐associated virus 5 (rAAV5) encoding a chimeric human tumour necrosis factorα (TNFα) soluble receptor I linked to a mouse immunoglobulin heavy chain Fc portion (TNF receptor I; TNFRI‐Ig).
Methods
Expression was under control of a nuclear factor kappa B (NFκB)‐responsive promoter and compared with a cytomegalovirus (CMV) promoter (rAAV5.NFκB‐TNFRI‐Ig and rAAV5.CMV‐TNFRI‐Ig, respectively).
Results
Fibroblast‐like synoviocytes transduced in vitro with rAAV5.NFκB‐TNFRI‐Ig were able to produce TNFRI‐Ig protein in response to several stimuli, and this was inhibited upon treatment with a specific NFκB blocking agent. A bioassay revealed that the synthesised TNFRI‐Ig was bioactive, showing a higher affinity for human than for rat TNFα. Transcription of the transgene and protein production were detectable in joints injected with both constructs. No dissemination of the vector was observed outside the joints. A significant reduction in paw swelling was seen in rats treated with rAAV5.NFκB‐TNFRI‐Ig. This clinical effect was accompanied by a decrease in pro‐inflammatory cytokine levels and an increase in IL10 expression in the synovium.
Conclusion
These results provide evidence that intra‐articular gene therapy using rAAV5 encoding TNFRI‐Ig may be a safe and feasible approach for the treatment of rheumatoid arthritis. The higher affinity for human TNFα suggests that in patients with rheumatoid arthritis the therapeutic effect might be even more pronounced than in rat adjuvant arthritis.
Keywords: AAV, gene therapy, TNF receptor I, arthritis, gene transfer
Rheumatoid arthritis is a chronic immune‐mediated disease characterised primarily by inflammation of the joints with concomitant destruction of both cartilage and bone1 Besides conventional therapy with disease‐modifying anti‐rheumatic drugs, novel approaches aimed at TNFα blockade have successfully entered clinical practice. It is now possible to reach 20% improvement (by American College of Rheumatology (ACR) criteria) in >60% of patients with rheumatoid arthritis treated with tumour necrosis factor (TNF)‐blocking agents.2,3
Most of these ACR‐20% responders, however, will still have some actively inflamed joints, and long‐term systemic treatment with anti‐TNF agents can result in serious adverse effects.4 Importantly, some patients have only a few symptomatic joints and are not considered candidates for receiving systemic anti‐TNF therapy. Local gene transfer might allow persistent production of a TNF inhibitor within the inflamed joint directly, and enhance the bioavailability at the site of inflammation when the right vector is used.
Recombinant adeno‐associated viruses (rAAV) are currently considered the most suitable vectors for gene therapy in chronic diseases because of their safety profile.5 These vectors are not pathogenic in humans, show expanded tropism and are able to establish long‐term production of the transgenic protein in animal models of arthritis.6,7 The therapeutic potential of rAAV expressing a TNF inhibitor has previously been demonstrated in two animal models of arthritis. Intra‐articular injection of rAAV type 2 (rAAV5) encoding the p55 TNF receptor (TNFR) in the joints of human TNFα transgenic mice decreased arthritis activity.8 A similar effect was shown in rats with arthritis induced by streptococcal cell wall after administration of an rAAV2 vector containing the rat p75TNF receptor–immunoglobulin Fc (TNFR:Fc) fusion gene.9 In a recent study it was shown that rAAV type 5 (rAAV5) may be superior in transducing rodent arthritic synovium compared with rAAV2, without inducing a significant immune response and is, therefore, an excellent candidate vector for synovial gene transfer.10,11
We combined this promising vector with a chimeric human soluble p55 TNFR‐Ig coupled to the Fc part of murine IgG1 (TNFRI‐Ig) to form the therapeutic gene, placed under control of a disease‐inducible nuclear factor kappa B (NFκB) responsive promoter compared with a cytomegalovirus (CMV) immediate early promoter. As we intend to develop intra‐articular gene therapy for rheumatoid arthritis, we evaluated the effects of these gene constructs on arthritis activity, cytokine levels, and the biodistribution after intra‐articular injection in the rat adjuvant arthritis model of rheumatoid arthritis.
Methods
Construction of expression vectors
The chimeric hTNFRI/mIgG gene was obtained and cloned into a pVAX2 plasmid, as described previously.12,13 This gene was subsequently inserted into a pAAV2‐CMV shuttle plasmid (Applied Viromics, Fremont, CA). As control vector, we used the CMV shuttle plasmid without transcription cassette. To obtain an NFκB‐driven construct, the CMV promoter was replaced by an NFκB‐responsive promoter. All constructs were confirmed by restriction‐site analysis and sequencing, and subsequently used for vector production.
Vector production
Recombinant AAV5 vectors were produced by the vector cores of the University of North Carolina (Chapel Hill, North Carolina, USA), as previously described14 and University Hospital of Nantes (France), supported by the Association Française contre les Myopathies, as described previously.15
Isolation of human primary fibroblast‐like synoviocytes
Synovial biopsies from patients with rheumatoid arthritis were enzymatically dispersed and fibroblast‐like synoviocytes (FLS) were cultured as described previously.16 The cells were used from passages 3 to 10. The medical ethics committee of the Academic Medical Center, University of Amsterdam, approved the protocol and patients gave written informed consent.
