Abstract
Objective
To examine rheumatoid arthritis (RA) with short disease duration over 10 years, and to identify factors that are associated with the course of pain, depression and anxiety.
Methods
A cohort of 238 patients with RA (age 20–70 years, mean disease duration 2.3 years, 68% rheumatoid factor positive) was followed with assessments at baseline and after 1, 2, 5 and 10 years. Self‐reported health status was assessed by pain on a 100 mm visual analogue scale, the Arthritis Impact Measurement Scales (AIMS), the 28‐item version of General Health Questionnaires, and the Health Assessment Questionnaire. We also examined the erythrocyte sedimentation ratio, grip strength (kg) and radiographic progression of the hands (van der Heijde modified Sharp score). Repeated measures analyses of variance were used to explore the effect of time on measures of outcome among completers, whereas repeated measures analyses using a mixed model were applied to identify factors that were longitudinally associated with pain, depression and anxiety.
Results
At the various assessment points 30% had a visual analogue scale pain score of ⩾40 mm, 5–13% had an AIMS depression score of ⩾4.0 and 20–30% had an AIMS anxiety score of ⩾4.0. The perceived level of pain was explained longitudinally by anxiety, disease activity, physical function and female gender, depression by high disease activity and anxiety, whereas anxiety was explained by low disease activity and depression.
Conclusion
More patients had increased levels of anxiety (20–30%) than increased levels of depression (5–13%). Several factors, including anxiety, but not depression, were associated with the course of pain.
Several longitudinal studies of patient‐cohorts with rheumatoid arthritis (RA) have been performed during the last decades. The most frequent focus has been on the course of physical function and damage,1,2,3,4,5 whereas few studies with a duration of 10 years or more have addressed pain and psychological health status as the primary outcomes.6
Patients with RA rank pain as the most important symptom to be improved.7,8 During the OMERACT sessions on patients' perspectives on outcome assessments, patients have reported that pain as well as other relevant outcomes should be addressed more extensively by researchers. This perspective of the patients is also supported by epidemiological data showing that the prevalence of depression in RA varies from 10% to 46%.9,10,11,12 Although depression and anxiety are associated they are two separate mental dimensions that need different treatment approaches and therefore should be studied as two different entities.13,14
Comprehensive data on health status, inflammatory activity as well as radiographic damage have been collected longitudinally in the Norwegian cohort of the European Research on Incapacitating Diseases and Social Support (EURIDISS) project. We have previously demonstrated that radiographic damage was longitudinally associated to the course of disability over 10 years in this cohort.5 Taking advantage of the comprehensive data collection in this cohort, we aimed to examine the longitudinal course of pain, and psychological health in patients with RA followed from early disease and over the next 10 years. More importantly, the aim of these analyses was to identify demographic, disease and health status variables that were associated longitudinally and independently with the course of pain, depression and anxiety.
Patients and methods
Patients
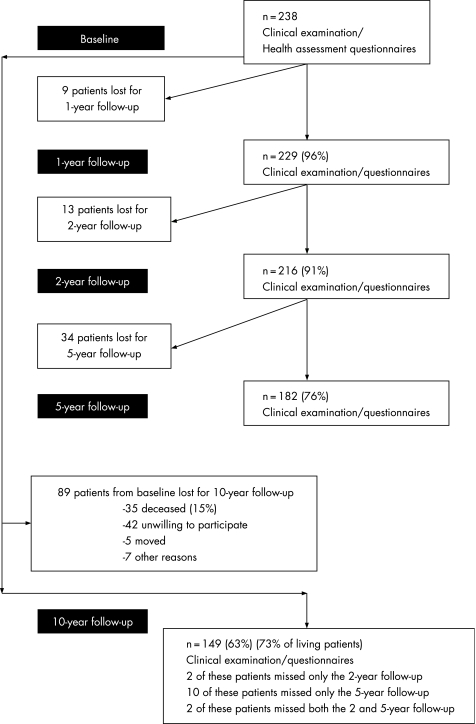
The sample procedure, the patient sample and the inclusion/exclusion criteria of the EURIDISS project have been described in detail elsewhere.15,16 In short, the number of patients included at baseline was 238 (175 females, 63 males), with mean age 52 years (SD 13.0), mean disease duration 2.3 years (SD 1.2, range 0–4) and 68% were rheumatoid factor (RF) positive. The patients were followed longitudinally with examinations after 1, 2, 5 and 10 years. All patients who participated in the baseline visit and who were still alive (n = 203), were asked to participate in the 10‐year follow‐up examination. The attrition rate among living patients was 27%. Power calculations performed in the planning phase recommended that each centre in the EURIDISS study should include 250 patients to ensure 150 patients at the end of the 2‐year follow‐up period. Norway included 238 patients, of whom 149 patients completed the 10‐year follow‐up, indicating maintained power. Patients gave written informed consent before participation, and the study was approved by the regional ethics committee. The sample of patients and withdrawals over time are illustrated in fig 1.
