Abstract
Objectives
To compare disease activity and the improvement of disease activity evaluated between by Disease Activity Score 28 using erythrocyte sedimentation rate (DAS28‐ESR) and by DAS28 using C‐reactive protein (DAS28‐CRP) in Japanese patients with rheumatoid arthritis (RA).
Methods
Data from 3073 RA patients registered in the large cohort database (NinJa: National Database of Rheumatic Diseases by iR‐net in Japan) of 2003 was used to calculate DAS28‐ESR and DAS28‐CRP and disease activities were evaluated. Improvements in disease activities were also evaluated according to the European League Against Rheumatism (EULAR) response criteria in 1482 RA patients whose DAS28‐ESR and DAS28‐CRP could be calculated from data for both 2002 and 2003.
Results
The mean value of DAS28‐CRP (3.59, SD 1.25) was significantly smaller than that of mean DAS28‐ESR (4.31, SD 1.32) (p < 0.0001). The number of patients who satisfied the criteria of remission was 297 (9.7%) in DAS28‐ESR versus 705 (22.9%) in DAS28‐CRP and the number of patients with high disease activity was 842 (27.4%) versus 357 (11.6%) for DAS28‐ESR and DAS28‐CRP, respectively; there was a significant difference between the two (p < 0.0001). Change of respective DAS28 was significantly correlated (ΔDAS28‐ESR −0.05, SD 1.14 versus ΔDAS28‐CRP −0.10, SD 1.10) (p < 0.0001); however, the number of “good response” patients was significantly different (p < 0.03) between DAS28‐ESR (97 patients, 6.5%) and DAS28‐CRP (136 patients, 9.2%).
Conclusions
DAS28‐CRP significantly underestimated disease activity and overestimated the improvement in disease activity compared with DAS28‐ESR. DAS28‐CRP should be evaluated using different criteria from that for DAS28‐ESR.
At present, the Disease Activity Score (DAS) is a major scoring system for evaluating disease activity of rheumatoid arthritis (RA). The initial development of DAS was reported by van der Heijde et al in 19901 and 1992,2 and then DAS was modified by a group of investigators from the Netherlands.3,4,5 The use of DAS is officially recommended by the European League Against Rheumatism (EULAR) for evaluating disease activity and the improvement in disease activity in clinical trials and also in daily clinical practice.
For reasons of convenience, the modified DAS including 28‐joint count (DAS28),3 instead of the original DAS based on the Ritchie articular index and 44‐swollen joint count,1,2 was proposed and the response criteria and the remission criteria for DAS28 were also reported.6 DAS28 is calculated according to the formula that is composed of the number of tender joints and swollen joints, patient's global assessment of disease activity on a visual analogue scale (VAS), and erythrocyte sedimentation rate (ESR), not C‐reactive protein (CRP). ESR tends to reflect disease activity of the past few weeks, whereas CRP reflects more short‐term changes in disease activity.7 Therefore, the advantage of CRP is that it is more sensitive to short‐term changes in disease activity. Furthermore, ESR can be influenced by confounding factors such as age, sex, fibrinogen levels, hypergammaglobulinemia, rheumatoid factor, and anemia.8,9 For these reasons, DAS28 using CRP instead of ESR was recently proposed by Fransen et al.10 and presented on the DAS website11 (To avoid confusion, we describe “DAS28‐ESR” for DAS28 using ESR and “DAS28‐CRP” for DAS28 using CRP in this paper.)
It was stated on the DAS website that the formula to calculate DAS28‐CRP gives a good estimation of DAS28‐ESR values on a group level,11 whereas it was noted in the EULAR handbook of clinical assessments in RA that “as the agreement between ESR and CRP is imperfect, disease activity scores calculated with ESR and CRP will deviate to some extent in most individuals”.12 At present, the criteria of disease activity and the response criteria for DAS28‐ESR are applied to DAS28‐CRP; however, no reports have so far validated them.
NinJa (National Database of Rheumatic Diseases by iR‐net in Japan; www.ninja‐ra.jp)13 is a nationwide cohort database of rheumatic diseases in Japan, and has collected data since 2002. For the database of RA, core information about disease activity (the number of tender and swollen joints, patient's and doctor's VAS, ESR, CRP, and modified health assessment questionnaire; mHAQ), treatment, complications, operation, and the incidence of malignancy and tuberculosis have been collected once a year. As of March 2006, 33 hospitals around Japan were participating in data collection and approximately 4000 RA patients had been registered.
