Abstract
Objectives
To describe a novel scoring system for the assessment of tenosynovitis by magnetic resonance imaging (MRI) in patients with rheumatoid arthritis, and assess its intra‐ and inter‐reader reliability in a multireader, longitudinal setting.
Methods
Flexor and extensor tenosynovitis were evaluated at the level of the wrist in 10 different anatomical areas, graded semi‐quantitatively from grade 0 to 3 (total score 0–30), based on the maximum width of post‐contrast enhancement within each anatomical area on axial T1‐weighted MR images. Ten sets of baseline and 1‐year follow‐up MR images of the wrists of patients with rheumatoid arthritis with early and established disease were scored independently by four readers twice on 2 consecutive days. Intra‐ and inter‐reader agreements were evaluated.
Results
The intrareader intraclass correlation coefficients (ICCs) were high for status scores (median ICCs 0.84–0.88) and slightly lower for change score (0.74). The smallest detectable difference (SDD) in % of the maximum score was 11.2–11.5% for status scores and 13.3% for change scores. Inter‐reader single‐measure ICCs were acceptable for both status scores (median 0.73–0.74) and change scores (0.67), while average‐measures ICCs were very high for both status and change score (all ⩾0.94). The median scoring time per patient (baseline and follow‐up images) was 7 min (range 3–10).
Conclusions
The introduced tenosynovitis scoring system demonstrates a high degree of multireader reliability, is feasible, and may be used as an adjuvant to the existing OMERACT RAMRIS score, allowing improved quantification of inflammatory soft tissue changes in patients with rheumatoid arthritis.
Tenosynovitis is a common problem in rheumatoid arthritis (RA), and is observed in a large proportion of these patients.1,2 It is not specific for RA, and has also been described in other rheumatological diseases, such as systemic lupus erythematosus.3 The tenosynovium produces proinflammatory cytokines and proteolytic enzymes that are important in the tissue degradation seen in RA.4 The proliferation of the tenosynovial lining can lead to impaired function due to scarring and adhesions. Flexor tenosynovitis in the hands has also been identified as a risk factor for destructive erosion of the joints.5 The ongoing tenosynovial inflammation may ultimately lead to tendon rupture, which is a serious complication that leads to reduced hand function.6
Magnetic resonance imaging (MRI) is a powerful imaging modality that is now widely used in both scientific research and clinical settings to visualise joints of patients with RA. MRI is able to image structural damage and soft tissue changes, ie, synovitis, bone oedema, damage to cartilage and bone, as well as tendon pathology. Patients are likely to have ongoing tendon disease if MRI evidence of high‐grade tendinopathy is identified in early disease.7 Clinicians should be aware of this so that preventive measures can be applied. If the tenosynovitis cannot be controlled by systemic drug therapy or local steroid injections, surgical intervention with tenosynovectomy or removal of bone spurs can prevent tendon ruptures and give long‐term relief.6,8,9 When a rupture has occurred, reconstruction has a reasonable chance of restoring hand function as long as the number of affected tendons is limited.10
The complexity and the amount of information provided by MRI pose a challenge, and the issue is how best to use this modality as a reliable outcome measure. The OMERACT MRI‐RA Working Group has for some years been working on this issue, and has developed a scoring system, the OMERACT RAMRIS, which includes a semiquantitative score for bone erosions, bone oedema and synovitis.11 RAMRIS has recently been validated in a multireader, longitudinal setting12, and the reliability of this scoring method is similar to that of the scoring methods used for conventional radiography, such as the Sharp and Larsen methods with modifications.13,14,15
Synovial inflammation is the primary lesion in RA, and studies suggest that progressive joint destruction does not occur in the absence of MRI synovitis.16,17,18 As the goal of modern drug therapy in early RA is to eliminate rather than inhibit radiographic erosive damage, future main outcome measures in both clinical trials and clinical practice should include a sensitive and robust measure of inflammation, such as MRI. Taking the frequent occurrence of tenosynovitis into account, scoring tenosynovitis in addition to the aspects covered by the RAMRIS, will allow a broader assessment of inflammation in patients with RA.