In vitro transduction of fibroblast‐like synoviocytes
FLS were seeded in 48‐wells plates at 3.5×104 cells/well in Dulbecco's modified Eagle's medium (DMEM)/10% fetal calf serum (FCS) (Gibco‐BRL, Grand Island, New York, USA). After 24 hours, rAAV5.TNFRI‐Ig constructs were added at a multiplicity of infection (MoI) of 2×103 in DMEM/10% FCS. As controls, rAAV5.GFP and a non‐expressing rAAV5 vector were used. Medium was replaced the next day and collected 48 hours later. For stimulation experiments, medium with 2% serum replacement was used (Sigma‐Aldrich Corp., St Louis, Missouri, USA). Forty‐eight hours after transduction medium was replaced with medium containing 1 μg/ml lipopolysaccharide (LPS), 100 ng/ml TNFα, or 100 ng/ml IL1β (R&D Systems, Minneapolis, Minnesota, USA) in the presence or absence of ammonium pyrrolidinedithiocarbamate (PDTC, 200 μM), a specific inhibitor of NFκB 17 (Calbiochem, San Diego, California, USA), and harvested after 24 hours.
TNFα neutralising activity in vitro
A bioassay was performed using TNFα‐sensitive WEHI‐164 subclone 13 cells (adapted from Eskandari et al18), to determine TNFR‐Ig neutralising capicity. Cells were resuspended at 1×106 cells/ml in RPMI‐1640/10% FCS, 2 mmol L‐glutamine and 40 mM LiCl, and 50 μl/well was dispensed into 96‐well culture plates. In parallel, medium from FLS was transduced with rAAV5. NFκB‐TNFRI‐Ig was incubated in serial dilutions for 1 hour with recombinant rat or human TNFα (62.5 pg/ml for human TNFα and 20 pg/ml for rat TNFα). Recombinant human and rat TNFα standards (0.05– 500 pg/ml) were used in duplicate in the appropriate wells and incubated at 37°C for 20 hours. The cell viability was assessed by addition of 10 μl/well of a 5 mg/ml solution of 3‐(4,5‐dimethylthiazol‐2‐yl)2,5‐diphenyltetrazolium bromide (MTT). Four hours later, plates were centrifuged and medium was discarded, 100 μl acidified isopropanol (0.01 mol/l HCl) was added and absorbance was read at 570 nm using 650 nm as a reference. The percentage of human or rat TNFα blockade by TNFRI‐Ig could be calculated using the corresponding standard curve and was corrected for the amount of TNFRI‐Ig protein present in the medium samples.
Local gene transfer
Pathogen‐free male Lewis rats (150–200 g) were obtained (Harlan Sprague Dawley Inc.; Horst, The Netherlands). The animal care and use committee of the University of Amsterdam (Amsterdam, The Netherlands) approved all experiments.
Rats were immunised at the base of the tail with 1 mg of Mycobacterium tuberculosis H37RA (Difco, Detroit, Michigan, USA) in 0.1 ml mineral oil on day 0.19 Paw swelling was measured daily by water displacement plethysmometry. The right ankle joints were injected intra‐articularly on day 12 after immunisation with 2×1010 viral molecules of rAAV5.NFκB‐TNFRI‐Ig, rAAV5.CMV‐TNFRI‐Ig, or empty vector in a total volume of 50 μl saline.20 Animals were killed 2 weeks later. Hind paws and organs were collected to evaluate biodistribution. The experiments were performed first with 6 animals per group (total of 18) and subsequently using 10 animals per group (total of 30). In both experiments, the groups were divided into two subgroups, one for histology (2 or 5 animals, respectively) and one for RNA and protein isolation (4 and 5 animals, respectively). For histology, organs and hind paws were fixed in 10% formalin, paws were decalcified and subsequently embedded in paraffin wax. For RNA and protein isolation, joints were snap‐frozen in liquid nitrogen.
Detection of human TNFRI mRNA by real‐time PCR
Ankle joints and organs were snap‐frozen in liquid nitrogen, pulverised, and homogenised in Trizol reagent (100 mg/ml) (Invitrogen). Total RNA was isolated from the aqueous phase according to the manufacturer's instructions and cDNA was synthesised.
For reverse transcriptase PCR, 5 μl of cDNA solution was amplified (AccuPrime SuperMix I; Invitrogen Life Technologies, Carlsbad, California, USA), 215 mmol/l of the forward TNFRI primer (specific for human TNFRI) (5′‐TCTACCTAGCAGGCCTCG‐3′) and 215 mmol/l of the reverse TNFRI‐Ig primer (5′‐GGAGCAGCTGAGGCAGTG‐3′) in a total volume of 50 μl. Rat glyceraldehyde phosphodehydrogenase (GAPDH) was used as control (forward: 5′‐CGGTGTCAACGGATTTGGC‐3′, reverse: 5′‐CCATGCCAGTGAGCTTCCC‐3′). Amplification was performed in a thermocycler (Bio‐Rad; Veenendaal, The Netherlands) as follows: 3 minutes at 95°C, 35 cycles of 94°C for 1 minute, 59°C for 90 seconds and 72°C for 1 minute, respectively, followed by a final extension phase at 72°C for 10 minutes. The PCR products were analysed by standard agarose gel electrophoresis.
