Abstract
Objectives
Correlation of serum trough infliximab levels and antibodies to infliximab (anti‐infliximab) with clinical response in ankylosing spondylitis.
Methods
In accordance with the international ASsessment in Ankylosing Spondylitis (ASAS) consensus statement, patients were treated with infliximab (5 mg/kg) every 6 weeks after a starting regimen. Preinfusion sera were collected at baseline, 24 and 54 weeks. At every visit, the 20% improvement response (ASAS‐20) was assessed and laboratory tests performed.
Results
24 of the 38 (63%) patients fulfilled ASAS‐20 response criteria after 24 weeks of treatment and 21 (53%) after 54 weeks. After 54 weeks, 11 (29%) patients showed undetectable serum trough infliximab levels and detectable anti‐infliximab; six of these patients developed an infusion reaction. Anti‐infliximab was found significantly more often (p = 0.04) in ASAS‐20 non‐responders compared with responders at week 54. Serum trough infliximab levels were significantly (p<0.0001) lower in patients with (mean 0.02 mg/l) than in those without (12.7 mg/l) anti‐infliximab.
Conclusions
In ankylosing spondylitis, high levels of serum trough infliximab correlated with a good clinical response. Detection of anti‐infliximab within 54 weeks is associated with undetectable serum trough infliximab levels, reduced response to treatment and increased risk of developing an infusion reaction.
Keywords: Spondylitis, ankylosing; tumor necrosis factor‐alpha; infliximab; antibodies to infliximab
Large randomised clinical trials have shown that tumour necrosis factor blocking agents such as infliximab are very effective in ankylosing spondylitis.1 It is unknown why >30% of patients with ankylosing spondylitis fail to respond, or why some initial responders lose responsiveness during treatment and in some cases even develop an infusion reaction.
The non‐responsiveness to infliximab might be due to the development of antibodies against it, which has been described in patients with rheumatoid arthritis and Crohn disease.2,3,4,5 In ankylosing spondylitis, we recently showed in a small group of patients that detection of anti‐infliximab was associated with undetectable serum trough infliximab levels, a reduced response to treatment and a higher risk of infusion reactions.6
The aim of this study was to evaluate these data in a larger group of patients with ankylosing spondylitis who were treated for a longer period of time and to specify the influence on infliximab levels.
Methods
All consecutive patients with ankylosing spondylitis (according to the 1984 modified New York Criteria7) who received treatment with infliximab in our centre were included in this study.
Disease activity was measured with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)8 and the ASsessment in Ankylosing Spondylitis 20% response criteria (ASAS‐20).9 Active disease was defined as a BASDAI score ⩾4. Response to treatment with infliximab was defined as fulfilment of the ASAS‐20 response criteria.
Patients with ankylosing spondylitis were treated with intravenous infliximab, 5 mg/kg bodyweight at baseline, weeks 2 and 6, and every 6 weeks thereafter. This treatment was initiated in accordance with the international ASAS consensus statement.9 In case of decrease of clinical response, the dose of infliximab was increased to 7.5 mg/kg.
At each visit, the presence of infections, side‐effects or infusion reactions, and the cause for discontinuation of therapy were recorded. Questionnaires and routine laboratory tests were obtained. Preinfusion sera were collected at baseline, weeks 24 and 54, before any dose escalation and at two consecutive visits after dose escalation. After 24 weeks of treatment, serum samples were collected from 15 patients to measure infliximab levels 2 weeks after the infliximab infusion.
Validated immunoassays (Sanquin Research, Amsterdam, the Netherlands) were used for detection of anti‐infliximab and serum trough infliximab levels.5 Trough serum infliximab levels were measured by ELISA, based on the principle that infliximab is captured through its ability to bind tumour necrosis factor‐α. The assay, which was described previously, was modified recently. It currently uses specific polyclonal rabbit antibodies to infliximab for detection instead of the monoclonal anti human IgG that was previously used. The sensitivity of detection is 0.0003 mg/l.