The aim of the present study was to introduce and describe a novel scoring system for the assessment of tenosynovitis, and assess the intra‐ and inter‐reader reliability of this scoring system in a multireader, longitudinal setting in patients with RA with both early and established disease.
Patients and methods
Patient selection and image evaluation
MR images of the dominant wrist in 10 patients with RA, which showed progression of either erosions or joint space narrowing on conventional hand radiographs were included in the study (four with early RA and six with established disease, all fulfilling the American College of Rheumatology 1987 criteria for RA). The median (range) disease duration for the patients with early RA was 3.5 months (0.8–11.9), and for those with established RA 7.4 years (5.1–9.0). The patients received conventional RA treatment during the 12‐month follow‐up (methotrexate n = 6, prednisolone n = 3, combination therapy with hydroxychloroquine and sulphasalazine n = 1). The regional ethics committee evaluated the study, and all enrolled patients gave informed consent. The median interval between the first and second scan was 12 months, and ranged from 12 to 14 months. The paired images were read in chronological order on large‐screen (21′′) radiological workstation monitors using a standard PACS software program (SECTRA IDS5, Sweden). This software package provides the readers with advanced features of image viewing, allowing the reader to adjust window/level settings, to zoom in/out, and to use a localiser that allows the accurate placement of specific lesions in two planes (axial and coronal), with the opportunity to measure distances and areas accurately.
The readers met for 2 days to perform the reading. The four readers had different levels of experience with MRI reading. MØ and BE were experienced MRI readers, and both had taken part in the development and testing of the OMERACT RAMRIS system; however, they had no previous experience in the tenosynovitis scoring system. EAH had some experience with the RAMRIS, and had previously used the tenosynovitis score in a pilot study, and thus had some experience using the score. NPK was familiar with MR‐reading, including assessment of tenosynovitis, but did not have any previous experience with neither the RAMRIS nor the tenosynovitis scoring system. The four readers met for a brief training session the evening before the exercise, to review the scoring method and for initial training of the readers not familiar with the tenosynovitis scoring system. All readers read images independently at four different workstations in four separate locations. A technician coded the image sets and removed patient names. This procedure was repeated for a second reading the consecutive day, with rearrangement of the image sets in a different order and with a different coding. The score sheets from day 1 were sealed in envelopes until the second reading was completed.
MRI sequences
MRI of the dominant wrist was performed at baseline and at 1 year, using a GE Signa 1.5T MRI scanner (General Electric (GE) Signa, Milwaukee, Wisconsin, USA) with a dedicated high‐resolution wrist phased array coil. The same scanner and wrist coil were used for both examinations. The hand was placed in the wrist coil at the patient's side with the coil anchored to the base tray to reduce motion artefacts. The MRI sequences in this study included the OMERACT recommended MRI core set of sequences,11 but only pre‐ and post‐contrast T1‐weighted axial images (slice thickness 3.0 mm) were used to evaluate the tenosynovitis. The image sequences were tested in a pilot study, and developed in collaboration with an experienced MR radiologist and a product specialist from GE. Details of the sequences can be found in a previous study.12 Experienced technicians reviewed the images immediately after acquisition, and a sequence was reobtained if the quality was not acceptable.
