Abstract
Objectives
Regulatory T cells (Tregs) exert their anti‐inflammatory activity predominantly by cell contact‐dependent mechanisms. A study was undertaken to investigate the regulatory capacity of autologous peripheral blood Tregs in contact with synovial tissue cell cultures, and to evaluate their presence in peripheral blood, synovial tissue and synovial fluid of patients with rheumatoid arthritis (RA).
Methods
44 patients with RA and 5 with osteoarthritis were included in the study. The frequency of interferon (IFN)γ‐secreting cells was quantified in synovial tissue cell cultures, CD3‐depleted synovial tissue cell cultures, synovial tissue cultures co‐cultured with autologous CD4+ and with CD4+CD25+ peripheral blood T cells by ELISPOT. Total CD3+, Th1 polarised and Tregs were quantified by real‐time PCR for CD3ε, T‐bet and FoxP3 mRNA, and by immunohistochemistry for FoxP3 protein.
Results
RA synovial tissue cell cultures exhibited spontaneous expression of IFNγ which was abrogated by depletion of CD3+ T cells and specifically reduced by co‐culture with autologous peripheral blood Treg. The presence of Treg in RA synovitis was indicated by FoxP3 mRNA expression and confirmed by immunohistochemistry. The amount of FoxP3 transcripts, however, was lower in the synovial membrane than in peripheral blood or synovial fluid. The T‐bet/FoxP3 ratio correlated with both a higher grade of synovial tissue lymphocyte infiltration and higher disease activity.
Conclusion
This study has shown, for the first time in human RA, the efficacy of autologous Tregs in reducing the inflammatory activity of synovial tissue cell cultures ex vivo, while in the synovium FoxP3+ Tregs of patients with RA are reduced compared with peripheral blood and synovial fluid. This local imbalance of Th1 and Treg may be responsible for repeated rheumatic flares and thus will be of interest as a target for future treatments.
Rheumatoid arthritis (RA) is characterised by autoimmune phenomena and destructive polyarticular arthritis with hyperplasia of the synovial lining cells, neovascularisation and leucocyte infiltration of the sub‐lining forming distinct anatomical patterns of secondary lymphoid tissues.1 The predominance of a Th1 lymphocyte profile together with defective peripheral immune tolerance appears to be pivotal in the pathogenesis of RA.2 High ratios of interferon γ (IFNγ) to interleukin 4 (IL‐4) have been found in synovial tissue and synovial fluid from patients with RA,3,4 while IFNγ/IL‐4 ratios were comparably low in the peripheral blood of patients with active disease.5
Various regulatory CD4+ T cell (Treg) subsets have been described in human pathology, including natural CD4+CD25+ naïve Treg,6 antigen‐induced CD4+CD25+ effector/memory Treg,7 IL‐10‐producing Tr18 and transforming growth factor β (TGF‐β)‐producing Th3 cells.9 Anti‐inflammatory mediators such as IL‐10 and TGF‐β may be abundantly present in inflamed RA joints, but they obviously fail to control the disease process sufficiently.10,11 Cellular peripheral tolerance mechanisms appear attractive for targeted treatment in autoimmunity.12,13 In comparison to a healthy state, Tregs in the peripheral blood are already reduced in early active RA,14 suggesting compromised Treg function in RA. In line with this observation, transfer of CD4+CD25+ Tregs markedly slowed down disease progression in the collagen‐induced arthritis model15 and in bone marrow transplanted children with juvenile idiopathic arthritis.16 However, high numbers of Tregs have been reported in the synovial fluid of patients with RA,17,18,19 and impaired Treg function in established active RA could be restored by successful blockade with tumour necrosis factor (TNF).20,21 These observations, together with resistance of synovial effector T cells against Treg, put the therapeutic options of Treg expansion and transfer not only in RA into perspective.22 In this investigation we found a potent regulatory effect of autologous peripheral blood Treg on synovial tissue cultures of patients with RA, which led us to search again for Treg in RA joints including inflamed tissue.
Patients and methods
Patients
Forty‐four patients with RA who fulfilled the revised American College of Rheumatology (ACR) criteria23 were consecutively recruited for the study when undergoing joint surgery. Synovial tissue from five patients with osteoarthritis was used as a control. The study protocol was approved by the Frankfurt University Hospital ethics committee and all patients gave their written informed consent. The characteristics of the patients with RA are summarised in table 1.