In this study, we compared disease activity and the improvement in disease activity evaluated by DAS28‐ESR and by DAS28‐CRP, and examined whether DAS28‐CRP can be evaluated by the same criteria as DAS28‐ESR in Japanese patients with RA using data from NinJa.
Methods
Data source
The data source employed was a nationwide observation cohort database of rheumatic diseases in Japan named NinJa.13,14NinJa started data collection in 2002, which has been performed annually. At the end of March in 2006, 33 institutions that are located throughout Japan and almost all of which are members of iR‐net (Division of Rheumatology, Immunologic Disorder Network, National Hospital Organization in Japan), were participating in NinJa. The annual data for each patient with RA consist of two components; one component is about information during the year (outcome, death, hospitalization, operation, malignancy, and tuberculosis), and the other component is about information collected on an arbitrary day in daily clinical practice (the number of tender and swollen joints, mHAQ, patient's global and pain VAS, doctor's VAS, ESR, CRP, DA28‐ESR, DAS28‐CRP, use of corticosteroids, disease‐modifying antirheumatic drugs, and non‐steroidal anti‐inflammatory drugs). All the hospitals participating in NinJa used a CRP test with a lower detection level of 1.0 mg/l. The CRP test was calibrated using a standardized method (CRM 470 of the International Federation of Clinical Chemistry). ESR was measured by the Westergren method.
So far, data of 1928 patients in NinJa 2002 (data for the period April 2002 to March 2003) and of 4026 patients in NinJa 2003 (data for the period April 2003 to March 2004) have been collected and analysed. The NinJa project was reviewed and approved by the National Hospital Organization research ethics committees, and all patients participating in the study provided informed consent.
Patients
For the cross‐sectional study (evaluation of disease activity), data of 3073 patients in NinJa 2003, which had both DAS28‐ESR and DAS28‐CRP, were utilized. Of the 3073 patients included in the study, 2583 (84.1%) were women, with a mean age (SD) of 61.1 (11.4) years (range 18–89), and mean disease duration (SD) of 13.5 (10.6) years (range 0–56). Most of the patients (n = 2638, 86.9%) were taking disease‐modifying antirheumatic drugs, 2046 patients (66.6%) were taking corticosteroids, and 2315 patients (75.3%) were using non‐steroidal anti‐inflammatory drugs.
For the longitudinal study (evaluation of the improvement in disease activity), 1482 patients whose DAS28‐ESR and DAS28‐CRP could be evaluated in both NinJa 2002 and 2003 were selected. Of the 1482 patients, 1270 (85.7%) were women, with a mean age (SD) of 61.7 (10.6) years (range 19–89), and mean disease duration (SD) of 15.7 (10.7) years (range 0–56) for the database of 2003.
Calculation and evaluation of disease activity and improvement in disease activity
According to the formula on the DAS website,11 DAS28‐ESR and DAS28‐CRP were calculated and both DAS28 values were categorized as follows: > 5.1, high disease activity; ⩽ 3.2, low disease activity; and < 2.6, remission. Improvement in disease activity was evaluated according to EULAR response criteria.6
Statistics
DAS28‐ESR and DAS28‐CRP values were compared by calculating correlation coefficients and by linear regression analysis. Bland–Altman plots were generated for assessment of the variation between DAS28‐ESR and DAS28‐CRP.15 Comparisons of the number of patients with each disease activity or with improvement in disease activity evaluated by DAS28‐ESR and by DAS28‐CRP were performed using Student's t‐test and the χ2 test. Data processing and analyses were conducted using SPSS software (Windows release 11.0; SPSS Inc., Chicago, Illinois, USA).