A radioimmunoassay was used for anti‐infliximab detection.5 Arbitrary units per ml (AU/ml) were expressed as absolute amounts of infliximab‐specific IgG (mg/l) 10 (1 AU = 12 ng of infliximab‐specific IgG). The cut‐off value for IgG anti‐infliximab was determined by assaying in our anti‐infliximab test 100 plasma samples from blood donors sent to Sanquin for IgG anti‐tetanus toxoid testing. The average result (AU/ml) + 6 SD was 12 AU/ml (0.144 mg/l).
The clinical data and presence of HLA B27 were used to correlate disease activity with serum trough infliximab levels and anti‐infliximab levels. Differences between groups were tested with the Mann–Whitney U test. Associations were calculated with logistic regression. The threshold for significance was set at p<0.05. The last observation was carried forward for patients who dropped out before week 54.
Results
Demographic and clinical characteristics of the 38 patients included are shown in table 1. Four patients were lost to follow‐up before week 54: one wanted to become pregnant, one preferred to be treated in a hospital nearby and two because of comorbidities.
Table 1 Demographic and clinical variables at baseline, week 24 and at week 54 of patients with ankylosing spondylitis (n = 38).
| Variables | Baseline | Week 24 | Week 54 | |||
|---|---|---|---|---|---|---|
| Male gender (%) | 26 (68) | — | — | |||
| Age (years) | 40 (10) | — | — | |||
| HLA B27+ (%) | 32 (84) | — | — | |||
| IBD (%) | 6 (16) | — | — | |||
| Use of corticosteroids (%) | 3 (8) | — | — | |||
| Use of other immunosuppressives (%) | 6 (16) | — | — | |||
| BASDAI | 6.4 (1.2) | 3.6 (2.6)‡ | 4.1 (3.0‡ | |||
| Morning stiffness† | 6.3 (2.2) | 3.0 (2.5)‡ | 3.5 (3.2)‡ | |||
| GDA | 6.8 (1.3) | 4.3 (2.9)‡ | 4.9 (3.4)§ | |||
| C‐reactive protein (normal<8.0 mg/l) | 37 (34.2) | 9.3 (10.7)‡ | 15.8 (21.1)¶ | |||
| Detectable serum trough infliximab (%) | 0 | 31 (82) | 27 (71) | |||
| Anti‐infliximab (%) | 0 | 7 (18) | 11 (29) |
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index (0–10 cm); GDA VAS, Global Disease Activity Visual Analogue Scale (0–10 cm); HLA B27, human lymphocyte antigen B27; IBD, inflammatory bowel disease.
Except when indicated otherwise, the values are the mean (SD).
†Mean of item 5+6 of the BASDAI (0–10 cm).
Compared with baseline: ‡p<0.001; §p = 0.005; ¶p = 0.003.
There was a significant decrease in BASDAI, morning stiffness, global disease activity and C‐reactive protein after 24 and 54 weeks of treatment (table 1) and all pre‐treatment samples showed undetectable infliximab levels and no anti‐infliximab. We did not detect anti‐infliximab in the presence of infliximab.
After 24 weeks, 24 patients (63%) met ASAS‐20 response criteria. Responders showed higher mean serum trough infliximab levels, and only two patients (8%) showed anti‐infliximab, compared with 5 (36%) of the non‐responders (p = 0.08).
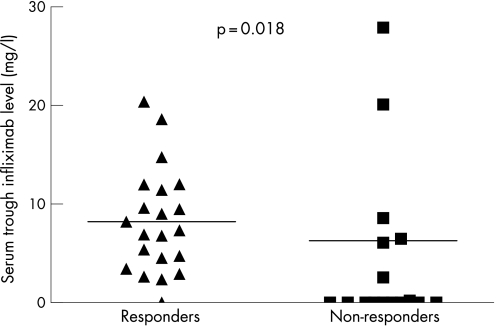
After 54 weeks of treatment, ASAS‐20 response criteria were met by 21 patients (53%). The mean serum trough infliximab level for responders was significantly (p<0.01) higher that that of the non‐responders (8.2 mg/l vs 6.3 mg/l; figure 1) and anti‐infliximab was significantly (p<0.04) more often found in non‐responders. Only 5% (1 of 21) of the responders showed anti‐infliximab, compared with 59% (10 of 17) of the non‐responders.