Real‐time PCR amplification mixtures contained 25 ng template cDNA, 2× SYBR Green I Supermix (Bio‐Rad) and 300 nmol/l primers for TNFRI (both rat and human; forward, 5′‐CGATTTGCTGTACCAAGTGC‐3′ and reverse, 5′‐TGAGGCAGTGTCTGAGGTG‐3′. As an internal reference gene, rat GAPDH was used (forward: 5′‐ATGCCATCACTGCCACTC‐3′, reverse 5′‐GGGTAGGAACACGGAAGG‐3′). Reactions were run on a real‐time thermal cycler (MiniOpticon; Bio‐Rad). The thermal profile consisted of 1 cycle at 95°C for 3 minutes, 40 cycles at 95°C for 15 seconds, and at 59°C for 45 seconds. Each assay included (in duplicate): a standard curve of five serial dilutions of TNFRI and GAPDH cDNA, a no‐template control and 25 ng of sample cDNA. Each run was followed by a melting curve. Single‐control normalisation for internal control gene and correction for primer efficiency were calculated as described earlier.21
Detection of human TNFRI‐Ig and rat TNFα by sandwich ELISA
Protein was isolated from crushed joints by adding 2 ml of lysis buffer (20 mmol/l HEPES, 0.5 mol/l NaCl, 0.25% Triton X and protease inhibitors) to 200 mg pulverised ankle joint, mixed by rotations for 4 hours at 4°C and then spun in a centrifuge. The amount of protein was detected in supernatants. The levels of human TNFRI‐Ig or rat TNFα in the medium and joint isolates were quantified according to the manufacturer's protocol (Duoset; R&D systems).
Joint histology
Arthritic paws were fixed in 10% buffered formalin, and decalcified in 15% EDTA. The paws were then embedded in paraffin and 5 μm sagittal serial sections of the ankle joints were cut. Tissue sections were stained with haematoxylin and eosin. The tissue was evaluated using a semiquantative scoring system (0–4) for synovial hyperplasia as described previously.22
Immunohistochemistry
Paraffin wax‐embedded sections (5 μm) were dewaxed and rehydrated in a gradient of alcohol, and endogenous peroxidase activity was inhibited using 0.1% sodium azide and 0.3% hydrogen peroxide in phosphate‐buffered saline. Antigen retrieval was performed by heating the sections 10 minutes at 121°C in 0.1 mol/l citric acid pH 6.0. Primary IgG antibodies (goat anti‐human TNFRI, goat anti‐rat TNFα, goat anti‐rat interleukin (IL)1β, goat anti‐rat IL10, and goat anti‐rat IL6; R&D Systems) were incubated overnight at 4°C, followed by incubation with horseradish peroxidase (HRP)‐conjugated swine anti‐goat immunoglobulin (Tago, Burlingame, California, USA) for 30 minutes. For control sections, the primary antibodies were omitted or an irrelevant antibody was used. Enhancement of the signal was performed with biotinylated tyramide (NEN Life Science Products, Boston, Massachusetts, USA) and HRP‐conjugated streptavidin (Dako, Hamburg, Germany) for 15 minutes.23 HRP activity was detected using hydrogen peroxide as substrate and 3‐amino‐9‐ethylcarbazole (Sigma) as chromogen. Sections were briefly counterstained with Mayer's haemalum solution and mounted in Kaiser's glycerol gelatin (Merck, Darmstadt, Germany).
Microscopy analysis
After immunohistochemical staining, the various markers in the synovial tissue of the ankle joints were scored semiquantitatively on a 5‐point scale (0, minimal expression; 4, abundant expression of a marker). All sections were analysed in a blinded manner by two independent observers. Minor differences between the observers were resolved by mutual agreement.24
Statistics
Differences between groups were determined by Kruskal–Wallis test, followed by a Mann–Whitney U rank sum test. A p value <0.05 was considered statistically significant. All analyses were done using SPSS version 11.5 (SPSS, Chicago, Illinois, USA).
Results
In vitro validation of TNFα blocking rAAV5 constructs
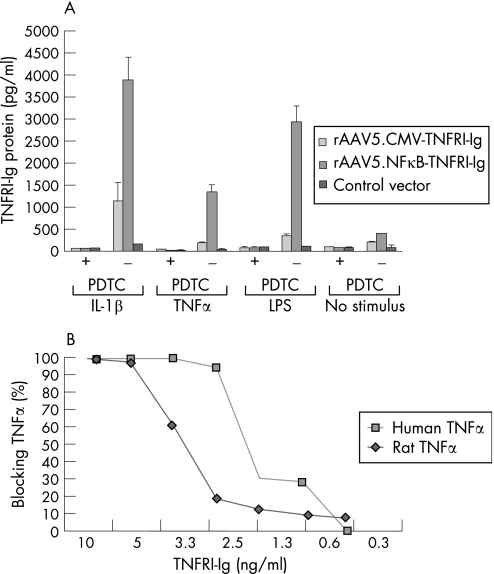
To test whether FLS, the main target cells for rAAV5 in vivo, were able to produce TNFRI‐Ig after rAAV5 transduction and if this production was responsive to NFκB after activation with inflammatory stimuli, we infected FLS with either rAAV5.NFκB‐TNFRI‐Ig or rAAV5.CMV‐TNFRI‐Ig vectors. The cells were stimulated with TNFα, IL1β or LPS in the presence or absence of the specific NFκB blocking agent PDTC (figure 1A). As control for transduction efficacy, a GFP expressing rAAV5 vector was used, showing an average of 60% positive cells. IL1β stimulation resulted in a significant increase of TNFRI‐Ig production using both constructs. However, the NFκB promoter‐driven construct showed a much higher protein production compared with the CMV promoter. The same trend, although to a lesser extent, was observed using TNFα or LPS as a stimulus. Importantly, induced production was effectively inhibited by blocking NFκB independent of the stimulus used.