Results
Comparison of disease activity between DAS28‐ESR and DAS28‐CRP
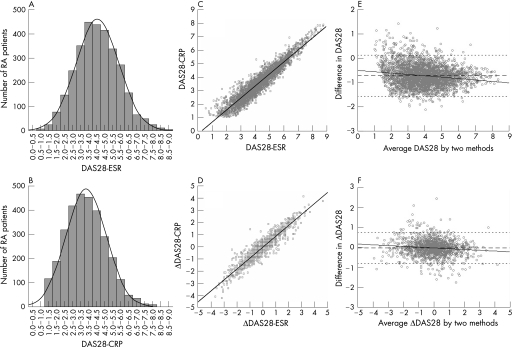
DAS28‐ESR and DAS28‐CRP values of 3073 patients in NinJa 2003 were normally distributed (fig 1A and B), although the peak of DAS28‐CRP was shifted to the left compared with that of DAS28‐ESR. The means (SD) of DAS28‐ESR and DAS28‐CRP were 4.31 (1.32) and 3.59 (1.25), respectively (table 1), and these differences were statistically significant (p < 0.0001). Linear regression analysis showed a significant correlation between DAS28‐ESR and DAS28‐CRP (DAS28‐CRP = 0.893 × DAS28‐ESR − 0.253, R2 = 0.893; p < 0.0001) (fig 1C). The Bland–Altman analysis showed that as the average value of DAS28 increases, the difference between DAS28‐CRP and DAS28‐ESR (DAS28‐CRP minus DAS28‐ESR) becomes larger (r = −0.059, 95% CI −0.070 to −0.047, p < 0.0001) (fig 1E).
Figure 1 Distribution, correlation and difference of DAS28‐ESR and DAS28‐CRP. Distribution of DAS28‐ESR (A) and DAS28‐CRP (B) scores from 3073 patients with rheumatoid arthritis in NinJa 2003. Correlation of the values of DAS28‐ESR and DAS28‐CRP from 3073 patients in NinJa 2003 (C), and correlation of the changes of DAS28‐ESR and DAS28‐CRP scores (Δ: score of 2003 minus that of 2002) from 1482 patients whose DAS28‐ESR and DAS28‐CRP could be evaluated in both NinJa 2002 and 2003 (D). Solid line indicates the regression line of DAS28‐CRP on DAS28‐ESR (C, D). Bland–Altman plot analysis of the absolute values of DAS28 (E) and the changes of DAS28 scores (F). The ordinate scale represents the difference between DAS28‐CRP and DAS28‐ESR (i.e. DAS28‐CRP minus DAS28‐ESR). The abscissa scale represents the average of DAS28‐ESR and DAS28‐CRP (i.e. DAS28‐ESR plus DAS28‐CRP divided by 2). The middle dashed line indicates the bias (mean difference), and the upper and lower dotted lines represent the limits of agreement (mean ± 2 SD). The solid line indicates the regression line of difference on average.
Table 1 Characteristics of patients in the cross‐sectional and longitudinal studies.
| Cross‐sectional NinJa 2003 | Longitudinal NinJa 2002–2003 | ||
|---|---|---|---|
| 2002 | 2003 | ||
| Patients (n) | 3073 | 1482 | |
| Age (years; mean [SD]) | 61.1 [11.4] | 61.7* [10.6] | |
| Women (%) | 2583 (84.1%) | 1270 (85.7%) | |
| Disease duration (years; mean [SD]) | 13.5 [10.6] | 15.7* [10.7] | |
| Disease activity score (mean [SD]) | |||
| DAS28‐ESR | 4.31† [1.32] | 4.29† [1.22] | 4.24† [1.30] |
| DAS28‐CRP | 3.59 [1.25] | 3.61[1.20] | 3.51 [1.23] |
| Disease activity characteristics (median [first; third quartile]) | |||
| Tender joint count (0–28) | 2 [0; 4] | 2 [1; 5] | 2 [1; 5] |
| Swollen joint count (0–28) | 1 [0; 4] | 2 [0; 5] | 1 [0; 4] |
| ESR (mm/h; normal <20) | 39 [21; 60] | 38 [20; 58] | 40 [22; 60] |
| CRP (mg/dl; normal <0.4) | 0.78 [0.23;2.09] | 0.82 [0.27; 2.30] | 0.72 [0.22; 1.98] |
| mHAQ score (range 0–3) | 0.50 [0.10; 1.10] | 0.50 [0.12; 1.00] | 0.60 [0.12; 1.25] |
| Patient's pain VAS (10 cm) | 3.7 [1.9; 5.5] | 3.4 [3.4; 5.4] | 3.8 [2.0; 5.8] |
| Patient's global VAS (10 cm) | 4.0 [2.0; 5.6] | 3.8 [2.0; 5.8] | 4.1 [2.1; 5.7] |
| Doctor's global VAS (10 cm) | 2.9 [1.6; 4.7] | 3.1 [2.0; 4.9] | 3.1 [1.9; 4.9] |
CRP, C‐reactive protein; DAS28, Disease Activity Score 28; ESR, erythrocyte sedimentation rate; VAS, visual analogue scale.