Table 1 Characteristics of patients with RA.
| Number of patients (M/F) | 44 (4/40) |
| Median (range) age (years) | 59 (22–80) |
| Duration of disease (years) | 10 (0.5–42) |
| RF (% positive; n = 38) | 85 |
| DAS 28 (n = 43)24 | 3.14 (1.07–5.5) |
| DMARD (%) | 76.7 |
| Anti‐TNF treatment (%) | 18.6 |
| Glucocorticoids (%) | 88.4 |
| NSAID or coxibs (%) | 60 |
| No treatment | 1/44 |
Unless otherwise indicated, values are given as median (range).
RF, rheumatoid factor; DAS, disease activity; DMARD, disease‐modifying antirheumatic drug; TNF, tumour necrosis factor; NSAID, non‐steroidal anti‐inflammatory drugs.
Drugs indicate current pharmacological treatment.
Peripheral blood and synovial fluid mononuclear cell preparation
Specimens for pairwise analyses were simultaneously obtained by vein puncture needle aspiration of the joint immediately before arthrotomy. Fluids were collected in NH4‐heparin containing S‐Monovettes (Sarstedt, Germany). Peripheral blood mononuclear cells and synovial fluid mononuclear cells were isolated by Ficoll Hypaque (Biochrom, Germany) density gradient centrifugation.
Preparation of synovial tissue for functional assays was done rapidly by injecting 1 mg/ml collagenase (Sigma‐Aldrich, Germany) into synovial tissue samples and incubating for 20 min at ambient temperature. The synovial tissue was subsequently minced and incubated for an additional 50 min at 37°C in collagenase 1%. The cell suspension was strained by 70 μm nylon filters (Falcon, USA), washed twice in phosphate‐buffered saline (PBS) and cultured in HAM F 10 medium (Invitrogen, Germany) containing 5% fetal calf serum and 1% penicillin/streptomycin at 37°C in 5% CO2 atmosphere over night.
Magnetic bead activated cell sorting
Synovial tissue cell cultures were CD3‐depleted using anti‐CD3 monoclonal antibody‐coated magnetic beads (Miltenyi Biotec, Gladbach, Germany) according to the manufacturer's instructions. A rosette kit (StemCell Technologies, USA) was applied for isolation of CD4+ peripheral blood T cells (fig 1) and CD25+ cells were enriched with an automated magnetic bead activated cell sorting system (autoMACS, Miltenyi Biotec, Germany). T cells were stained with 10 μl anti‐CD4‐PE/CY5, anti‐CD3‐ECD, anti‐CD45‐FITC (all BD Pharmingen, USA) or anti‐CD25‐PE (Miltenyi Biotec, Germany), incubated for 10 min at room temperature and measured by flow cytometry (Beckman Coulter, USA).
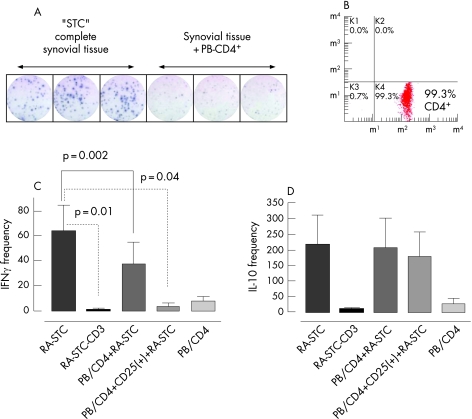
Figure 1 Regulatory effect of autologous peripheral blood (PB) T cells on synovial tissue cells (STC) in patients with rheumatoid arthritis (RA). (A) Representative interferon γ (IFNγ) ELISPOT experiment, done in triplicate, with substantial reduction of spontaneously IFNγ‐secreting cells/104 STC in the presence of PB CD4+ T cells. Each blue dot represents a single cytokine‐secreting cell. (B) Flow cytometry of PB T cells showing the purity of untouched CD4+ PB T cells selected with the rosette technique. (C) Quantitative analyses of spontaneously IFNγ‐secreting cells/104 STC. (D) Quantitative analyses of spontaneously IL‐10‐secreting cells per 104 STC. Columns represent mean (SE) values of 13 and 5 patients with RA, respectively, each examined at least in duplicate. CD3 depletion abolished IFNγ and IL‐10 secretion almost completely (n = 3). Co‐culture of STC with CD4+ led to a moderate reduction in IFNγ secreting cells and co‐culture of STC with CD4+CD25+ PB T cells led to a robust reduction in IFNγ secreting cells (n = 16).