Figure 1 The patient sample at various time points.
At baseline (10‐year follow‐up) 56% (41%) of the completers used traditional regimens with disease‐modifying antirheumatic drugs (DMARDs), 25% (36%) used prednisolone, and 52% (17%) used NSAIDs. In addition, 12% of patients were treated with anti‐tumour necrosis factor drugs (infliximab or etarencept) either as monotherapy (5%) or in combination with methotrexate (7%), and 27% used coxibs at the 10‐year follow‐up. Of the completers 19% had never used DMARDs.
Self‐reported questionnaires
At each examination patients filled in self‐report questionnaires, including pain on a visual analogue scale (VAS, 100 mm), Arthritis Impact Measurement Scales (AIMS),17 the 28‐item version of the General Health Questionnaire (GHQ)18,19 and the Health Assessment Questionnaire (HAQ).20
AIMS17 includes scales on pain, psychological status (depression and anxiety), physical disability and social activity (score 0–10, 10 = worse health). The patient global assessment of the impact of arthritis was assessed by one item from the AIMS.
Global psychological distress was measured with the 28‐item version of GHQ consisting of seven questions in each subscale measuring symptoms of depression, anxiety, social dysfunction and somatisation (each question scored on a Likert scale ranging from 0 to 3, giving a range from 0 to 21 for each of the four subscales).18,19 The GHQ‐28 score (range 0–28) is calculated after recoding the scores on the Likert scales into a dichotomous response scale. A GHQ‐28 score above 11 is required to characterise a patient with chronic somatic disease as “psychologically distressed”.12
HAQ (range 0–3) examines physical disability within eight dimensions of daily living, allowing upgrading of scores for devices or help from another person.20
Clinical examination
The same rheumatologist (LMS) assessed the patients clinically at baseline and at the 1‐ and 2‐year follow‐up, a second rheumatologist (TU) performed the clinical examination at the 5‐year follow‐up, and a third rheumatologist examined the patients after 10 years (SØ). Reliability for joint counts was tested between examiners, demonstrating moderate inter‐examiner agreement with weighted κ for tender joints of 0.45 (LMS and TU, 5‐year follow‐up) and 0.49 (TU and SØ, 10‐year follow‐up).
The medical examination at baseline, 1, 2, 5 and 10 years included the Ritchie articular index,21 measurement of grip strength (hand‐held JAMAR® Dynamometer, kg, average of right and left arms),22 and acute phase reactants (erythrocyte sedimentation rate (ESR) and C‐reactive protein).
Investigators' global assessment of disease activity was indicated on a 100 mm visual analogue scale (VAS). IgM‐RF was analysed at baseline for all patients and was defined as positive if IgM‐RF was 10 IU/ml or higher. Radiographic damage was examined by a modified Sharp score.5
Statistical analyses
Analyses in completers (n = 149)
A two‐tailed t‐test was applied for group comparisons of continuous variables and Pearson's χ2 test for categorical variables. Mean value with SD was calculated for each continuous variable. Repeated measures analyses of variance (ANOVA) with sex as factor and age as covariate were used to process data from all five examinations and to explore the effect of time on measures of outcome among completers.
Model assumptions were tested using Jackknife residuals and Cook's d. The Simes' procedure was used for correcting p‐values in the testing of multiple hypotheses in the ANOVA analyses.23 The proportion of patients with clinical important involvement in physical function, pain, depression and anxiety at each examination point were calculated, based on the following cut‐off points: VAS pain ⩾40 mm; AIMS depression ⩾4.0 and AIMS anxiety ⩾4.0; HAQ ⩾1.0.24,25,26,27,28 Pearson's correlation coefficient (r) was calculated between values at all time points for each measure to assess the fluctuations over time for individual patients.
Analyses to explain pain, depression and anxiety longitudinally
Repeated measures analyses using a mixed model approach29 were applied in three different models, each with either pain, depression or anxiety as dependent variables. In each model, the two of these variables that were not the dependent variable, were considered as potential explanatory variables together with physical function, disease activity (ESR) and damage (modified Sharp score). Age, sex, disease duration and RF, were entered as covariates in each of the three models. To examine the models for multicollinearity we used a pertubation analysis, where the influence of the single regressors was recorded revealing no abnormalities. The main advantages of the mixed model approach are that patients with missing data on single time‐points are retained in the analyses, and that unequal time intervals and high within‐patients correlation are handled appropriately.30 Polynomial models were tried out in the analysis, but it turned out that the association was linear as the higher order coefficients all were non‐significant.
The robustness of the findings was tested by repeating the analyses with different dependent variables within the same dimension of health. Similarly, we tested the pain model with different measures of physical function as explanatory variables.