Figure 1 In vitro validation of promoter and transgene function. Fibroblast‐like synoviocytes (FLS) were transduced with rAAV5 containing the NFκB.TNFRI‐Ig or the CMV‐TNFRI‐Ig gene. After 24 hours, interleukin (IL)1β (100 ng/ml), tumour necrosis factor (TNF)α (100 ng/ml) or lipopolysaccharide (LPS; 1 μg/ml) was added to the culture medium in the presence or absence of the nuclear factor kappa B (NFκB) blocking agent pyrrolidinedithiocarbamate (PDTC; 200 μmol/l). (A) Forty‐eight hours later, the supernatants were collected and an ELISA was performed to analyse the amount of TNFRI‐Ig protein. The capacity of produced TNFRI‐Ig to bind and neutralise human and rat TNFα was tested in a TNFα bioassay. (B) The percentage of TNFα neutralisation by TNFRI‐Ig is directly related to the percentage of the number of surviving cells and expressed as percentage of TNFα blocking. The presented results are performed in triplicate and are representative for two independent experiments.
Both rat and human TNFα were effectively neutralised by TNFRI‐Ig produced by FLS after transduction with the rAAV5 vector as tested in a bioassay (figure 1B). However, 50% of the human TNFα was already neutralised at 2.3 ng/ml TNFRI‐Ig, whereas it took 3.1 ng/ml TNFRI‐Ig to neutralise 50% of rat TNFα, indicating a higher affinity of human TNFRI‐Ig for human TNFα compared with rat TNFα.
Local delivery and expression of the TNFRI‐Ig gene
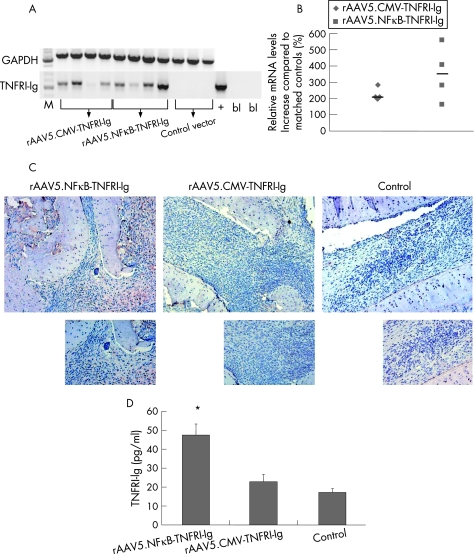
After intra‐articular injection of the rAAV5 vectors, human TNFRI‐Ig RNA was found in the joints treated with rAAV5.NFκB‐TNFRI‐Ig and rAAV5.CMV‐TNFRI‐Ig, but not in control vector‐treated joints (figure 2A). This was quantified by real‐time PCR, showing increased levels compared with controls (mean (SD) 342.30 (86.4)% and 228.35 (16.6)% for rAAV5.NFκB‐TNFRI‐Ig and rAAV5.CMV‐TNFRI‐Ig, respectively (figure 2B). In addition, joint sections were immunohistochemically stained to detect human TNFRI‐Ig protein (figure 2C). TNFRI‐Ig positive cells were observed in the synovium of animals injected with rAAV5.NFκB‐TNFRI‐Ig and to a lesser extent in those injected with rAAV5.CMV‐TNFRI‐Ig. The control group showed no staining. This was confirmed by ELISA, showing a significant increase in TNFRI‐Ig production in rats treated with rAAV5.NFκB‐TNFRI‐Ig (47.53 (5.72) pg/ml versus 17.12 (1.98) pg/ml, p<0.05 compared with control), whereas those treated with rAAV5.CMV‐TNFRI‐Ig showed a trend towards lower levels of TNFRI‐Ig (22.87(3.89) pg/ml versus 17.12 (1.98) pg/ml; NS) (figure 2D).
Figure 2 Human tumour necrosis factor receptor type I (TNFRI)‐Ig gene transcription in the ankle joints of rats with adjuvant arthritis after local recombinant adeno‐associated virus (rAAV5) gene therapy. Total RNA was extracted from crushed joints 14 days after intra‐articular injection and cDNA was synthesised. Reverse‐transcriptase PCR was performed using primers specific for the human TNFRI‐Ig gene. Plasmid containing the human TNFRI‐Ig gene was used as positive control (+), and a no‐template control as blanks (bl). (A) Rat glyceraldehyde phosphodehydrogenase (GAPDH) was used as a reference gene (n = 4/group). A real‐time PCR was performed to calculate the percentage TNFRI‐Ig upregulation. (B) Obtained threshold cycle (Ct) values were normalised to GAPDH levels and expressed as percentage increase compared with matched controls as individual data points (median). All treated animals significantly overexpressed the transgene in the joints (*p<0.05) (n = 4/group). (C) Ankle joints were embedded in paraffin wax, sectioned and immunohistochemically stained for human TNFRI‐Ig and counterstained with Mayer's haemalum (n = 2/group). Representative pictures are shown (original magnification 100× (upper panels), 200× (lower panels)). (D) Protein was isolated from injected joints and an ELISA for TNFRI was performed. Intra‐articular injection of rAAV5.NFκB‐TNFRI‐Ig resulted in the highest levels of TNFRI‐Ig in joint protein isolates (p<0.05), whereas the cytomegalovirus (CMV)‐driven construct only resulted in TNFRI‐Ig levels slightly above those in controls (n = 4/group).