ELISPOT assays
Synovial tissue cultures and peripheral blood mononuclear cell fractions were seeded in equal densities of 104 cells per well in sterile polyvinylidene fluoride microtitre plates (Millipore, USA) pre‐coated with anti‐IFNγ or anti‐IL‐10 antibody (Mabtech AB, Sweden) and cultured for 36 h at 37°C in a 5% CO2 atmosphere. Biotin‐labelled secondary monoclonal antibodies were added after removing the cell layer and thorough washing. Plates were dried for 2 h at ambient temperature and subsequently stained for 15 min with streptavidin alkaline phosphatase complexes and 5‐bromine‐4‐chlor‐3‐indoxylphos‐phate/nitroblue‐tetrazoliumchloride solution (BCIP/NBT, Mabtech, Sweden). Cytokine spots were quantified (A•EL•VIS GmbH, Hannover, Germany) and the mean spot number per well from duplicate or triplicate experiments was calculated. Net counts were established after background subtraction.
Real‐time PCR
Acknowledging the relevance of transcription factors for gene expression and differentiation of lymphocytes, T‐bet and FoxP3 transcripts were quantified. FoxP3, the most specific Treg marker,25 is present in naïve natural and memory Tregs.6,7 T‐bet is mainly expressed in differentiated Th1 cells and closely linked to IFNγ expression.26 In brief, synovial tissue was immediately stored in RNAlater (QIAGEN, Hilden, Germany), homogenised after removal from RNAlater, peripheral blood mononuclear cells were disrupted and total RNA was isolated with RNeasy Mini Kit (QIAGEN) or TRIzol Reagent (Invitrogen, USA). First strand cDNA synthesis was performed using 1 μg total RNA by Thermoscript RT (Invitrogen, USA). Quantitative PCR was performed by pre‐designed TaqMan gene expression assays (Applied Biosystems, California, USA) for human T‐bet (Hs00203436), FoxP3 (Hs00203958), CD3ε transcripts (Hs00167894), a constitutively expressed gene in CD3+ T cells, and eukaryotic 18S rRNA (4333760T). Probes were labelled with FAM/MGB fluorescent dye and amplified with Absolute QPCR ROX Mix (ABgene, Epsorn, UK) in an ABI Prism 7700 sequence detector (Applied Biosystems, California, USA) in 25 μl reaction volume. The expression was defined by calculating the mean cycle number of triplicate experiments.
Immunohistochemistry for FoxP3 in RA synovial tissue
Specimens were immediately embedded in OCT compound (TissueTek TT 4583; Sakura Finetech, Torrance, California, USA) and snap frozen in liquid nitrogen. Sections 7 μm thick were prepared, fixed in acetone, washed in ddH2O and subsequently in PBS. Endogenous peroxidase activity was blocked using 0.1% H2O2. Slides were incubated for 30 min in blocking solution (2% horse serum in PBS) and incubated overnight at 4°C with 10 μg/ml mouse monoclonal anti‐FoxP3 antibody (AbCam, Cambridge, UK) or an isotype‐matched mouse IgG1 antibody (DakoCytomation, Glostrup, Denmark). Sections were incubated for 30 min with biotinylated goat anti‐mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania, USA) secondary antibody in PBS. Immune complexes were stained with horseradish peroxidase‐conjugated streptavidin complex (Vectastain Elite ABC kit; Vector, Burlingame, California, USA) and aminoethylcarbazole chromogen substrate (DakoCytomation, Glostrup, Denmark). Nuclei were counterstained with haematoxylin.
Statistical analyses
The results are presented as mean (SE) unless otherwise stated. Group comparisons were calculated by the Mann‐Whitney U test for untailed groups and by the Wilcoxon signed rank test for tailed groups. The significance of correlations was estimated by the Pearson test. Statistical analyses were done with Graph Pad Prism 4.0 Software.