*Age and disease duration at the time of data collection in 2003.
†The mean value of DAS28‐ESR was significantly larger than that of DAS28‐CRP (p < 0.0001).
The difference in the mean values between DAS28‐ESR (4.36 [SD 1.31]) and DAS28‐CRP (3.61 [SD 1.26]) in women was statistically larger than that in men (4.02 [SD 1.41] versus 3.50 [SD 1.25], p < 0.0001; table 2). Furthermore, differences in the mean values between DAS28‐ESR and DAS28‐CRP tended to be larger as disease duration and age increased (table 2).
Table 2 Effects of sex, disease duration, and age on the differences between DAS28‐ESR and DAS28‐CRP.
| n (%) | DAS28‐ESR | DAS28‐CRP | Difference (z) | |
|---|---|---|---|---|
| Sex | ||||
| Women | 2583 (84.1) | 4.36 [1.31]* | 3.61 [1.26] | 0.75** |
| Men | 490 (15.9) | 4.02 [1.41] | 3.50 [1.25] | 0.52 |
| Disease duration (x) (years, women) | ||||
| 0∼2 | 306 (11.8) | 4.22 [1.42] | 3.50 [1.37] | 0.72 |
| 3∼5 | 385 (14.9) | 4.14 [1.31] | 3.41 [1.26] | 0.73 |
| 6∼10 | 469 (18.2) | 4.29 [1.26] | 3.57 [1.20] | 0.72 |
| 11∼15 | 441 (17.1) | 4.54 [1.34] | 3.79 [1.26] | 0.75 |
| 16∼20 | 336 (13.0) | 4.42 [1.24] | 3.64 [1.20] | 0.78 |
| 21∼25 | 246 (9.5) | 4.43 [1.24] | 3.63 [1.24] | 0.80 |
| 26∼? | 400 (15.5) | 4.49 [1.27] | 3.69 [1.24] | 0.80 |
| Disease duration (x) (years, men) | ||||
| 0∼2 | 104 (21.2) | 4.09 [1.42] | 3.59 [1.28] | 0.50 |
| 3∼5 | 79 (16.1) | 3.70 [1.57] | 3.34 [1.30] | 0.36 |
| 6∼10 | 118 (24.1) | 3.94 [1.32] | 3.42 [1.13] | 0.52 |
| 11∼15 | 66 (13.4) | 4.22 [1.49] | 3.67 [1.43] | 0.55 |
| 16∼20 | 40 (8.2) | 4.39 [1.35] | 3.74 [1.24] | 0.65 |
| 21∼25 | 40 (8.2) | 3.84 [1.27] | 3.23 [1.15] | 0.61 |
| 26∼? | 43 (8.8) | 4.20 [1.31] | 3.59 [1.18] | 0.61 |
| Age (y) (years, women) | ||||
| ∼40 | 159 (6.2) | 4.16 [1.41] | 3.56 [1.31] | 0.60 |
| 41∼50 | 228 (8.8) | 4.26 [1.37] | 3.55 [1.28] | 0.71 |
| 51∼60 | 780 (30.2) | 4.32 [1.29] | 3.56 [1.25] | 0.76 |
| 61∼70 | 911 (35.3) | 4.41 [1.30] | 3.64 [1.25] | 0.77 |
| 71∼80 | 443 (17.2) | 4.47 [1.26] | 3.68 [1.23] | 0.79 |
| 81∼? | 62 (2.4) | 4.39 [1.23] | 3.57 [1.17] | 0.82 |
| Age (y) (years, men) | ||||
| ∼40 | 23 (4.7) | 3.39 [1.63] | 3.38 [1.24] | 0.01 |
| 41∼50 | 38 (7.8) | 3.79 [1.43] | 3.41 [1.21] | 0.38 |
| 51∼60 | 118 (24.1) | 4.05 [1.33] | 3.63 [1.13] | 0.42 |
| 61∼70 | 171 (34.9) | 4.01 [1.46] | 3.44 [1.32] | 0.57 |
| 71∼80 | 127 (25.9) | 4.26 [1.34] | 3.60 [1.25] | 0.66 |
| 81∼ | 13 (2.7) | 3.38 [1.25] | 2.71 [1.30] | 0.67 |
CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate.
*Mean [SD].