Anti‐arthritic effects of locally transduced TNFRI‐Ig gene
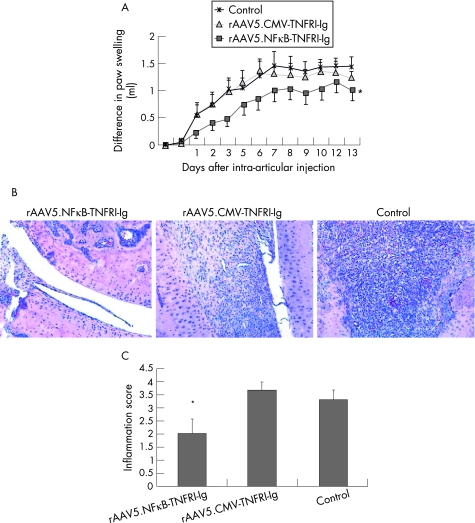
In the rats treated with rAAV5.NFκB‐TNFRI‐Ig, significantly less paw swelling was observed, as tested by differences in area under the curve from the day of treatment until the end of the experiment compared with controls (p<0.05). There was a trend towards less paw swelling in rats treated with the rAAV5 vector containing the TNFRI‐Ig gene under control of a CMV promoter, but this difference did not reach statistical significance (figure 3A). Consistent with these findings, we found a reduction in synovial inflammation only in animals injected with rAAV5.NFκB‐TNFRI‐Ig compared with controls (2.0 (0.57) versus 3.3 (0.33), respectively, p<0.05) (figure 3B and 3C).
Figure 3 Effects of constitutive local TNF blockade on paw swelling and inflammation in rats with adjuvant arthritis. To evaluate the effects of rAAV5.CMV‐TNFRI‐Ig or rAAV5.NFκB‐TNFRI‐Ig gene therapy on clinical arthritis, 10 rats per group were injected intra‐articularly into the right ankle joint with the therapeutic or control vector on day 12 after arthritis induction. (A) Paw swelling was measured by water displacement plethysmometry and expressed as Δ paw swelling (n = 10/group) (*p<0.05, as tested by difference in area under the curve from the day of treatment until the end of the experiment). Results are representative of two separate experiments. (B) At the end of the experiment, joints were removed, embedded in paraffin wax and examined by routine histology. Tissue sections were stained with haematoxylin and eosin (n = 5/group) original magnification 100×. (C) The tissue was evaluated using a semiquantative scoring system (0–4) for synovial hyperplasia and expressed as mean (SEM). Rats treated with rAAV5.NFκB‐TNFRI‐Ig displayed significantly less synovial hyperplasia (*p<0.05).
Changes in cytokine profile after rAAV5.TNFRI‐Ig gene therapy
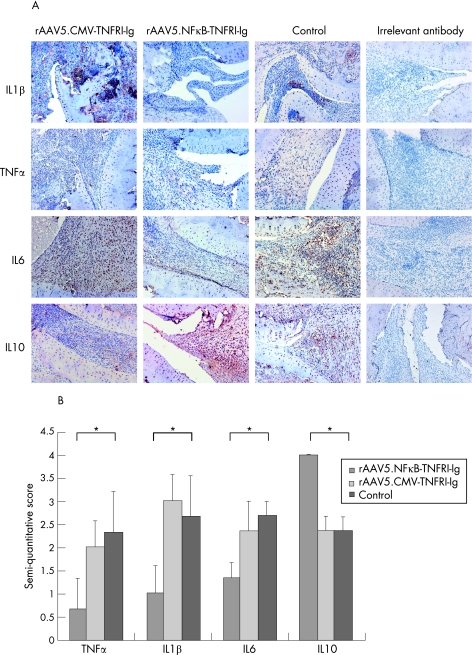
The joints were immunohistochemically stained to detect TNFα, IL1β, IL6, and IL10 (figure 4A). TNFα levels were significantly (p<0.05) lower in the synovial tissue of animals treated with rAAV5.NFκB‐TNFRI‐Ig (0.66 (0.88)), but not in animals treated with rAAV5.CMV‐TNFRI‐Ig (2 (0.57)), compared with controls (2.33 (0.88)) (figure 4B). The same consistent pattern was observed for IL1β (1 (0.57) and 3 (0.58) versus 2.66 (0.89) for rAAV5.NFκB‐TNFRI‐Ig and rAAV5.CMV‐TNFRI‐Ig compared with control) and IL6 (1.3 (0.34) and 2.33 (0.67) versus 2.67 (0.33) for rAAV5.NFκB‐TNFRI‐Ig and rAAV5.CMV‐TNFRI‐Ig compared with control). Interestingly, the anti‐inflammatory cytokine IL10 was significantly increased in rats treated with rAAV5.NFκB‐TNFRI‐Ig compared with controls (4.0 (0) versus 2.33 (0.33)), whereas rAAV5.CMV‐TNFRI‐Ig injected animals showed no difference in IL10 levels.
Figure 4 Cytokine levels in rat synovial tissue. At the end of the experiment, ankle joints were collected, embedded in paraffin wax and immunohistochemically stained with anti‐rat interleukin (IL)1β IL6 and IL10 and tumour necrosis factor (TNF)α, antibodies and counterstained with Mayer's haemalum. An irrelevant antibody was used as control. (A) Representative pictures are shown (original magnification 100×). (B) The images were semiquantitatively scored (0–4) by two independent observers (*p<0.05). Values are expressed as mean (SEM) (n = 5/group).