Results
Spontaneous expression of IFNγ and IL‐10 in synovial tissue cultures
ELISPOT assays allowed detection of IFNγ in synovial tissue cultures without artificial stimulation (fig 1A). The mean (SE) frequency of IFNγ spots was 65 (20)/104 cells (n = 13) in RA‐derived synovial tissue cultures and 7 (2.5)/104 cells in OA‐derived synovial tissue cultures (n = 5, p = 0.03). In contrast, the frequency of IL‐10‐secreting cells was similar in RA and OA synovial tissue cultures (218 (94)/104 cells (n = 5) vs 161 (86)/104 cells (n = 4), p = 0.9). CD3 depletion almost completely abrogated IFNγ production in RA synovial tissue cultures (mean frequency 1 (0.5)/104 cells per well (n = 3), p = 0.01), indicating that IFNγ in synovial tissue cultures was predominantly released by CD3+ T lymphocytes (fig 1C). CD3+ depletion also strongly reduced IL‐10‐secreting synovial tissue cultures (fig 1D).
Reduction of IFNγ secretion of synovial tissue cultures by autologous CD4+CD25+ peripheral blood T cells
“Untouched” CD4+ cells were isolated from peripheral blood (fig 1B) and co‐cultured with corresponding synovial tissue cultures. The number of intrinsically IFNγ‐secreting CD4+ peripheral blood T cells was low (8 (3)/104 cells per well), and co‐culture led to substantially reduced IFNγ frequency in autologous synovial tissue cultures (38 (17)/104 cells per well, p = 0.002); the regulatory effect was even more pronounced when enriched CD4+CD25+ Tregs were used (IFNγ spots 3.5 (2.5)/104 cells per well (n = 16), p<0.05, fig 1C). In contrast, spontaneous IL‐10 secretion in RA synovial tissue cultures was not significantly modified by co‐culture with total CD4+ or CD4+CD25+ cells (218 (94) vs 206 (96) vs 178 (79)/104 cells), while their intrinsic IL‐10 frequency (25 (19)/104 cells) was relatively low in CD4+ peripheral blood T cells (fig 1D).
Localisation of FoxP3‐positive Treg in synovitis
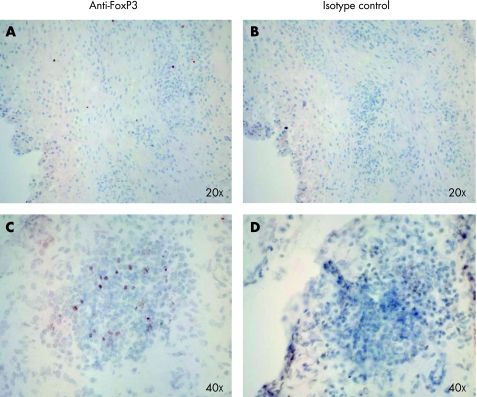
The remarkable regulatory capacity of autologous peripheral blood Tregs on synovial tissue cultures led us to search thoroughly for the presence of Tregs in RA synovial tissue. Immunohistochemistry revealed that Tregs were sparsely distributed throughout the entire sub‐lining tissue. FoxP3‐positive cells could also be detected at the basal layer of the hyperplastic synovial lining, and some clusters of these cells were seen in areas of lymphoid aggregates (fig 2).
Figure 2 Immunohistochemistry of FoxP3‐positive cells in rheumatoid arthritis synovial tissue. (A) FoxP3 staining was sparsely distributed in the sub‐lining tissue. (C) A tendency for higher numbers of Tregs was observed in synovial aggregates. (B) and (D) Control staining with an irrelevant primary antibody.
T‐bet, FoxP3 and CD3ε mRNA expression in RA synovium
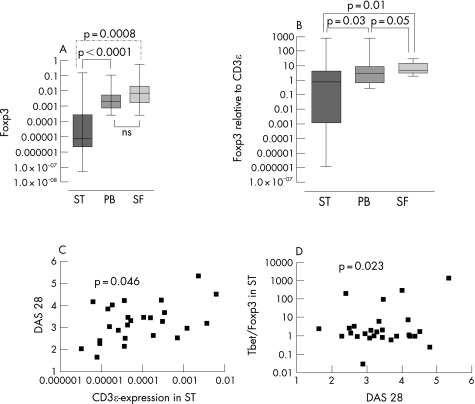
Quantitative analysis of transcription factors prototypic for Th1 and Treg showed a close correlation between the T‐bet/FoxP3 mRNA ratio and CD3ε levels in synovial tissue (r = 0.95; n = 32, p<0.0001). To clarify whether there was a preponderance of Th1 compared with Tregs exclusively in the synovial tissue, we divided the patients into two equivalent groups according to their high or low T‐bet/FoxP3 mRNA ratio in synovial tissue (n = 16; p<0.0001). We next looked at the corresponding ratios in synovial fluid and peripheral blood and found that the high T‐bet/FoxP3 ratio in synovial tissue was not seen in peripheral blood and synovial fluid samples (n = 8; p>0.05 for both). Confirming this imbalance in the group with a high T‐bet/FoxP3 ratio in the synovial tissue compartment, a significantly lower ratio was measured in the synovial fluid (n = 20, p = 0.014). The amount of FoxP3 transcripts in synovial tissue was significantly lower than in peripheral blood (p<0.0001) and synovial fluid (p = 0.0008) after normalisation to 18S rRNA and CD3ε transcripts (p = 0.03 vs peripheral blood, p = 0.01 vs synovial fluid; fig 3A, B).