†Differences in the mean values between DAS28‐ESR and DAS28‐CRP in women were statistically larger than those in men (p < 0.0001)
x, Disease duration; y, age; z, difference between DAS28‐ESR and DAS28‐CRP.
Women: z = 0.0024x + 0.718 (R2 = 0.004, p = 0.002), z = 0.0041y + 0.502 (R2 = 0.013, p < 0.001).
Men: z = 0.0059x + 0.456 (R2 = 0.013, p = 0.01), z = 0.0141y – 0.369 (R2 = 0.100, p < 0.001).
The number of patients categorized by each criteria of disease activity, i.e. remission (DAS28 < 2.6) and low disease activity criteria (DAS28 ⩽ 3.2) included 297 (9.7%) and 653 (21.2%) patients, respectively, evaluated by DAS28‐ESR, whereas there were 705 (22.9%) and 1218 (39.6%) patients, respectively, by DAS28‐CRP. On the other hand, patients categorized by high disease activity criteria (DAS28 > 5.1) included 842 (27.4%) patients by DAS28‐ESR criteria, and 357 (11.6%) patients by DAS28‐CRP criteria (table 3). These differences were statistically significant (p < 0.0001).
Table 3 Comparison of disease activity between DAS28‐ESR and DAS28‐CRP.
| DAS28‐CRP (y) | ||||||
|---|---|---|---|---|---|---|
| y<2.6 | 2.6⩽y⩽3.2 | 3.2<y⩽5.1 | 5.1<y | Total (%) | ||
| DAS28‐ESR (x) | x<2.6 | 282 (9.2) | 13 (0.4) | 2 (0.07) | 0 | 297 (9.7)* |
| 2.6⩽x⩽3.2 | 251 (8.2) | 83 (2.7) | 22 (0.72) | 0 | 356 (11.6%) | |
| 3.2<x⩽5.1 | 172 (5.6) | 417 (13.6) | 986 (32.1) | 3 (0.98) | 1578 (51.4) | |
| 5.1<x | 0 | 0 | 488 (15.9) | 354 (11.5) | 842 (27.4)* | |
| Total (%) | 705 (22.9) | 513 (16.7) | 1498 (48.7) | 357 (11.6) | 3073 | |
CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate.
*The number of patients with remission and high disease activity evaluated by DAS28‐ESR was significantly different from that by DAS28‐CRP (p < 0.0001).
Comparison of the improvement in disease activity between DAS28‐ESR and DAS28‐CRP
The improvement in disease activity was examined using data from 1482 patients whose DAS28‐ESR and DAS28‐CRP could be evaluated in both NinJa 2002 and 2003. Linear regression analysis showed a significant correlation between the differences in DAS28 values in each DAS28 between NinJa 2002 and 2003 (p < 0.0001), which could be fitted to the equation: ΔDAS28‐CRP = 0.902 × ΔDAS28‐ESR − 0.053, R2 = 0.879 (fig 1D). The means (SD) of ΔDAS28‐ESR and ΔDAS28‐CRP were −0.05 (1.14) and −0.10 (1.10), respectively, and the difference was statistically significant (p < 0.0001). The Bland–Altman analysis showed that as an absolute value of the average of ΔDAS28 increases, that of the difference between ΔDAS28‐CRP and ΔDAS28‐ESR (ΔDAS28‐CRP minus ΔDAS28‐ESR) becomes larger (r = −0.039, 95% CI −0.057 to −0.020, p < 0.0001) (fig 1F).
The improvements in disease activity evaluated by DAS28‐ESR and DAS28‐CRP were compared using the EULAR response criteria (table 4). The numbers of “moderate” and “no” response patients were comparable between DAS28‐ESR and DAS28‐CRP; however, the number of “good response” patients was significantly different (p < 0.03) between DAS28‐ESR (97 patients, 6.5%) and DAS28‐CRP (136 patients, 9.2%).
Table 4 Comparison of the improvement of disease activity between DAS28‐ESR and DAS28‐CRP.
| DAS28‐CRP | |||||
|---|---|---|---|---|---|
| Good | Moderate | No response | Total (%) | ||
| DAS28‐ESR | Good | 71 (4.8) | 20 (1.4) | 6 (0.4) | 97 (6.5)* |
| Moderate | 64 (4.3) | 178 (12.0) | 40 (2.7) | 282 (19.0%) | |
| No response | 1 (0.07) | 85 (5.7) | 1017 (68.6) | 1103 (74.4) | |
| Total (%) | 136 (9.2) | 283 (19.1) | 1063 (71.7) | 1482 | |
CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate.