Figure 1 Serum trough infliximab level for responders (n = 21; 8.2 mg/l) and non‐responders (n = 17; 6.3 mg/l) according to the ASAS‐20 response criteria, at week 54 (p = 0.018).
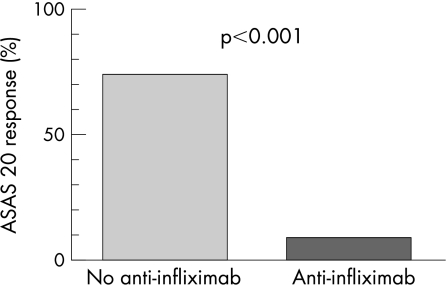
In total, 9% (1 of 11) patients with detectable anti‐infliximab was classified as a responder at week 54, compared with 74% (20 of 27) of patients without anti‐infliximab (figure 2).
Figure 2 Percentage of patients (n = 38) with (9%) and without (74%) ATI fulfilling ASAS‐20 response criteria at week 54 *p<0.001).
After correction for probable confounding variables such as gender and human leucocyte antigen B27 (HLA‐B27), the absence of anti‐infliximab remained a significant determinant for ASAS‐20 response, with an odds ratio (OR) of 100 (95% CI 5.2 to 1000). Remarkably, the presence of anti‐infliximab was significantly associated with the absence of HLA‐B27 (OR = 7.1; 95% CI 1.1 to 47.6; Pearson χ2, p = 0.03).
Two weeks after the infusion of week 24, significantly lower infliximab levels were measured (20 mg/l compared with 51 mg/l; p<0.01) in patients who developed anti‐infliximab within 54 weeks of treatment.
In 12 patients, dose was increased within the 54 weeks, because of insufficient clinical response. Nine (75%) of these patients showed anti‐infliximab antibodies. Increase in dose did not result in a significant increase of the serum trough infliximab level (p = 0.33), or a significant decrease in the anti‐infliximab level (p = 0.90) and BASDAI (p = 0.39). However, 2 of 12 patients reported longer duration of effect.
Infusion reactions occurred in six patients. Most reactions were mild, and all patients recovered after supportive therapy. Treatment with infliximab was stopped in each case. Every infusion reaction was preceded by development of anti‐infliximab and consequently undetectable serum trough infliximab levels. All antibodies to infliximab consisted of IgG1 and IgG4 subtypes. Although these infusion reactions resemble a type 1 allergic reaction, no IgE was detected. One patient's pre‐infusion serum contained an anti‐infliximab level of 6.4 g/l, indicating that approximately half of his total serum IgG consisted of infliximab‐specific antibodies.
Discussion
A good clinical response of ankylosing spondylitis to treatment with infliximab was correlated with the presence of high serum trough infliximab levels and the absence of anti‐infliximab antibodies, and inefficacy with the reverse. Moreover, these data demonstrate that anti‐infliximab antibodies precede an infusion reaction.
The mechanism of the decrease in efficacy can be explained by the lower serum trough infliximab levels, probably caused by enhanced clearance due to immune complex formation of anti‐infliximab antibodies and infliximab. A recent study in RA showed an enhanced clearance as a consequence of this process and an accumulation in the macrophage–phagocyte system (liver and spleen).11 Indeed, in those patients with ankylosing spondylitis who developed detectable anti‐infliximab within 54 weeks of treatment with infliximab, a significantly lower infliximab level was found 2 weeks after infusion compared with patients who did not develop anti‐infliximab.