Figure 3 Transcript quantification representing total (CD3ε), Th1 polarised (T‐bet) and regulatory T cells (FoxP3) in peripheral blood (PB), synovial tissue (ST) and synovial fluid (SF). (A) FoxP3 expression and (B) FoxP3 expression relative to CD3ε was significantly lower in ST than in PB and in SF. (C) The clinical disease activity score DAS 28 correlated with the grade of T cell infiltration in ST represented by CD3ε mRNA expression (r = 0.4, n = 31, p<0.05). (D) DAS 28 could also be correlated with the T‐bet/FoxP3 mRNA expression ratio in ST, implying an excess of Th1 cells over Treg in phases of high inflammatory activity (r = 0.4, n = 31, p = 0.02).
Correlation of synovial tissue T cell mRNA levels with clinical disease activity
CD3ε mRNA expression in synovial tissue correlated significantly with DAS 28 (r = 0.4, n = 31, p = 0.045, fig 3C). Indicating that most of these CD3ε positive cells may exhibit an inflammatory profile, DAS 28 also correlated with T‐bet/FoxP3 mRNA ratios (r = 0.4, n = 31, p = 0.02, fig 3D). Further comparison of mRNA levels with clinical and treatment data in our cohort revealed that disease duration had no influence on the expression of FoxP3. In addition, there was no correlation between anti‐TNF treatment or steroid dosage and the expression of FoxP3 or FoxP3/CD3ε in synovial tissue (r = −0.04, p = 0.8 for FoxP3; r = −0.01, p = 0.9 for FoxP3/CD3ε).
Discussion
This study has shown that IFNγ, a pro‐inflammatory mediator critically important for innate and adaptive immune processes, is expressed in primary synovial cell cultures predominantly by CD3+ T cells. Their frequency could be precisely quantified using the ELISPOT technique without applying artificial stimuli. The frequency of IFNγ (6/1000) and IL‐10 (20/1000) secreting T cells determined by this method in synovial tissue was in a similar range as was previously found by in situ detection methods,27 and our results in the peripheral blood show good agreement with a previous ELISPOT study.28
We observed reduced IFNγ secretion in synovial tissue cultures following co‐culture with peripheral blood Treg. Ongoing work will try to elucidate whether the naïve or memory phenotype of Treg is responsible for this key finding. In this context, we were able for the first time to show Treg in RA synovial tissue by FoxP3 immunohistochemistry, but found expression of FoxP3 transcripts significantly lower here than in synovial fluid or peripheral blood using quantitative transcript analyses. Our data are in agreement with a previous report of scarce CD25 expression in T cell infiltrates of synovial biopsies but a higher frequency of CD25+ T cells in the synovial fluid in active states of early arthritis.29 Following these data, a quantitative Treg deficiency in the RA synovium, either by restricted access or by impaired persistence in an inflamed milieu, can be postulated. Mechanisms potentially shortening the half‐life of Treg tissue have recently been published,21,30 but the cause of insufficient supplementation remains elusive. Exemplary flow cytometric analyses of synovial tissue culture also failed to reveal appreciable numbers of CD4+/CD25high cells, although these cells could easily be detected in corresponding peripheral blood samples (data not shown). As the staining of surface molecules might have been affected by the tissue preparation, we continued our quantitative analyses with intracellular Th1 and Treg markers. T‐bet expression is tightly correlated with IFNγ expression and migration properties of activated effector T cells.31 We found an increased T‐bet/FoxP3 transcript ratio in RA synovial tissues which could be correlated with the amount of CD3ε mRNA and clinical disease activity. These observations argue in favour of the hypothesis that CD3ε‐positive T cells infiltrating inflamed joint tissue underlie a selective distribution in favour of Th1 versus Tregs in active disease.