Anatomy of the wrist tendons
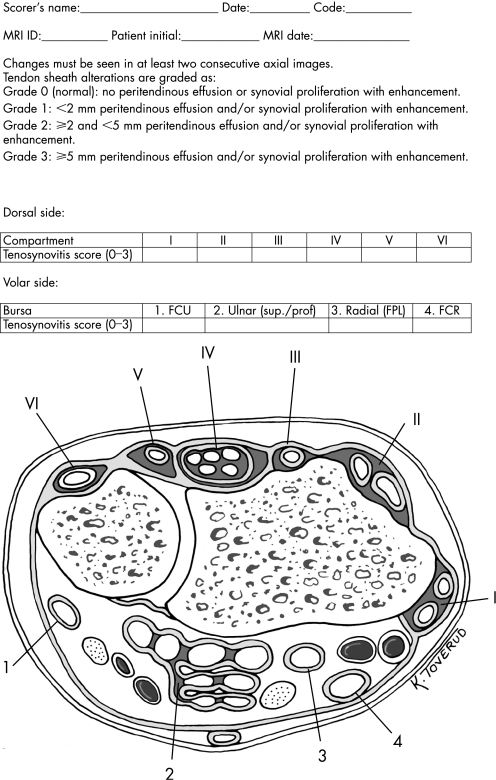
On the dorsal side the extensor tendons are stabilised by the extensor retinaculum, with septations dividing the extensor tendons into six discrete compartments (for details see fig 1 score sheet with illustration of anatomy of the wrist). The compartments contain the following tendons: (I) extensor pollicis brevis, abductor pollicis longus; (II) extensor carpi radialis brevis, extensor carpi radialis longus; (III) extensor pollicis longus; (IV) extensor digitorum communis, extensor indicus proprius; (V) extensor digiti quinti proprius; (VI) extensor carpi ulnaris. On the volar side the tendons can be divided into four discrete anatomical regions: (1) the flexor carpi ulnaris tendon (located ulnar to the carpal tunnel); (2) the flexor digitorum superficialis and profundus tendons (in the carpal tunnel, enclosed in a common sheath—the ulnar bursa); (3) the flexor pollicis longus tendon (located dorsally and radially to the median nerve as it passes through the carpal tunnel, and entering a continuous sheath that becomes the radial bursa); (4) the flexor carpi radialis (localised radially to the tendons enclosed in the ulnar bursa). Thus, the extensor and flexor tendons can be divided into 10 separate anatomical areas that can be evaluated for tenosynovitis.
Figure 1 Score sheet for tenosynovitis in a wrist with RA. Extensor compartments denoted in Roman numbers from I to VI: (I) extensor pollicis brevis, abductor pollicis longus; (II) extensor carpi radialis brevis, extensor carpi radialis longus; (III) extensor pollicis longus; (IV) extensor digitorum communis, extensor indicus proprius; (V) extensor digiti quinti proprius; (VI) extensor carpi ulnaris. Flexor tendon areas denoted in Arabic numbers from 1 to 4: (1) flexor carpi ulnaris; (2) ulnar bursa, including flexor digitorum profundus and superficialis tendon quartets; (3) flexor pollicis longus (tendon) in radial bursa; (4) flexor carpi radialis.
The tenosynovitis scoring system
Both flexor and extensor tenosynovitis are evaluated. The 10 anatomical areas to be scored for tenosynovitis (described above) are evaluated between the radioulnar joint and the hook of the hamate. Tenosynovitis is visualised on MRI as tendon sheath fluid, sheath thickening and enhancement after intravenous contrast injection. As small amounts of fluid can be seen in normal tendon sheets,18 it is essential that the tenosynovitis is visible in at least two consecutive axial slices within the tendon sheet to be scored as abnormal. Tendon sheath abnormalities are graded semiquantitatively from grade 0 to grade 3, reflecting the maximum width (in mm) of enhancement within each anatomical area as described below:
Grade 0 (normal): no peritendinous effusion or synovial proliferation with enhancement.
Grade 1: <2 mm peritendinous effusion and/or synovial proliferation with enhancement.
Grade 2: ⩾2 and <5 mm peritendinous effusion and/or synovial proliferation with enhancement.
Grade 3: ⩾5 mm peritendinous effusion and/or synovial proliferation with enhancement.