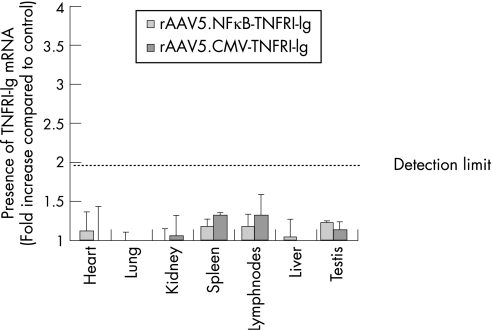
Biodistribution of transgene transcription after intra‐articular rAAV5 injection
At the end of experiment, organs and blood were collected to examine spreading of the virus using real time PCR. No significant copies of the transgene were found in the tissues examined. A slight increase in human TNFRI‐Ig mRNA was found only in spleen and draining lymph nodes compared with control group. However, a ΔCt value of one, resulting in a twofold increase in detectable transcripts, is considered below the detection limit of this method (figure 5). In addition, no TNFRI‐Ig protein was found in plasma using a specific ELISA (data not shown).
Figure 5 Biodistribution of transgene transcription after intra‐articular injection. To assess the presence of the transgene, heart, lung, kidney, spleen, draining lymph nodes, liver, and testis were excised and homogenised 14 days after intra‐articular injection. RNA was isolated and cDNA was synthesised (n = 5/group). Real‐time PCR was performed using transgene specific primers. The resulting threshold cycle (Ct) values were normalised to glyceraldehyde phosphodehydrogenase (GAPDH) levels and expressed as fold increase compared with control (mean (SEM)).
Discussion
The present study shows that intra‐articular injection of a human TNFRI‐Ig expressed by an rAAV5 vector with an NFκB inducible promoter in rats with adjuvant arthritis results in secreted and bioactive TNFRI‐Ig, able to block TNFα in the injected joint, providing a therapeutic effect.
TNFα is a key mediator in rheumatoid arthritis and systemic administration of soluble forms of its receptors has successfully entered clinical practice.25,26 TNFRI has a higher affinity for TNFα, and plays a predominant role in the induction of cellular responses by TNFα. Studies with TNFRI knockout mice have shown that TNF signalling through TNFRI controls the severity of experimental arthritis.27 In addition, treatment with soluble TNFRI was effective in a rat model of arthritis28 as well as in mouse models of sepsis29 and uveitis.30 Results in clinical trials with patients with rheumatoid arthritis treated with a TNFRI:Fc fusion protein were also encouraging, although variable. This could be explained by the formation of non‐neutralising antibodies and the short half‐life of the compound used.31 In addition, the systemic dosages might have failed to achieve intra‐articular therapeutic levels.
Local overexpression of this TNF inhibitor via gene therapy might maximise the therapeutic potential. Previous work using the TNFRI‐Ig gene from a plasmid electrotransferred into muscle at the onset of the disease showed a beneficial effect in murine CIA.12 Although this would prevent multiple injections in patients, it does not have the advantage of local treatment. Using a similar gene expressed from an adenoviral construct, a moderate, transient effect was reported after intra‐articular injection in mice,32,33 rats33 and rabbits.34 An immune response to the adenoviral vector was identified as a potential cause for this short‐lived effect. To overcome this problem, the less immunogenic rAAV2 was used, coding for the monomeric form of the TNFRI, resulting in inhibition of arthritis after intra‐articular injection in TNFα transgenic mice.8 The monomeric form of the receptor, however, only binds one subunit of the TNFα trimer, hence only weakly prevents the action of this cytokine.35 In addition, monomeric forms are rapidly cleared from the circulation.
We combined the potency of the TNFRI‐Ig gene with the superior efficacy of an rAAV5 vector driven by an NFκB and CMV promoter, and evaluated the therapeutic effects in an animal model of rheumatoid arthritis. The promoter function and biological activity of TNFRI‐Ig was confirmed in vitro as well as in vivo. One single injection of rAAV5 containing the gene for TNFRI‐Ig into arthritic joints of rats resulted in reduction of arthritis severity, consistent with the observed neutralisation of intra‐articular TNFα. In line with the effect on paw swelling, a significant reduction in synovial hyperplasia was observed accompanied by a decrease in pro‐inflammatory cytokines such as TNFα, IL1β and IL6 and an increase in the anti‐inflammatory cytokine IL10, thereby disengaging the cytokine cascade that leads to synovial inflammation. Importantly, no significant transgene transcripts were detected outside the joints, adding to the safety profile of this local approach.