Treg‐mediated tolerance against arthritogenic peptides in humans requires cell‐cell contact.32 Prevention of disease in a murine model of type 1 diabetes was associated with Treg localisation in the target tissue or in draining lymph nodes, and their regulatory function was mediated by direct contact with dendritic cells.33,34 Alternative paracrine mechanisms of Treg function would require at least adjacent localisation.35 Tregs are thus required on the site of inflammation or the draining lymphoid tissue to display sufficient suppression, and their relative absence/disappearance in synovial tissue would be a sustainable explanation for the recurrent imbalance of Th1 and Treg activity leading to repeated disease flares in RA. Although the published data are complex and the molecular mechanisms may differ between natural naïve and effector/memory Tregs,36,37,38,39,40,41,42,43 therapeutic targeting of the migratory Treg appears to be an attractive goal.
Acknowledgements
The authors thank Martina Herrero San Juan for her continuous technical support during the years of this study.
Abbreviations
IFNγ - interferon γ
IL - interleukin
RA - rheumatoid arthritis
TNF - tumour necrosis factor
Treg - regulatory T cell
Footnotes
This work was supported by a Tandem grant of the Clinic of the Goethe University (awarded to FB and AH) and by funds of the Dr Hans Schleussner Foundation of Immune Pharmacology awarded to HHR.
Competing interests: None.
References
- 1.Weyand C M, Kang Y M, Kurtin P J, Goronzy J J. The power of the third dimension: tissue architecture and autoimmunity in rheumatoid arthritis. Curr Opin Rheumatol 200315259–266. [DOI] [PubMed] [Google Scholar]
- 2.Skapenko A, Leipe J, Lipsky P E, Schulze‐Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther 20057(Suppl 2)S4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berner B, Akca D, Jung T, Muller G A, Reuss‐Borst M A. Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J Rheumatol 2000271128–1135. [PubMed] [Google Scholar]
- 4.Yin Z, Siegert S, Neure L, Grolms M, Liu L, Eggens U.et al The elevated ratio of interferon gamma‐/interleukin‐4‐positive T cells found in synovial fluid and synovial membrane of rheumatoid arthritis patients can be changed by interleukin‐4 but not by interleukin‐10 or transforming growth factor beta. Rheumatology 1999381058–1067. [DOI] [PubMed] [Google Scholar]
- 5.Kawashima M, Miossec P. mRNA quantification of T‐bet, GATA‐3, IFN‐gamma, and IL‐4 shows a defective Th1 immune response in the peripheral blood from rheumatoid arthritis patients: link with disease activity. J Clin Immunol 200525209–214. [DOI] [PubMed] [Google Scholar]
- 6.Valmori D, Merlo A, Souleimanian N E, Hesdorffer C S, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest 20051151953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Jin W, Hardegen N, Lei K J, Li L, Marinos N.et al Conversion of peripheral CD4+25‐ naïve T cells to CD4+CD25+ regulatory T cells by TGF‐beta induction of transcription factor Foxp3. J Exp Med 20031981875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roncarolo M G, Bacchetta R, Bordignon C, Narula S, Levings M K. Type 1 T regulatory cells. Immunol Rev 200118268–79. [DOI] [PubMed] [Google Scholar]
- 9.Weiner H L. Induction and mechanism of action of transforming growth factor‐beta‐secreting Th3 regulatory cells. Immunol Rev 2001182207–214. [DOI] [PubMed] [Google Scholar]
- 10.Katsikis P D, Chu C Q, Brennan F M, Maini R N, Feldmann M. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med 19941791517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennan F M, Chantry D, Turner M, Foxwell B, Maini R, Feldmann M. Detection of transforming growth factor‐beta in rheumatoid arthritis synovial tissue: lack of effect on spontaneous cytokine production in joint cell cultures. Clin Exp Immunol 199081278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shevach E M. Regulatory/suppressor T cells in health and disease. Arthritis Rheum 2004502721–2724. [DOI] [PubMed] [Google Scholar]
- 13.van Roon J A, Bijlsma J W, Lafeber F P. Diversity of regulatory T cells to control arthritis. Best Pract Res Clin Rheumatol 200620897–913. [DOI] [PubMed] [Google Scholar]