The data were analysed using the Statistical package of Social Sciences (SPSS), version 12.0.1., and Statistical Analysis System (SAS) version 9.1.3. for the mixed models. The level of significance was set to 0.05 unless otherwise stated.
Results
Comparison between completers and non‐completers
The patients who completed the follow‐up were younger than non‐completers (mean (SD) 50.2 (12.5) vs.54.6 (13.4) years, p = 0.01) and less physically disabled (mean (SD) HAQ score 0.86 (0.61) vs. 1.05 (0.67), p = 0.03). Completers and non‐completers were comparable for disease duration (mean (SD) 2.2 (1.2) vs. 2.3 (1.1) years), gender (females 76% vs. 70%), positive RF (69% vs. 67%), ESR (mean (SD) 25.3 (19.7) vs.27.0 (19.9) mm/h), VAS pain score (mean (SD) 31.4 (24.5) vs. 35.8 (24.2), AIMS depression score (mean (SD) 2.0 (1.7) vs. 2.4 (1.7)) and AIMS anxiety score (mean (SD) 3.1 (2.1) vs. 3.5 (2.0)) Completers and living non‐completers had similar levels for all health status variables indicating that differences in health status between completers and non‐completers could be due to deceased patients (data not shown).
Level of pain, psychological, social and physical health status
On a group level, the patients maintained their health status with respect to pain, psychological and social function, whereas physical disability increased slightly from 5 to 10 years, and the radiographic damage increased steadily over the entire 10‐year period (table 1). However, pain, psychological, social and physical function for individual patients fluctuated considerably over time, as indicated by moderate correlation coefficients (from 0.3 to 0.7) between the scores over time (data not shown).
Table 1 Course of rheumatoid arthritis over 10 years.
| Mean scores (SD) | Baseline | 1 year | 2 years | 5 years | 10 years | p‡ |
|---|---|---|---|---|---|---|
| (n = 149) | (n = 149) | (n = 145)* | (n = 137)† | (n = 149) | ||
| Pain, global scores | ||||||
| Pain VAS (0–100) | 31.4 (24.5) | 28.7 (24.4) | 30.3 (24.4) | 29.6 (21.1) | 33.6 (23.2) | 0.99 |
| Pain AIMS (0–10) | 4.6 (2.4) | 4.3 (2.5) | 4.4 (2.5) | 4.6 (2.3) | 4.7 (2.4) | 0.99 |
| AIMS impact (0–10) | 3.8 (1.9) | 3.3 (2.3) | 3.6 (2.2) | 3.7 (2.1) | 3.9 (2.4) | 0.28 |
| Physician global (0–100) | – | – | 32.6 (20.4) | 16.1 (17.9) | 22.5 (16.4) | 0.01 |
| Psychosocial function | ||||||
| AIMS depression (0–10) | 2.0 (1.7) | 1.7 (1.5) | 1.8 (1.5) | 1.9 (1.6) | 2.0 (1.7) | 0.43 |
| AIMS anxiety (0–10) | 3.1 (2.1) | 2.7 (1.9) | 2.7 (1.9) | 2.9 (1.9) | 2.8 (2.0) | 0.96 |
| AIMS social activity (0–10) | 4.0 (1.7) | 3.7 (1.7) | 3.8 (1.9) | 3.7 (1.8) | 3.7 (1.8) | 0.07 |
| GHQ somatisation (0–21) | 7.5 (3.9) | 7.3 (4.3) | 7.2 (4.1) | 6.3 (3.7) | 6.1 (3.7) | 0.99 |
| GHQ depression (0–21) | 2.3 (3.4) | 2.0 (3.4) | 1.6 (2.7) | 2.0 (3.7) | 1.8 (3.2) | 0.004 |
| GHQ anxiety (0–21) | 6.7 (4.0) | 5.9 (4.4) | 6.3 (4.4) | 5.8 (4.6) | 5.3 (3.9) | 0.99 |
| GHQ social (0–21) | 8.0 (3.4) | 8.1 (3.3) | 8.2 (3.2) | 7.8 (2.8) | 8.1 (3.1) | 0.92 |
| GHQ‐28 (0–28) | 5.9 (6.0) | 5.6 (6.1) | 5.4 (6.0) | 4.8 (6.2) | 4.6 (5.8) | 0.21 |
| GHQ‐28 >11 (%) | 17.0 | 16.9 | 17.9 | 14.9 | 15.9 | |
| Physical function | ||||||
| HAQ (0–3) | 0.86 (0.61) | 0.85 (0.62) | 0.85 (0.65) | 0.86 (0.60) | 0.91 (0.70) | 0.03 |
| AIMS physical (0–10) | 1.8 (1.4) | 1.6 (1.2) | 1.8 (1.4) | 2.0 (1.3) | 2.1 (1.7) | 0.99 |
| Grip strength, average (kg) | 20.6 (12.2) | 20.5 (12.3) | 20.6 (12.8) | 20.0 (12.3) | 19.8 (11.7) | 0.99 |
| Disease activity | ||||||
| Ritchie articular index (0–78) | 9.16 (5.6) | 8.1 (6.3) | 8.5 (6.5) | 5.8 (6.3) | 13.0 (9.6) | 0.99 |
| Erythrocyte sedimentation rate | 25.3 (19.7) | 21.2 (15.0) | 19.8 (15.1) | 18.9 (15.1) | 17.8 (12.3) | 0.99 |
| C‐reactive protein | 11.5 (16.5) | 8.0 (11.2) | 9.9 (12.0) | 12.6 (8.8) | 7.6 (8.7) | 0.99 |
| Radiographic damage | ||||||
| Modified Sharp (0–280)§ | 7.2 (12.7) | 10.8 (16.0) | 14.5 (18.9) | 24.7 (27.5) | 36.0 (36.6) | 0.02 |
*Four of the 149 completers missed the 2 years data and †12 of the 149 completers missed the 5 years data.