Despite the severity of the animal model used, adjuvant arthritis in rats, a clear clinical effect was observed, indicating the effectiveness of local gene therapy using this gene construct. In a clinical setting, an even more pronounced effect might be expected, owing to the higher affinity we observed for human TNFα compared with rat TNFα. Although both CMV and NFκB promoter driven constructs were proven bioactive in vitro and in vivo, only the transgene under control of the disease inducible promoter resulted in a significant therapeutic effect. The exact mechanism for this remains unclear, but it could involve the ”on demand” high TNFRI‐Ig production induced when pro‐inflammatory factors are upregulated. In fact, we found higher production of the therapeutic protein in vitro after stimulation of the NFκB‐responsive promoter, and this was NFκB‐dependent. The difference between TNFRI‐Ig protein production as detected by the ELISA after IL1β or LPS and TNFα stimulation could partly be explained by the fact that TNFRI‐Ig protein secreted into the culture medium after TNFα stimulation can bind exogeneous added TNFα present in the medium and neutralise its activity. The ELISA used can only detect free and not bound TNFRI. In addition, we also observed lower levels of both TNFRI‐Ig mRNA and protein in vivo using the CMV‐driven construct compared with the NFκB‐driven construct. Looking at TNFRI‐Ig mRNA levels, the variation in animals treated with rAAV5.CMV‐TNFRI‐Ig is minimal compared with those treated with rAAV5.NFκB. This might reflect the disease‐induced transcription during the variable course of arthritis in vivo using the NFκB promoter. These are of course endpoint measurements, giving little information about the kinetics of production during the course of disease.
In conclusion, this work supports the feasibility of local gene therapy using an rAAV5 vector to deliver a therapeutic gene directly to the inflamed joint. Additionally, disease‐regulated transgene expression for physiologically responsive gene therapy might be feasible and could contribute to the safety and effectiveness of the gene‐therapy approach. Thus, this study provides the rationale for further research towards clinical development of rAAV5.NFκB‐TNFRI‐Ig intra‐articular gene therapy.
Acknowledgements
This research was supported by the European Community's FP6 funding. This publication reflects only the author's views. The European Community is not liable for any use that may be made of the information herein.
Abbreviations
ACR - American College of Rheumatology
CMV - cytomegalovirus
Ct - threshold cycle (Ct)
DMEM - Dulbecco's modified Eagle's medium
FCS - fetal calf serum
FLS - fibroblast‐like synoviocyte
GADPH - glyceraldehyde phosphodehydrogenase
HRP - horseradish peroxidase
IL - interleukin
LPS - lipopolysaccharide
NFκB - nuclear factor kappa B
PDTC - pyrrolidinedithiocarbamate
rAAV - recombinant adeno‐associated virus
TNFR - tumour necrosis factor receptor
References
- 1.Tak P P, Bresnihan B. The pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis. Arthritis Rheum 2000432619–2633. [DOI] [PubMed] [Google Scholar]
- 2.Bathon J M, Martin R W, Fleischmann R M, Tesser J R, Schiff M H, Keystone E C.et al A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 20003431586–1593. [DOI] [PubMed] [Google Scholar]
- 3.Lipsky P E, van der Heijde D M, St Clair E W, Furst D E, Breedveld F C, Kalden J R.et al Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti‐Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 20003431594–1602. [DOI] [PubMed] [Google Scholar]
- 4.Gomez‐Reino J J, Carmona L, Valverde V R, Mola E M, Montero M D. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active‐surveillance report. Arthritis Rheum 2003482122–2127. [DOI] [PubMed] [Google Scholar]
- 5.Adriaansen J, Vervoordeldonk M J, Tak P P. Gene therapy as a therapeutic approach for the treatment of rheumatoid arthritis: innovative vectors and therapeutic genes. Rheumatology (Oxford) 200645656–668. [DOI] [PubMed] [Google Scholar]
- 6.Grieger J C, Samulski R J. Adeno‐associated virus as a gene therapy vector: vector development, production and clinical applications. Adv Biochem Eng Biotechnol 200599119–145. [PubMed] [Google Scholar]
- 7.Rabinowitz J E, Samulski J. Adeno‐associated virus expression systems for gene transfer. Curr Opin Biotechnol 19989470–475. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H G, Xie J, Yang P, Wang Y, Xu L, Liu D.et al Adeno‐associated virus production of soluble tumor necrosis factor receptor neutralizes tumor necrosis factor alpha and reduces arthritis. Hum Gene Ther 2000112431–2442. [DOI] [PubMed] [Google Scholar]
- 9.Chan J M, Villarreal G, Jin W W, Stepan T, Burstein H, Wahl S M. Intraarticular gene transfer of TNFR:Fc suppresses experimental arthritis with reduced systemic distribution of the gene product. Mol Ther 20026727–736. [DOI] [PubMed] [Google Scholar]
- 10.Adriaansen J, Tas S W, Klarenbeek P L, Bakker A C, Apparailly F, Firestein G S.et al Enhanced gene transfer to arthritic joints using adeno‐associated virus type 5: implications for intra‐articular gene therapy. Ann Rheum Dis 2005641677–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apparailly F, Khoury M, Vervoordeldonk M J, Adriaansen J, Gicquel E, Perez N.et al Adeno‐associated virus pseudotype 5 vector improves gene transfer in arthritic joints. Hum Gene Ther 200516426–434. [DOI] [PubMed] [Google Scholar]
- 12.Bloquel C, Bessis N, Boissier M C, Scherman D, Bigey P. Gene therapy of collagen‐induced arthritis by electrotransfer of human tumor necrosis factor‐alpha soluble receptor I variants. Hum Gene Ther 200415189–201. [DOI] [PubMed] [Google Scholar]