*The number of patients with “good” response evaluated by DAS28‐ESR was significantly smaller than that by DAS28‐CRP (p < 0.03).
Relationship between ESR and CRP
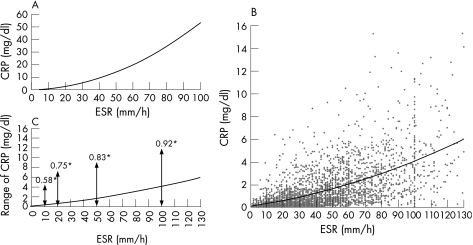
To address the differences between DAS28‐ESR and DAS28‐CRP noted above, the formula of DAS28‐ESR was compared with that of DAS28‐CRP. If DAS28‐ESR and DAS28‐CRP were equivalent, that is, the relationship of 0.70 × ln (ESR) = 0.36 × ln (CRP + 1) + 0.96 (CRP: mg/l) held true, the relationship between ESR and CRP would be as shown in Figure 2A. The curve on Figure 2A indicated that an ESR of 50 mm/h is approximately equivalent to a CRP of 14 mg/dl. Next, we plotted the raw values of ESR and CRP of patients tested above (fig 2B). A best‐fit procedure suggested a curvilinear relationship (CRP = 0.0002 × ESR2 + 0.0214 × ESR + 0.1014, R2 = 0.372, p < 0.0001), and an ESR of 50 mm/h was approximately equivalent to a CRP of 1.7 mg/dl. However, the actual relationship between ESR and CRP varies. For example, an ESR of 50 mm/h can be equivalent to 0 or 9 mg/dl CRP, which according to the DAS28‐CRP formula indicates an absolute difference in DAS28‐CRP of 0.83 (fig 2C).
Figure 2 Relationship between erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP). Plot of the relationship between ESR and CRP in the condition that the correlation formula of DAS28‐ESR and DAS28‐CRP was correct, that is, the relationship of 0.70 × ln (ESR) = 0.36 × ln (CRP + 1) + 0.96 (CRP: mg/l) held true (A). Plot of the distribution of ESR and CRP in rheumatoid arthritis patients tested in this study (B). The solid line indicates the curvilinear regression line of ESR and CRP (CRP = 0.0002 × ESR2 + 0.0214 × ESR + 0.1014, R2 = 0.372, p < 0.0001). Range of CRP for a representative value of ESR in patients (C). Solid line is the same as drawn in (B). *Absolute difference in DAS28‐CRP while ESR is fixed at 10, 20, 50, and 100 mm/h.
Discussion
In this study, using NinJa, a large observational cohort of RA patients in Japan, we showed that values of DAS28‐CRP are significantly lower than those of DAS28‐ESR, and that it is inappropriate to evaluate DAS28‐CRP using the criteria of evaluation for DAS28‐ESR. In several previous studies, although the backgrounds of patients were diverse, both DAS28‐ESR and DAS28‐CRP were calculated and the mean values were presented as follows: DAS28‐ESR versus DAS28‐CRP: 6.29 (SD 1.16) versus 5.97 (SD 1.05) in early RA,16 2.89 (SD 1.00) versus 2.67 (SD 0.84) in stable RA, and 4.73 (SD 1.18) versus 4.44 (SD 1.08) in active RA;17 the medians (first and third quartile) were 4.09 (2.99; 5.17) versus 3.78 (2.71; 4.82) in a routine cohort study and 5.62 (4.81; 6.44) versus 4.67 (4.04; 5.50) in an inception cohort study.18 Although DAS28‐CRP tends to be lower than DAS28‐ESR, except in the original paper that reported the development of DAS28‐CRP (DAS28‐ESR versus DAS28‐CRP: 4.38 [SD 1.47] versus 4.41 [SD 1.27]),10 surprisingly, whether the criteria of disease activity and the response criteria for DAS28‐ESR could be applied to DAS28‐CRP has never been questioned.