‡Repeated measures analyses of variance (ANOVA), p‐values corrected with use of Simes' procedure.
§9–15% missed hand radiographs the four first time points, only 1% missed hand radiographs at the 10‐year examination.
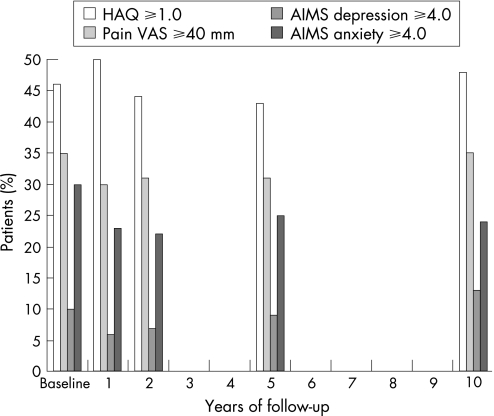
The stability of pain over time on a group level was demonstrated both for pain VAS and AIMS (table 1). At all points in the time during the 10‐year follow‐up 30–35% of the patients had VAS pain scores ⩾40 mm (fig 2).
Figure 2 Proportions of patients (%) with clinically important affection in disability, pain, depression and anxiety (n = 149).
Psychological health status was also stable over 10 years, except for the GHQ depression subscale score, which improved during follow‐up (table 1). AIMS depression score ⩾4.0 was seen in 9.7% of patients at baseline, in 5.4% at 1‐year follow‐up and in 12.6% at 10‐year follow‐up. At baseline, 30% of the patients had increased levels in AIMS anxiety score (⩾4.0), whereas the proportions during follow‐up varied between 23% and 25% (fig 2). Of the patients 15–18% could be characterised as “psychological distressed” during follow‐up based on the GHQ‐28 (score >11) (table 1). Using Simes' procedure for correcting p‐values in the testing of multiple hypothesis only one (AIMS social activity) of the five initially statistically significant findings in table 1 disappeared.
Women had higher depression and anxiety scores than males at every follow‐up examination (statistically significance difference for AIMS depression at baseline, AIMS anxiety at 10‐year follow‐up and for GHQ anxiety at baseline) (data not shown).
Explanation of pain
Anxiety, female gender, disease activity (ESR), and physical function (grip strength) independently explained the course of pain over 10 years (table 2). AIMS depression and modified Sharp score, although, in the initial model, were not significant contributors. Replacement of VAS pain with AIMS pain as the dependent variable did not alter the results. As an example of interpretation, VAS pain increased with 3.9 mm and AIMS pain increased with 0.33 units for each unit increase in AIMS anxiety. We also replaced grip strength by HAQ‐score, and then by AIMS physical, and we achieved similar associations as those presented in table 2.
Table 2 Explanatory factors for the longitudinal course of pain.
| VAS pain (0–100) | AIMS pain (0–10) | |||
|---|---|---|---|---|
| Beta (SE Beta) | p‐value | Beta (SE Beta) | p‐value | |
| Anxiety (AIMS, units) | 3.90 (0.39) | <0.0001 | 0.33 (0.04) | <0.0001 |
| Grip strength (kg) | −0.71 (0.10) | <0.0001 | −0.08 (0.01) | <0.0001 |
| ESR (mm/h) | 0.24 (0.04) | <0.0001 | 0.03 (0.005) | <0.0001 |
| Sex (female versus male) | 12.99 (2.69) | 0.0001 | 1.24 (0.28) | 0.0001 |
The results after repeated measures analyses using a mixed model approach adjusted for age, rheumatoid factor and disease duration. AIMS depression and modified Sharp score, although, in the initial model, were not statistically significant.