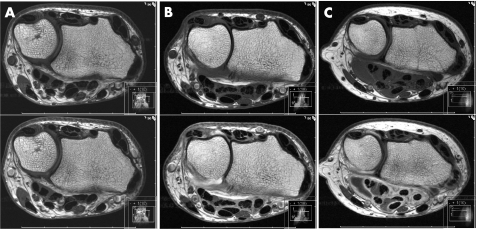
The extent of the synovial enhancement is measured at the point of maximal thickness, perpendicular to the tendon surface. This grading scheme is based on a modification of a scheme that has been applied in another study of tenosynovitis.19 Missing tendons, for example due to physiological anatomical variants, are given a score of zero. The range of the total score is from zero to 30. The score sheet that was used including a detailed illustration of the anatomy of this region is provided in fig 1. Examples of different grades of tenosynovitis in patients with RA are found in fig 2. The readers recorded the time consumed scoring each image set according to the tenosynovitis scoring system.
Figure 2 Pre‐contrast (top) and post‐contrast (bottom) axial T1‐weighted MR images illustrating: (A) no tenosynovitis; (B) grade 1 tenosynovitis in extensor compartments IV and VI, and flexor anatomical areas 2, 3 and 4; (C) grade 2 and 3 tenosynovitis in flexor anatomical areas 3 and 2, respectively.
Statistical methods
Intrareader and inter‐reader reliability were evaluated using a two‐way mixed effect model, and single measure and average measure intraclass correlation coefficients (ICC) were calculated for both status scores and change scores. The average measure ICC corrects for the number of readers and was calculated for the inter‐reader reliability. The ICC is presented as median (range) for intra‐reader reliability due to the low number of values, and as mean with 95% CI for the inter‐reader reliability analyses. ICC values are comparable with κ values; scores above 0.60 are considered good, and scores above 0.80 very good. Sensitivity to change was assessed by calculating the smallest detectable difference (SDD), derived from the limits of agreement method described by Bland and Altman.20 The SDD represents the smallest change score that can be discriminated from the measurement error of the scoring method, and is expressed in the same units of measurement as calculated for the score. Using SDD as the threshold level for relevant progression of joint damage ensures that an observed change with 95% confidence exceeds the measurement error. Minimal detectable change (MDC) is a way to express the SDD as a percentage of the maximum score of the method, to allow comparisons with other radiographic and clinical measures. An SDD of zero is perfect agreement, and there is no convention regarding any upper limit; however, a MDC of less than 20% is generally accepted to reflect a high potential to detect changes.20 All statistical analyses were performed using the Statistical Package for the Social Sciences for Windows, version 12 (SPSS, Chicago, Illinois, USA).
Results
A total of 10 sets of MR images from wrist joints in patients with RA were scored by four readers. The intra‐reader single‐measure ICC, the SDD and the MDC are presented in table 1.
Table 1 Intra‐reader agreement of tenosynovitis scores (two‐way mixed effect model, single measure ICCs, SDDs and MDC. Values are medians and ranges).
| Measure | Baseline | 1‐year follow‐up | Change score |
|---|---|---|---|
| Intra‐reader ICC | 0.84 (0.57–0.95) | 0.88 (0.73–0.93) | 0.74 (0.62–0.85) |
| SDD | 3.44 (2.48–5.86) | 3.37 (2.52–4.41) | 4.00 (1.86–5.15) |
| MDC (%) | 11.5 (8.26–19.5) | 11.2 (8.41–14.7) | 13.3 (6.2–17.2) |
ICC, intraclass correlation coefficients; SDD, smallest detectable difference; MDC (%), minimal detectable change, defined as the SDD expressed as a percentage of the maximum score.
The intrareader ICCs were generally high for status scores (median baseline ICCs 0.84 and follow‐up ICC 0.88) and slightly lower for change score (median ICC 0.74). The SDD and MDC of the tenosynovitis scoring system were acceptable and comparable with similar measures, ie the OMERACT RAMRIS score for MR images and Larsen and Sharp scores for conventional radiographs.12,13,14,15
Table 2 provides the inter‐reader single measure and average measure ICCs for baseline, 1‐year follow‐up and change scores.