As stated above, the values of DAS28‐ESR and those of DAS28‐CRP were comparable in the original report; however, it is not clear why other studies including ours did not show similar results. It may be that the relationship between ESR and CRP is different among patients examined in different studies. ESR is well known to be affected by sex, age, immunoglobulin levels, and abnormal size or shape of red blood cells,8,9 whereas CRP is less affected by these factors. Therefore, the relationship between ESR and CRP might differ with various factors, such as the ratio of women to men, age, disease activity (which has an influence on anemia), and the ratio of patients with Sjögren's syndrome that often accompanies hypergammaglobulinemia in RA patients in such studies. Our results support the findings that sex, disease duration, and age could affect the relationship between DAS28‐ESR and DAS28‐CRP (table 2). It is interesting, but not clear, whether the race of patients also has an influence on this relationship. Further investigations will be needed.
In the two previous reports about the relationship between ESR and CRP in RA, similar results were obtained; an ESR of 48 mm/h approximated to a CRP of 2.45 mg/dl19 and to 2.7 mg/dl.20 On the basis of these results, it is not acceptable to propose a relationship between ESR and CRP when an ESR of 50 mm/h is almost equivalent to a CRP of 14 mg/dl, if the formula of DAS28‐ESR and that of DAS28‐CRP are equivalent (fig 2A). As noted, it may be inappropriate to compare DAS28‐CRP with DAS28‐ESR directly. However, our results show that there is a significant correlation between DAS28‐ESR and DAS28‐CRP (fig 1C) and that DAS28‐CRP values are systematically shifted to the left compared with DAS28‐ESR (fig 1A and B). Therefore, DAS28‐CRP can be a more useful and reliable score if the appropriate cut‐off points of disease activity and those of response criteria for DAS28‐CRP are set. As noted above, DAS28‐CRP was developed by the modification of DAS28‐ESR, which had also previously been developed by modification of the original DAS. Similarly, the criteria of disease activity and the response criteria for DAS28‐ESR had been proposed by modification of those of the original DAS, which was developed in a cohort of patients in the early stages of RA. Our study also has a tendency to validate DAS28‐CRP because there is no external standard, such as treatment changes or the patient's or the doctor's perspective. Therefore, the original criteria of disease activity and the original response criteria for DAS28‐CRP should be studied and validated among various kinds of RA patient groups.
Although the changes of DAS28 values in DAS28‐ESR and DAS28‐CRP were very similar and were significantly correlated (fig 1D), DAS28‐CRP overestimated the EULAR response criteria compared with DAS28‐ESR (table 3). This may be because of differences in the current values of DAS28. Unlike American College of Rheumatology improvement criteria,21 the EULAR response criteria include changes in disease activity as well as current disease activity. Therefore, revising the threshold of disease activity may make it possible to evaluate the improvement in disease activity of DAS28‐CRP.
In conclusion, both DAS28‐ESR and DAS28‐CRP are useful outcome measures in RA; however, DAS28‐CRP significantly underestimated disease activity and overestimated the improvement in disease activity compared with DAS28‐ESR. DAS28‐CRP should be evaluated using different criteria from those for DAS28‐ESR.
Acknowledgements
The authors wish to acknowledge the assistance of the following clinicians who have referred patients to NinJa 2002 and 2003: Dr Kenji Ichikawa, Dr Norio Tamura, Dr Makoto Sueishi, Dr Hajime Yamagata, Dr Toshihito Mori, Dr Atsuhito Seki, Dr Takuo Juji, Dr Akio Mimori, Dr Yukihiko Saeki, Dr Kunikazu Ogawa, Dr Kazuhito Shinohara, Dr Yusuke Ohta, Dr Akira Okamoto, Dr Yasuhiko Yoshinaga, Dr Akinori Matsumori, Dr Hisaaki Miyahara, Dr Eiichi Suematsu, Dr Satoru Motokawa, Dr Shigeru Yoshizawa, and Dr Yoshiki Shiohira.
Abbreviations
CRP - C‐reactive protein
DAS28 - Disease Activity Score 28
ESR -
erythrocyte sedimentation rate -
mHAQ - modified health assessment questionnaire
RA - rheumatoid arthritis
VAS - visual analogue scale
Footnotes
Sources of support: This work was supported in part by grants from the Ministry of Health, Labour and Welfare, Japan.