Explanation of depression
Depression, measured both with AIMS and GHQ, were longitudinally explained by the level of anxiety, physical function (grip strength) and age (table 3). High disease activity (ESR) was additionally an independent explanatory factor for AIMS depression, while RF positivity was an explanatory factor for depression measured with the GHQ. Modified Sharp score and AIMS pain, although in the initial model, were not significant contributors to the course of depression.
Table 3 Explanatory factors for the longitudinal course of depression.
| AIMS depression (0–10) | GHQ depression (0–21) | |||
|---|---|---|---|---|
| Beta (SE Beta) | p‐value | Beta (SE Beta) | p‐value | |
| Anxiety (AIMS or GHQ, units) | 0.65 (0.02) | <0.0001 | 0.46 (0.02) | <0.0001 |
| Grip strength (kg) | −0.008 (0.004) | 0.04 | −0.04 (0.01) | 0.0002 |
| ESR (mm/h) | 0.005 (0.002) | 0.02 | NS | |
| Age (years) | 0.01 (0.003) | 0.0003 | 0.04 (0.01) | <0.0001 |
| Sex (female vs. male) | NS | 0.65 (0.35) | 0.06 | |
| Rheumatoid factor (RF+ versus RF−) | NS | 0.75 (0.28) | 0.001 | |
The results after repeated measures analyses using a mixed model approach adjusted for disease duration. Modified Sharp score and AIMS pain, although in the initial model, were not statistically significant.
Explanation of anxiety
Level of depression was longitudinally associated with the course of anxiety. However, low disease activity was also an independent explanatory factor (table 4). These results were similar if either AIMS anxiety or GHQ anxiety was used as the dependent variable in the model. The level of pain was associated to the course of anxiety measured by AIMS, but not GHQ. Male gender was longitudinally associated with anxiety measured with AIMS, but not with GHQ, whereas an inverse association was found between age and GHQ anxiety (but not with AIMS anxiety) (table 4). Grip strength and modified Sharp score were not associated to the course of anxiety.
Table 4 Explanatory factors for the longitudinal course of anxiety.
| AIMS anxiety (0–10) | GHQ anxiety (0–21) | |||
|---|---|---|---|---|
| Beta (SE Beta) | p‐value | Beta (SE Beta) | p‐value | |
| Depression (AIMS or GHQ, units) | 0.87 (0.02) | <0.0001 | 0.72 (0.03) | <0.0001 |
| ESR (mm/h) | −0.007 (0.002) | 0.04 | −0.02 (0.007) | 0.001 |
| Pain (AIMS, units) | 0.09 (0.01) | 0.0001 | NS | |
| Sex (female versus male) | −0.29 (0.12) | 0.01 | NS | |
| Age (years) | NS | −0.05 (0.05) | 0.0001 | |
The results after repeated measures analyses using a mixed model approach adjusted for rheumatoid factor and disease duration. Grip strength and modified Sharp score, although in the initial model, were not statistically significant.
Discussion
The patients on a group‐level maintained their health status during the observation period (1992–2002), in which traditional DMARDs were the predominant disease modifying treatment options. At all time points during the 10‐year follow‐up about one‐third of the patients had clinically important levels of pain, and a larger proportion of patients had clinically important increased levels of anxiety (20–30%) than depression (5–13%). The perceived level of pain was longitudinally explained by anxiety, disease activity, physical function and female gender, but not by depression. Anxiety and depression were longitudinally interrelated, but the level of physical functioning as well as age were also independent explanatory factors of the course of depression. Radiographic damage was not longitudinally associated to any of the primary outcomes in these analyses, but have previously been shown to be associated to the course of physical disability in the same cohort of patients.5
The fact that the patients on a group level maintained their pain and psychological health status in spite of progression in radiographic damage and physical disability confirms the “well being homeostasis” described by Cummins31 and corroborates with findings in other RA studies.6,24 In agreement with Hawley and Wolfe,24 we found little change in the mean psychological scores over years, although individual variability in anxiety and depression scores from time to time was observed. The percentage of patients with depression varied from 9.7% at baseline, to 5.4% at 1‐year follow‐up increasing to 12.6% at 10‐year follow‐up. In a meta‐analysis Dickens et al10 concluded that major depressive disorders affect between 13 and 17% of patients with RA. We found that the mean and median levels of the AIMS anxiety scores were higher than the AIMS depression scores during the 10‐year follow‐up, and this difference has also been found by others cross‐sectionally or over shorter follow‐up periods.24,26,32 There is no general agreement on clinical important cut‐off point for the AIMS anxiety subscale, but we decided to use ⩾4 as the cut‐off point for anxiety, i.e. the same as for the AIMS depression subscale.25
No other study has explored the longitudinal influence of both depression and anxiety on pain in a 10‐year perspective in RA. In an observational study over 3 years Hawley and Wolfe24 found that depression was longitudinally associated with pain, whereas we found that anxiety was independently and longitudinally associated with pain. For clinicians, both anxiety and depression seem to be associated to pain and we therefore addressed the aspect of multicollinearity in the statistical analyses without finding a reason to change the model. We did not find depression to be a significant contributor in the initial model of longitudinal associations. In addition to anxiety we found female gender, physical function and disease activity to be explanatory factors for pain longitudinally. These results support multifactorial causes of pain and the importance of a comprehensive understanding of factors influencing chronic pain. Thus, patients with RA suffering from refractory pain should be managed in a multidisciplinary clinical setting accounting for the multifactorial nature of pain perception.33
In accordance with our findings, El‐Miedany and El‐Rasheed32 described that depression was an independent explanatory factor for anxiety and vice versa, and that grip strength was an independent explanatory factor for depression. In our study, disease activity (ESR) was independently and longitudinally associated with depression, in agreement with previous findings.9,34 On the other hand, the level of disease activity was inversely related to the course of anxiety. The present results indicate that the relationship between inflammatory activity and psychological health status is weak and also inconsistent.32,35,36
Some studies have demonstrated that women are more prone to high depression scores compared with men37; however, it is not clear that this connotes higher psychological distress or less of a reporting bias.38,39 We found that this gender difference was statistically significant only for AIMS depression at baseline.