Table 2 Inter‐reader agreement of tenosynovitis scores (two‐way mixed effect model, single measure ICCs and average measure ICCs. Values are mean and 95%CI).
| Score | Measure | ICC | 95% CI |
|---|---|---|---|
| Baseline T1 | Sm ICC | 0.73 | 0.52–0.90 |
| Avm ICC | 0.96 | 0.90–0.99 | |
| 1‐year follow‐up T2 | Sm ICC | 0.74 | 0.53–0.91 |
| Avm ICC | 0.96 | 0.90–0.99 | |
| Change score | Sm ICC | 0.67 | 0.45–0.88 |
| Avm ICC | 0.94 | 0.87–0.98 |
SmICC, single measure intraclass correlation coefficients; AvmICC; average measure intraclass correlation coefficients.
Inter‐reader single‐measure ICCs were acceptable for both status scores (baseline ICC 0.73 and follow‐up ICC 0.74) and change score (median ICC 0.67), while the average measures ICCs were very high for both status and change score (all ICCs >0.94). An overview of the distribution of scores is shown in table 3, including the mean with 95% confidence intervals and median with minimum and maximum values for tenosynovitis scores for baseline, 12‐month follow‐up and change.
Table 3 Mean, median, 95% confidence intervals (CI) and minimum and maximum values from duplicate assessments from four readers for all patients at baseline, 1‐year follow‐up and change scores.
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Mean | 2.25 | 4.38 | 11.30 | 7.63 | 4.75 | 8.13 | 9.25 | 8.13 | 7.25 | 1.75 |
| Median | 2.00 | 3.00 | 11.50 | 8.00 | 4.50 | 8.50 | 10.00 | 8.00 | 8.00 | 1.50 | |
| 95%CI lower | 1.66 | 1.45 | 8.94 | 6.37 | 3.28 | 7.18 | 6.93 | 5.39 | 5.21 | 1.01 | |
| 95%CI upper | 2.84 | 7.30 | 13.50 | 8.88 | 6.21 | 9.07 | 11.60 | 10.80 | 9.29 | 2.49 | |
| Min | 1.00 | 0.00 | 7.00 | 5.00 | 3.00 | 6.00 | 4.00 | 4.00 | 3.00 | 1.00 | |
| Max | 3.00 | 10.00 | 15.00 | 9.00 | 7.00 | 9.00 | 12.00 | 13.00 | 10.00 | 3.00 | |
| 1 year | Mean | 1.50 | 2.75 | 8.00 | 5.50 | 6.50 | 4.25 | 9.50 | 6.00 | 10.50 | 2.38 |
| Median | 1.00 | 1.50 | 8.50 | 5.50 | 6.50 | 4.50 | 9.00 | 4.50 | 10.50 | 2.50 | |
| 95%CI lower | 0.86 | 0.00 | 5.72 | 4.50 | 6.05 | 2.72 | 7.36 | 3.32 | 9.02 | 1.49 | |
| 95%CI upper | 2.13 | 5.64 | 10.30 | 6.50 | 6.95 | 5.78 | 11.60 | 8.68 | 11.90 | 3.26 | |
| Min | 1.00 | 0.00 | 4.00 | 4.00 | 6.00 | 2.00 | 7.00 | 3.00 | 7.00 | 1.00 | |
| Max | 3.00 | 9.00 | 11.00 | 7.00 | 7.00 | 7.00 | 13.00 | 11.00 | 13.00 | 4.00 | |
| Change | Mean | −0.75 | −1.63 | −3.25 | −2.13 | 1.75 | −3.88 | 0.25 | −2.13 | 3.25 | 0.63 |
| Median | −1.00 | −1.00 | −3.50 | −2.50 | 2.00 | −3.50 | 0.50 | −2.50 | 4.00 | 0.50 | |
| 95%CI lower | −1.34 | −2.71 | −4.49 | −3.06 | 0.28 | −5.32 | −1.21 | −3.93 | 1.92 | 0.00 | |
| 95%CI upper | −0.16 | −0.54 | −2.00 | −1.18 | 3.21 | −2.43 | 1.71 | −0.31 | 4.57 | 1.25 | |
| Min | −2.00 | −4.00 | −5.00 | −3.00 | −1.00 | −7.00 | −3.00 | −5.00 | 0.00 | 0.00 | |
| Max | 0.00 | 0.00 | 0.00 | 0.00 | 4.00 | −2.00 | 3.00 | 2.00 | 5.00 | 2.00 |
The mean tenosynovitis score ranged from 1.50 to 11.3, and for individual assessments from a minimum of 0 to a maximum score of 15 (out of 30). The complete scoring of one patient (baseline and follow‐up images) was completed in median 7 min, ranging from 3 to 10 min.