References
- 1.van der Heijde D M, van't Hof M A, van Riel P L, Theunisse L A, Lubberts E W, van Leeuwen M A.et al Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis 199049916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Heijde D M, van't Hof M A, van Riel P L, van Leeuwen M A, van Rijswijk M H, van de Putte L B. Validity of single variables and composite indices for measuring disease activity in rheumatoid arthritis. Ann Rheum Dis 199251177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevoo M L, van't Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B, van Riel P L. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 4.van Gestel A M, Prevoo M L, van't Hof M A, van Rijswijk M H, van de Putte L B, van Riel P L. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 19963934–40. [DOI] [PubMed] [Google Scholar]
- 5.Prevoo M L, van Gestel A M, van T Hof M A, van Rijswijk M H, van de Putte L B, van Riel P L. Remission in a prospective study of patients with rheumatoid arthritis. American Rheumatism Association preliminary remission criteria in relation to the disease activity score. Br J Rheumatol 1996351101–1105. [DOI] [PubMed] [Google Scholar]
- 6.Welsing P M, van Riel P L. The Nijmegen inception cohort of early rheumatoid arthritis. J Rheumatol 200431(Suppl 69)14–21. [PubMed] [Google Scholar]
- 7.van Leeuwen M A, van Rijswijk M H, van der Heijde D M, Te Meerman G J, van Riel P L, Houtman P M.et al The acute‐phase response in relation to radiographic progression in early rheumatoid arthritis: a prospective study during the first three years of the disease. Br J Rheumatol 199332(Suppl 3)9–13. [DOI] [PubMed] [Google Scholar]
- 8.Talstad I, Scheie P, Dalen H, Roli J. Influence of plasma proteins on erythrocyte morphology and sedimentation. Scand J Haematol 198331478–484. [DOI] [PubMed] [Google Scholar]
- 9.Kushner I. C‐reactive protein in rheumatology. Arthritis Rheum 1991341065–1068. [DOI] [PubMed] [Google Scholar]
- 10.Fransen J, Welsing P M, de Keijzer R M, van Riel P L. Disease activity scores using C‐reactive protein: CRP may replace ESR in the assessment of RA disease activity [abstract]. Ann Rheum Dis 200462(Suppl 1)151 [Google Scholar]
- 11.DAS‐SCORE NL. Home of the DAS: Disease activity score in rheumatoid arthritis, http://www.das‐score.nl/www.das‐score.nl/index.html (accessed 15 February 2007)
- 12.van Riel P L. ed. Disease activity. EULAR handbook of clinical assessment in rheumatoid arthritis. The Netherlands: Van Zuiden Communications BV, 200437
- 13.NinJa: National Database of Rheumatic Disease by iR‐net in Japan. http://www.ninja‐ra.jp/C_TOP‐E.html (accessed 15 February 2007)
- 14.Yamanaka H, Tohma S. Potential impact of observational cohort studies in Japan on rheumatoid arthritis research and practice. Mod Rheumatol 20061675–76. [DOI] [PubMed] [Google Scholar]
- 15.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 19861307–310. [PubMed] [Google Scholar]
- 16.Bathon J M, Martin R W, Fleischmann R M, Bingham C O, Whitmore J B, Eickenhorst T. Disease activity scores using CRP versus ESR and the relationship between EULAR and ACR responses in patients with early rheumatoid arthritis [abstract]. Ann Rheum Dis 200564(Suppl 3)173 [Google Scholar]
- 17.Soubrier M, Zerkak D, Gossec L, Ayral X, Roux C, Dougados M. Which variables best predict change in rheumatoid arthritis therapy in daily clinical practice? J Rheumatol 2006331243–1246. [PubMed] [Google Scholar]
- 18.Aletaha D, Nell V P, Stamm T, Uffmann M, Pflugbeil S, Machold K.et al Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 20057R796–R806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfe F. Comparative usefulness of C‐reactive protein and erythrocyte sedimentation rate in patients with rheumatoid arthritis. J Rheumatol 1997241477–1485. [PubMed] [Google Scholar]
- 20.Paulus H E, Ramos B, Wong W K, Ahmed A, Bulpitt K, Park G.et al Equivalence of the acute phase reactants C‐reactive protein, plasma viscosity, and Westergren erythrocyte sedimentation rate when used to calculate American College of Rheumatology 20% improvement criteria or the Disease Activity Score in patients with early rheumatoid arthritis. Western Consortium of Practicing Rheumatologists. J Rheumatol 1999262324–2331. [PubMed] [Google Scholar]
- 21.Felson D T, Anderson J J, Boers M, Bombardier C, Furst D, Goldsmith C.et al American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 199538727–735. [DOI] [PubMed] [Google Scholar]