Longitudinal studies lasting more than 10 years have shown that the physical functioning of patients with RA deteriorates with time.1,2,3,4,40,41,42 Most of the patients in our cohort received medical treatment for RA before baseline examination explaining the absence of the typical “J‐shaped” curve with an initial fall in HAQ scores followed by an increase the following years.43,44 The average annual increase of 0.01 units in HAQ score between 5 and 10 years is comparable with results from studies reporting the least average annual increase in patient‐reported disability levels.40,45,46,47
The most important strengths in this study are the longitudinal design with five waves of data collection and the comprehensive data collection. The results appeared to be consistent across different instruments within the same dimension, but less so for anxiety than for pain and depression.
The numbers of missing data except from radiographs were below 10% at each time point. Another limitation is that swollen joint counts were not assessed at baseline and at 1‐year follow‐up preventing us from using DAS28 as a marker for disease activity in the longitudinal analyses. Thus we used ESR, which was available at all time points. Because fatigue was not included in the protocol when we initiated the study in 1991 we were unfortunately unable to address the endpoint of fatigue, which has been considered very relevant and important by the patients who have attended the patients' perspective workshop on outcome assessments at the OMERACT meetings.
In conclusion, this 10‐year observational study showed that the patients with RA on a group level maintained their health status regarding pain and psychological health in spite of progression in radiographic damage. The longitudinal analyses contribute to the understanding of the complexity of explanatory factors for the course of pain and psychological health. It is well accepted that pain is a major symptom in patients with RA, and this study demonstrates that a number of disease and demographic factors contribute to the course of pain. The clinical interpretation of this finding is that a broad and multidisciplinary approach is required for the appropriate management of pain in patients with RA suffering from refractory pain. These study results further suggest that anxiety may be a more frequent health problem than depression in patients with RA, and perhaps also more important as anxiety but not depression was independently associated to the course of pain.
Abbreviations
AIMS - Arthritis Impact Measurement Scales
DMARD - disease‐modifying antirheumatic drugs
ESR - erythrocyte sedimentation rate
GHQ - General Health Questionnaire
HAQ - Health Assessment Questionnaire RA, rheumatoid arthritis
RF - rheumatoid factor
VAS - visual analogue scale
Footnotes
Funding: This study has been financed with grants from the Norwegian Women Public Health Association and the Norwegian Foundation for Health and Rehabilitation.
Competing interests: None.