Discussion
In this study we assessed the intra‐ and inter‐reader agreement of a novel tenosynovitis score in a longitudinal setting, allowing us to assess the reliability of this measure both for measuring status and progression. The reason for developing such a scoring system was based on work performed under the OMERACT umbrella. The OMERACT MRI in RA group has suggested MRI definitions of the important RA joint pathologies (synovitis, bone oedema and erosions), a “core set” of basic MRI sequences, and a scoring system for RA, namely the RAMRIS. Potential future research areas suggested at OMERACT 7 included “other structures/types of pathologies (e.g. cartilage, tenosynovitis)”.22 We have chosen to focus on tenosynovitis, and developed a simple scoring system that may be used as an adjuvant to RAMRIS.
Our scoring system graded tenosynovitis semiquantitatively from 0 to 3, ie the same range as for RAMRIS synovitis score. Tenosynovitis is evaluated on T1‐weighted axial images pre‐ and post‐contrast enhancement and these MRI sequences are part of the RAMRIS core set of sequences, ie no extra sequences are required. Tehranzadeh et al23 recently described that contrast‐enhanced T1‐weighted images allowed earlier detection of tenosynovitis in acute and subacute stages compared with T2‐weighted sequences, and that the majority of patients demonstrated higher tenosynovitis scores using contrast‐enhanced T1‐weighted sequences. Thus, it appears that the standard RAMRIS sequences are sufficient for evaluating tenosynovitis. McQueen et al24 have previously described an extensive scoring system for the evaluation of tendinopathy, which comprises tendon signal change, tendon size and tendon sheath signal change. To evaluate these measures, it is necessary to obtain sequences not included in the RAMRIS core set. As the RAMRIS is already quite extensive and time consuming, we felt it was important to develop a simple scoring system that can easily be evaluated within the framework of the RAMRIS core set of MRI sequences, and only requires minimal time to score. Ostendorf et al3 has previously described a semiquantitative scoring system for evaluating tenosynovitis in systemic lupus erythematosus, where proliferative and oedematous tenosynovitis is scored separately as “not present”, “slight”, “moderate” or “severe”. Although our tenosynovitis scoring system also is semiquantitative, each level of scoring is precisely defined quantitatively in millimetres as described in the Methods section, and can easily be measured using standard MRI imaging software, thus minimising bias caused by the reader's judgement.
We decided to focus on the wrist area in the development of the tenosynovitis score, as both the finger and wrist tendons are visible in this area, and we aimed at including all relevant tendons. The reason for not including evaluation of the distal part of the finger tendons is for feasibility issues—it is difficult to obtain high‐quality MRIs with a large field‐of‐view that allows simultaneous examination of both the wrist area and the fingers, and it would be time‐consuming to evaluate the finger tendons separately both proximally and distally. Tendons that lie in the same anatomical compartment were scored as one entity, and the results were not weighted. The fact that both the intra‐ and inter‐reader ICCs were so high, despite three of the readers had never used this scoring method previously, underlines the feasibility of this scoring system. The one reader that had experience with the use of this method had a slightly higher intra‐reader ICC, which implies that the ICCs found in this study may be further improved by training.