Often, the infliximab dose is increased in ankylosing spondylitis when responsiveness decreases, but reasons for dose escalation in ankylosing spondylitis are not yet well defined. In our small sample, no clear increase in serum trough infliximab level after dose escalation was shown.
Another option is to try to prevent anti‐infliximab formation with the concomitant administration of other immunosuppressive drugs such as methotrexate; however, this medication is not efficacious in ankylosing spondylitis.12
Remarkably, absence of HLA B27 shows significant correlation with anti‐infliximab formation. Further genetic evaluation will be performed to unravel this interesting observation.
It also has to be investigated whether coadministration of immunosuppressive drugs inhibits anti‐infliximab formation, and whether infliximab levels can be used for determination of the optimum dose of infliximab in ankylosing spondylitis.
In accordance with our previous report, the efficacy of infliximab in ankylosing spondylitis is clearly related to infliximab levels and the formation of anti‐infliximab antibodies. Detection of anti‐infliximab antibodies within 54 weeks is associated with undetectable serum trough infliximab levels, reduced response to treatment and increased risk of development of an infusion reaction.
Abbreviations
AU - arbitrary unit
ASAS - ASsessment in Ankylosing Spondylitis
BASDAI - Bath Ankylosing Spondylitis Disease Activity Index
HLA - human leucocyte antigen
References
- 1.van der Heijde D. Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo‐controlled trial (ASSERT), Arthritis Rheum 200552582–591. [DOI] [PubMed] [Google Scholar]
- 2.Baert F, Noman M, Vermeire S, Van A G, D' H G, Carbonez A.et al Influence of immunogenicity on the long‐term efficacy of infliximab in Crohn's disease. N Engl J Med 2003348601–608. [DOI] [PubMed] [Google Scholar]
- 3.Hanauer S B, Feagan B G, Lichtenstein G R, Mayer L F, Schreiber S, Colombel J F.et al Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 20023591541–1549. [DOI] [PubMed] [Google Scholar]
- 4.St Clair E W, Wagner C L, Fasanmade A A, Wang B, Schaible T, Kavanaugh A.et al The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double‐blind, placebo‐controlled trial. Arthritis Rheum 2002461451–1459. [DOI] [PubMed] [Google Scholar]
- 5.Wolbink G J, Vis M, Lems W, Voskuyl A E, de G E, Nurmohamed M T.et al Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum 200654711–715. [DOI] [PubMed] [Google Scholar]
- 6.de Vries M K, Wolbink G J, Stapel S O, de Groot E R, Dijkmans B A, Aarden L A.et al Inefficacy of infliximab in ankylosing spondylitis is correlated with antibody formation. Ann Rheum Dis 200766133–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Linden S. Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 198427361–368. [DOI] [PubMed] [Google Scholar]
- 8.Garrett S, Jenkinson T, Kennedy L G, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994212286–2291. [PubMed] [Google Scholar]
- 9.Braun J, Pham T, Sieper J, Davis J, van der L S, Dougados M.et al International ASAS consensus statement for the use of anti‐tumour necrosis factor agents in patients with ankylosing spondylitis. Ann Rheum Dis 200362817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuurman J, Perdok G J, Mueller G A, Benjamin D C, Yong T K, Chapman M D.et al Mouse/human chimeric IgG1 and IgG4 antibodies directed to the house dust mite allergen Der p 2: use in quantification of allergen specific IgG. Clin Exp Allergy 1997271095–1102. [DOI] [PubMed] [Google Scholar]
- 11.van der Laken C J, Voskuyl A E, Roos J C, Stigter van W M, de Groot E R, Wolbink G.et al Imaging and serum analysis of immune complex formation of radiolabelled infliximab and anti‐infliximab in responders and non‐responders to therapy for rheumatoid arthritis. Ann Rheum Dis 200766253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haibel H, Brandt H C, Song I H, Brandt A, Listing J, Rudwaleit M.et al No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16‐week open‐label trial. Ann Rheum Dis 200766419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]