References
- 1.Capell H A, Murphy E A, Hunter J A. Rheumatoid arthritis: workload and outcome over 10 years. Q J Med 199179461–476. [PubMed] [Google Scholar]
- 2.Drossaers‐Bakker K W, De Buck M, Van Zeben D, Zwinderman A H, Breedveld F C, Hazes J M. Long‐term course and outcome of functional capacity in rheumatoid arthritis: the effect of disease activity and radiologic damage over time. Arthritis Rheum 1999421854–1860. [DOI] [PubMed] [Google Scholar]
- 3.Gordon P, West J, Jones H, Gibson T. A 10 year prospective followup of patients with rheumatoid arthritis 1986–96. J Rheumatol 2001282409–2415. [PubMed] [Google Scholar]
- 4.Lindqvist E, Saxne T, Geborek P, Eberhardt K. Ten year outcome in a cohort of patients with early rheumatoid arthritis: health status, disease process, and damage. Ann Rheum Dis 2002611055–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ødegård S, Landewé R, Van Der Heijde D, Kvien T K, Mowinckel P, Uhlig T. Association of early radiographic damage with impaired physical function in rheumatoid arthritis: a ten‐year, longitudinal observational study in 238 patients. Arthritis Rheum 20065468–75. [DOI] [PubMed] [Google Scholar]
- 6.Strating M M, Suurmeijer T P, Van Schuur W H. Disability, social support, and distress in rheumatoid arthritis: results from a thirteen‐year prospective study. Arthritis Rheum 200655736–744. [DOI] [PubMed] [Google Scholar]
- 7.Heiberg T, Kvien T K. Preferences for improved health examined in 1,024 patients with rheumatoid arthritis: pain has highest priority. Arthritis Rheum 200247391–397. [DOI] [PubMed] [Google Scholar]
- 8.Mckenna F, Wright V. Pain and rheumatoid arthritis. Ann Rheum Dis 198544805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel‐Nasser A M, Abd El‐Azim S, Taal E, El‐Badawy S A, Rasker J J, Valkenburg H A. Depression and depressive symptoms in rheumatoid arthritis patients: an analysis of their occurrence and determinants. Br J Rheumatol 199837391–397. [DOI] [PubMed] [Google Scholar]
- 10.Dickens C, McGowan L, Clark‐Carter D, Creed F. Depression in rheumatoid arthritis: a systematic review of the literature with meta‐analysis. Psychosom Med 20026452–60. [DOI] [PubMed] [Google Scholar]
- 11.Pincus T. Psychological factors and rheumatoid arthritis. Int J Adv Rheumatol. 2003;1,58–63.
- 12.Smedstad L M, Moum T, Vaglum P, Kvien T K. The impact of early rheumatoid arthritis on psychological distress. a comparison between 238 patients with RA and 116 matched controls. Scand J Rheumatol 199625377–382. [DOI] [PubMed] [Google Scholar]
- 13.Clark D A, Steer R A, Beck A T. Common and specific dimensions of self‐reported anxiety and depression: implications for the cognitive and tripartite models. J Abnorm Psychol 1994103645–654. [PubMed] [Google Scholar]
- 14.Wittchen H U, Kessler R C, Pfister H, Lieb M. Why do people with anxiety disorders become depressed? A prospective‐longitudinal community study. Acta Psychiatr Scand 2000(Suppl)14–23. [PubMed]
- 15.Smedstad L M, Vaglum P, Kvien T K, Moum T. The relationship between self‐reported pain and sociodemographic variables, anxiety, and depressive symptoms in rheumatoid arthritis. J Rheumatol 199522514–520. [PubMed] [Google Scholar]
- 16.Smedstad L M, Moum T, Guillemin F, Kvien T K, Finch M B, Suurmeijer T P.et al Correlates of functional disability in early rheumatoid arthritis: a cross‐sectional study of 706 patients in four European countries. Br J Rheumatol 199635746–751. [DOI] [PubMed] [Google Scholar]
- 17.Meenan R F, Gertman P M, Mason J H. Measuring health status in arthritis. The Arthritis Impact Measurement Scales. Arthritis Rheum 198023146–152. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg D. Use of the General Health Questionnaire in clinical work. Br Med J (Clin Res Ed) 19862931188–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg D P, Hillier V F. A scaled version of the General Health Questionnaire. Psychol Med 19799139–145. [DOI] [PubMed] [Google Scholar]
- 20.Fries J F, Spitz P, Kraines R G, Holman H R. Measurement of patient outcome in arthritis. Arthritis Rheum 198023137–145. [DOI] [PubMed] [Google Scholar]
- 21.Ritchie D M, Boyle J A, Mcinnes J M, Jasani M K, Dalakos T G, Grieveson P.et al Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med 196837393–406. [PubMed] [Google Scholar]
- 22.Escalante A, Haas R W, Del R I. Measurement of global functional performance in patients with rheumatoid arthritis using rheumatology function tests. Arthritis Res Ther 20046R315–R325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rødland E A. Simes' procedure is “Valid On Average”. Biometrika 200693742–746. [Google Scholar]
- 24.Hawley D J, Wolfe F. Anxiety and depression in patients with rheumatoid arthritis: a prospective study of 400 patients. J Rheumatol 198815932–941. [PubMed] [Google Scholar]
- 25.Hawley D J, Wolfe F. Depression is not more common in rheumatoid arthritis: a 10‐year longitudinal study of 6,153 patients with rheumatic disease. J Rheumatol 1993202025–2031. [PubMed] [Google Scholar]
- 26.Söderlin M k, Hakala M, Nieminen P. Anxiety and depression in a community‐based rheumatoid arthritis population. Scand J Rheumatol 200029177–183. [DOI] [PubMed] [Google Scholar]
- 27.Uhlig T, Kvien T K, Glennas A, Smedstad L M, Forre O. The incidence and severity of rheumatoid arthritis, results from a county register in Oslo, Norway. J Rheumatol 1998251078–1084. [PubMed] [Google Scholar]
- 28.Wiles N J, Scott D G, Barrett E M, Merry P, Arie E, Gaffney K.et al Benchmarking: The five year outcome of rheumatoid arthritis assessed using a pain score, the Health Assessment Questionnaire, and the Short Form‐36 (SF‐36) in a community and a clinic based sample. Ann Rheum Dis 200160956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzmaurice G, Laird N, Ware J.Applied longitudinal analysis. New York: Wiley, 2004187–234.