Modern treatment strategies in patients with RA consist of early aggressive therapy, and the aim is total suppression of joint inflammation, the elimination of radiographic progression and no functional disability. This aim is within reach with new drugs and treatment strategies, but early initiation of therapy as well as sensitive monitoring of disease progression and changes in inflammatory activity are required. MRI has superior sensitivity to detect joint inflammation and structural damage compared with conventional methods,24,25,26 and, potentially, has the desired sensitivity in such a setting.
In this multireader study we have found that this novel tenosynovitis scoring system demonstrates adequate reliability, in line with existing scoring systems, is feasible, and may be used as an adjuvant to the existing OMERACT RAMRIS score, to be able to better quantify soft tissue changes reflecting inflammation in RA. Further prospective studies are needed to explore the sensitivity to change of this scoring system, as well as the clinical relevance of the MRI tenosynovitis score. An additional important item is to examine whether the combination of the RAMRIS synovitis and bone marrow oedema scores together with this novel tenosynovitis score is a more responsive marker of inflammatory activity in RA than each of the single components alone.
Acknowledgements
We thank research nurse Margareth Sveinsson for collecting clinical data, research coordinator Tone Omreng for organising the data collection, technician Marianne Ytrelid for technical assistance, medical illustrator Kari Toverud (MS CMI) for help with fig 1 and Petter Mowinckel (MSc) for statistical advice.
Abbreviations
CI - confidence interval
ICC - intraclass correlation coefficients
MDC - minimal detectable change
MRI - magnetic resonance imaging
RA - rheumatoid arthritis
SDD - smallest detectable difference
Footnotes
Funding: This study was supported in part by grants from The Research Council of Norway, The Norwegian Rheumatism Association, The Norwegian Women Public Health Association, Grethe Harbitz Legacy and Marie and Else Mustad's Legacy.
Competing interests: None
References
- 1.Gray R G, Gottlieb N L. Hand flexor tenosynovitis in rheumatoid arthritis. Prevalence, distribution, and associated rheumatic features. Arthritis Rheum 1977201003–1008. [DOI] [PubMed] [Google Scholar]
- 2.Boutry N, Larde A, Lapegue F, Solau‐Gervais E, Flipo R M, Cotten A. Magnetic resonance imaging appearance of the hands and feet in patients with early rheumatoid arthritis. J Rheumatol 200330671–679. [PubMed] [Google Scholar]
- 3.Ostendorf B, Scherer A, Specker C, Modder U, Schneider M. Jaccoud's arthropathy in systemic lupus erythematosus: differentiation of deforming and erosive patterns by magnetic resonance imaging. Arthritis Rheum 200348157–165. [DOI] [PubMed] [Google Scholar]
- 4.Jain A, Nanchahal J, Troeberg L, Green P, Brennan F. Production of cytokines, vascular endothelial growth factor, matrix metalloproteinases, and tissue inhibitor of metalloproteinases 1 by tenosynovium demonstrates its potential for tendon destruction in rheumatoid arthritis. Arthritis Rheum 2001441754–1760. [DOI] [PubMed] [Google Scholar]
- 5.Mottonen T T. Prediction of erosiveness and rate of development of new erosions in early rheumatoid arthritis. Ann Rheum Dis 198847648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ertel A N. Flexor tendon ruptures in rheumatoid arthritis. Hand Clin 19895177–190. [PubMed] [Google Scholar]
- 7.McQueen F, Beckley V, Crabbe J, Robinson E, Yeoman S, Stewart N. Magnetic resonance imaging evidence of tendinopathy in early rheumatoid arthritis predicts tendon rupture at six years. Arthritis Rheum 200552744–751. [DOI] [PubMed] [Google Scholar]
- 8.Stirrat C R. Treatment of tenosynovitis in rheumatoid arthritis. Hand Clin 19895169–175. [PubMed] [Google Scholar]