- 13.Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor‐IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med 19911741483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao X, Li J, Samulski R J. Production of high‐titer recombinant adeno‐associated virus vectors in the absence of helper adenovirus. J Virol 1998722224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fromes Y, Salmon A, Wang X, Collin H, Rouche A, Hagege A.et al Gene delivery to the myocardium by intrapericardial injection. Gene Ther 19996683–688. [DOI] [PubMed] [Google Scholar]
- 16.van Holten J, Reedquist K, Sattonet‐Roche P, Smeets T J, Plater‐Zyberk C, Vervoordeldonk M J.et al Treatment with recombinant interferon‐beta reduces inflammation and slows cartilage destruction in the collagen‐induced arthritis model of rheumatoid arthritis. Arthritis Res Ther 20046R239–R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreck R, Meier B, Mannel D N, Droge W, Baeuerle P A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med 19921751181–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eskandari M K, Nguyen D T, Kunkel S L, Remick D G. WEHI 164 subclone 13 assay for TNF: sensitivity, specificity, and reliability. Immunol Invest 19901969–79. [DOI] [PubMed] [Google Scholar]
- 19.Tak P P, Gerlag D M, Aupperle K R, van de Geest D A, Overbeek M, Bennett B L.et al Inhibitor of nuclear factor kappaB kinase beta is a key regulator of synovial inflammation. Arthritis Rheum 2001441897–1907. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen K H, Boyle D L, McCormack J E, Chada S, Jolly D J, Firestein G S. Direct synovial gene transfer with retroviral vectors in rat adjuvant arthritis. J Rheumatol 1998251118–1125. [PubMed] [Google Scholar]
- 21.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A.et al Accurate normalization of real‐time quantitative RT‐PCR data by geometric averaging of multiple internal control genes. Genome Biol 20023RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joosten L A, Helsen M M, Saxne T, van De Loo F A, Heinegard D, van Den Berg W B. IL1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen‐induced arthritis, whereas TNF‐alpha blockade only ameliorates joint inflammation. J Immunol 19991635049–5055. [PubMed] [Google Scholar]
- 23.Adriaansen J, Kuhlman R R, Holten J V, Kaynor C, Vervoordeldonk M J, Tak P P. Intraarticular interferon‐beta gene therapy ameliorates adjuvant arthritis in rats. Hum Gene Ther 200617996–998. [DOI] [PubMed] [Google Scholar]
- 24.Tak P P, Thurkow E W, Daha M R, Kluin P M, Smeets T J, Meinders A E.et al Expression of adhesion molecules in early rheumatoid synovial tissue. Clin Immunol Immunopathol 199577236–242. [DOI] [PubMed] [Google Scholar]
- 25.Moreland L W. Inhibitors of tumor necrosis factor: new treatment options for rheumatoid arthritis. Cleve Clin J Med 199966367–374. [DOI] [PubMed] [Google Scholar]
- 26.Smith C A, Davis T, Anderson D, Solam L, Beckmann M P, Jerzy R.et al A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science 19902481019–1023. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y X, Zhang H, Chiu B, Payne U, Inman R D. Tumor necrosis factor receptor p55 controls the severity of arthritis in experimental Yersinia enterocolitica infection. Arthritis Rheum 1999421662–1672. [DOI] [PubMed] [Google Scholar]
- 28.McComb J, Gould T, Chlipala E, Sennelo G, Frazier J, Kieft G.et al Antiarthritic activity of soluble tumor necrosis factor receptor type I forms in adjuvant arthritis: correlation of plasma levels with efficacy. J Rheumatol 1999261347–1351. [PubMed] [Google Scholar]
- 29.Evans T J, Moyes D, Carpenter A, Martin R, Loetscher H, Lesslauer W.et al Protective effect of 55‐ but not 75‐kD soluble tumor necrosis factor receptor‐immunoglobulin G fusion proteins in an animal model of gram‐negative sepsis. J Exp Med 19941802173–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson M, Liversidge J, Forrester J V, Dick A D. Neutralizing tumor necrosis factor‐alpha activity suppresses activation of infiltrating macrophages in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci 2003443034–3041. [DOI] [PubMed] [Google Scholar]
- 31.Furst D E, Weisman M, Paulus H E, Bulpitt K, Weinblatt M, Polisson R.et al Intravenous human recombinant tumor necrosis factor receptor p55‐Fc IgG1 fusion protein, Ro 45‐2081 (lenercept): results of a dose‐finding study in rheumatoid arthritis. J Rheumatol 2003302123–2126. [PubMed] [Google Scholar]
- 32.Quattrocchi E, Walmsley M, Browne K, Williams R O, Marinova‐Mutafchieva L, Buurman W.et al Paradoxical effects of adenovirus‐mediated blockade of TNF activity in murine collagen‐induced arthritis. J Immunol 19991631000–1009. [PubMed] [Google Scholar]
- 33.Le C H, Nicolson A G, Morales A, Sewell K L. Suppression of collagen‐induced arthritis through adenovirus‐mediated transfer of a modified tumor necrosis factor alpha receptor gene. Arthritis Rheum 1997401662–1669. [DOI] [PubMed] [Google Scholar]
- 34.Ghivizzani S C, Lechman E R, Kang R, Tio C, Kolls J, Evans C H.et al Direct adenovirus‐mediated gene transfer of interleukin 1 and tumor necrosis factor alpha soluble receptors to rabbit knees with experimental arthritis has local and distal anti‐arthritic effects. Proc Natl Acad Sci U S A 1998954613–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adam D, Kessler U, Kronke M. Cross‐linking of the p55 tumor necrosis factor receptor cytoplasmic domain by a dimeric ligand induces nuclear factor‐kappa B and mediates cell death. J Biol Chem 199527017482–17487. [PubMed] [Google Scholar]