- 30.Symmons D P. Methodological issues in conducting and analyzing longitudinal observational studies in rheumatoid arthritis. J Rheumatol 200469(Suppl)30–34. [PubMed] [Google Scholar]
- 31.Cummins R A. Objective and subjective quality of life: an interactive model. Social Indicators Res 20005255–72. [Google Scholar]
- 32.El‐Miedany Y M, El‐Rasheed A H. Is anxiety a more common disorder than depression in rheumatoid arthritis? Joint Bone Spine 200269300–306. [DOI] [PubMed] [Google Scholar]
- 33.Strand E B, Zautra A J, Thoresen M, Ødegård S, Uhlig T, Finset A. Positive affect as a factor of resilience in the pain‐negative affect relationship in patients with rheumatoid arthritis. J Psychosom Res 200660477–484. [DOI] [PubMed] [Google Scholar]
- 34.Mindham R H, Bagshaw A, James S A, Swannell A J. Factors associated with the appearance of psychiatric symptoms in rheumatoid arthritis. J Psychosom Res 198125429–435. [DOI] [PubMed] [Google Scholar]
- 35.Gardiner B M. Psychological aspects of rheumatoid arthritis. Psychol Med 198010159–163. [DOI] [PubMed] [Google Scholar]
- 36.Mcfarlane A C, Brooks P M. An analysis of the relationship between psychological morbidity and disease activity in rheumatoid arthritis. J Rheumatol 198815926–931. [PubMed] [Google Scholar]
- 37.Dowdy S W, Dwyer K A, Smith C A, Wallston K A. Gender and psychological well‐being of persons with rheumatoid arthritis. Arthritis Care Res 19969449–456. [DOI] [PubMed] [Google Scholar]
- 38.Kvien T K, Uhlig T, Ødegård S, Heiberg M S. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci 20061069212–222. [DOI] [PubMed] [Google Scholar]
- 39.Tousignant M, Brosseau R, Tremblay L. Sex biases in mental health scales: do women tend to report less serious symptoms and confide more than men? Psychol Med 198717203–215. [DOI] [PubMed] [Google Scholar]
- 40.Leymarie F, Jolly D, Sanderman R, Briancon S, Marchand A C, Guillemin F.et al Life events and disability in rheumatoid arthritis: a European cohort. Br J Rheumatol 1997361106–1112. [DOI] [PubMed] [Google Scholar]
- 41.Pincus T, Callahan L F, Sale W G, Brooks A L, Payne L E, Vaughn W K. Severe functional declines, work disability, and increased mortality in seventy‐five rheumatoid arthritis patients studied over nine years. Arthritis Rheum 198427864–872. [DOI] [PubMed] [Google Scholar]
- 42.Rasker J J, Cosh J A. The natural history of rheumatoid arthritis: a fifteen year follow‐up study. The prognostic significance of features noted in the first year. Clin Rheumatol 1984311–20. [DOI] [PubMed] [Google Scholar]
- 43.Kobelt G, Jonsson L, Lindgren P, Young A, Eberhardt K. Modeling the progression of rheumatoid arthritis: a two‐country model to estimate costs and consequences of rheumatoid arthritis. Arthritis Rheum 2002462310–2319. [DOI] [PubMed] [Google Scholar]
- 44.Wiles N, Dunn G, Barrett E, Silman A, Symmons D. Associations between demographic and disease‐related variables and disability over the first five years of inflammatory polyarthritis: a longitudinal analysis using generalized estimating equations. J Clin Epidemiol 200053988–996. [DOI] [PubMed] [Google Scholar]
- 45.Callahan L F, Pincus T, Huston JW I I I, Brooks R H, Nance E P., Jr Kaye JJ. Measures of activity and damage in rheumatoid arthritis: depiction of changes and prediction of mortality over five years, Arthritis Care Res 199710381–394. [DOI] [PubMed] [Google Scholar]
- 46.Ward M M, Leigh J P, Fries J F. Progression of functional disability in patients with rheumatoid arthritis. Associations with rheumatology subspecialty care. Arch Intern Med 19931532229–2237. [PubMed] [Google Scholar]
- 47.Ward M M, Lubeck D, Leigh J P. Longterm health outcomes of patients with rheumatoid arthritis treated in managed care and fee‐for‐service practice settings. J Rheumatol 199825641–649. [PubMed] [Google Scholar]