- 9.Ryu J, Saito S, Honda T, Yamamoto K. Risk factors and prophylactic tenosynovectomy for extensor tendon ruptures in the rheumatoid hand. J Hand Surg [Br ] 199823658–661. [DOI] [PubMed] [Google Scholar]
- 10.Wilson R L, DeVito M C. Extensor tendon problems in rheumatoid arthritis. Hand Clin 199612551–559. [PubMed] [Google Scholar]
- 11.Ostergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B.et al OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA‐MRI scoring system. J Rheumatol 2003301385–1386. [PubMed] [Google Scholar]
- 12.Haavardsholm E A, Ostergaard M, Ejbjerg B J, Kvan N P, Uhlig T A, Lilleas F G.et al Reliability and sensitivity to change of the OMERACT rheumatoid arthritis magnetic resonance imaging score in a multireader, longitudinal setting. Arthritis Rheum 2005523860–3867. [DOI] [PubMed] [Google Scholar]
- 13.Sharp J T, Lidsky M D, Collins L C, Moreland J. Methods of scoring the progression of radiologic changes in rheumatoid arthritis. Correlation of radiologic, clinical and laboratory abnormalities. Arthritis Rheum 197114706–720. [DOI] [PubMed] [Google Scholar]
- 14.Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn (Stockh) 197718481–491. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijde D M. Plain X‐rays in rheumatoid arthritis: overview of scoring methods, their reliability and applicability. Baillieres Clin Rheumatol 199610435–453. [DOI] [PubMed] [Google Scholar]
- 16.Ostergaard M, Hansen M, Stoltenberg M, Gideon P, Klarlund M, Jensen K E.et al Magnetic resonance imaging‐determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum 199942918–929. [DOI] [PubMed] [Google Scholar]
- 17.McGonagle D, Conaghan P G, O'Connor P, Gibbon W, Green M, Wakefield R.et al The relationship between synovitis and bone changes in early untreated rheumatoid arthritis: a controlled magnetic resonance imaging study. Arthritis Rheum 1999421706–1711. [DOI] [PubMed] [Google Scholar]
- 18.Conaghan P G, O'Connor P, McGonagle D, Astin P, Wakefield R J, Gibbon W W.et al Elucidation of the relationship between synovitis and bone damage: a randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis Rheum 20034864–71. [DOI] [PubMed] [Google Scholar]
- 19.Valeri G, Ferrara C, Ercolani P, De Nigris E, Giovagnoni A. Tendon involvement in rheumatoid arthritis of the wrist: MRI findings. Skeletal Radiol 200130138–143. [DOI] [PubMed] [Google Scholar]
- 20.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 19868476307–310. [PubMed] [Google Scholar]
- 21.Lassere M, McQueen F, Ostergaard M, Conaghan P, Shnier R, Peterfy C.et al OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Exercise 3: an international multicenter reliability study using the RA‐MRI Score, J Rheumatol 2003301366–1375. [PubMed] [Google Scholar]
- 22.Ostergaard M, McQueen F M, Bird P, Ejbjerg B, Lassere M N, Peterfy C G.et al Magnetic resonance imaging in rheumatoid arthritis: advances and research priorities. J Rheumatol 2005322462–2464. [PubMed] [Google Scholar]
- 23.Tehranzadeh J, Ashikyan O, Anavim A, Tramma S. Enhanced MR imaging of tenosynovitis of hand and wrist in inflammatory arthritis. Skeletal Radiol 200635814–822. [DOI] [PubMed] [Google Scholar]
- 24.McQueen F M, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan P L.et al Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals a high prevalence of erosions at four months after symptom onset. Ann Rheum Dis 199857350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Backhaus M, Kamradt T, Sandrock D, Loreck D, Fritz J, Wolf K J.et al Arthritis of the finger joints: a comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast‐enhanced magnetic resonance imaging. Arthritis Rheum 1999421232–1245. [DOI] [PubMed] [Google Scholar]
- 26.Ostergaard M, Hansen M, Stoltenberg M, Jensen K E, Szkudlarek M, Pedersen‐Zbinden B.et al New radiographic bone erosions in the wrists of patients with rheumatoid arthritis are detectable with magnetic resonance imaging a median of two years earlier. Arthritis Rheum 2003482128–2131. [DOI] [PubMed] [Google Scholar]