- 14.Lawson C A, Brown A K, Bejarano V, Douglas S H, Burgoyne C H, Greenstein A S.et al Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology 2006451210–1217. [DOI] [PubMed] [Google Scholar]
- 15.Morgan M E, Flierman R, van Duivenvoorde L M, Witteveen H J, van E W, van Laar J M.et al Effective treatment of collagen‐induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum 2005522212–2221. [DOI] [PubMed] [Google Scholar]
- 16.de Kleer I, Vastert B, Klein M, Teklenburg G, Arkesteijn G, Yung G P.et al Autologous stem cell transplantation for autoimmunity induces immunologic self‐tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood 20061071696–1702. [DOI] [PubMed] [Google Scholar]
- 17.Cao D, Malmstrom V, Baecher‐Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol 200333215–223. [DOI] [PubMed] [Google Scholar]
- 18.van Amelsfort J M, Jacobs K M, Bijlsma J W, Lafeber F P, Taams L S. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum 2004502775–2785. [DOI] [PubMed] [Google Scholar]
- 19.Ruprecht C R, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med 20052011793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrenstein M R, Evans J G, Singh A, Moore S, Warnes G, Isenberg D A.et al Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti‐TNFalpha therapy. J Exp Med 2004200277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valencia X, Stephens G, Goldbach‐Mansky R, Wilson M, Shevach E M, Lipsky P E. TNF downmodulates the function of human CD4+CD25hi T‐regulatory cells. Blood 2006108253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Boehmer H. Can studies of tolerance ever lead to therapy? Ann Rheum Dis 200665(Suppl 3)iii41–iii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 24.Prevoo M L, Kuper I H, van't Hof M A, van Leeuwen M A, van de Putte L B, van Riel P L. Validity and reproducibility of self‐administered joint counts. A prospective longitudinal followup study in patients with rheumatoid arthritis. J Rheumatol 199623841–845. [PubMed] [Google Scholar]
- 25.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 20032991057–1061. [PubMed] [Google Scholar]
- 26.Szabo S J, Sullivan B M, Stemmann C, Satoskar A R, Sleckman B P, Glimcher L H. Distinct effects of T‐bet in TH1 lineage commitment and IFN‐gamma production in CD4 and CD8 T cells. Science 2002295338–342. [DOI] [PubMed] [Google Scholar]
- 27.Kang Y M, Zhang X, Wagner U G, Yang H, Beckenbaugh R D, Kurtin P J.et al CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med 20021951325–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ronnelid J, Berg L, Rogberg S, Nilsson A, Albertsson K, Klareskog L. Production of T cell cytokines at the single‐cell level in patients with inflammatory arthritides: enhanced activity in synovial fluid compared to blood. Br J Rheumatol 1998377–14. [DOI] [PubMed] [Google Scholar]
- 29.Smeets T J, Dolhain R J E M, Miltenburg A M, de Kuiper R, Breedveld F C, Tak P P. Poor expression of T cell‐derived cytokines and activation and proliferation markers in early rheumatoid synovial tissue. Clin Immunol Immunopathol 19988884–90. [DOI] [PubMed] [Google Scholar]
- 30.Peng G, Guo Z, Kiniwa Y, Voo K S, Peng W, Fu T.et al Toll‐like receptor 8‐mediated reversal of CD4+ regulatory T cell function. Science 20053091380–1384. [DOI] [PubMed] [Google Scholar]
- 31.Lord G, Rao R M, Choe H, Sullivan B M, Lichtman A H, Luscinskas F W, Glimcher L H. T‐bet is required for optimal pro‐inflammatory CD4+ T cell trafficking. Blood 20051063432–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Bilsen J H, van Dongen H, Lard L R, van der Voort E I, Elferink D G, Bakker A M.et al Functional regulatory immune responses against human cartilage glycoprotein‐39 in health vs. proinflammatory responses in rheumatoid arthritis. Proc Natl Acad Sci USA 200410117180–17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green E A, Choi Y, Flavell R A. Pancreatic lymph node‐derived CD4+CD25+ Treg cells: highly potent regulators of diabetes that require TRANCE‐RANK signals. Immunity 200216183–191. [DOI] [PubMed] [Google Scholar]
- 34.Tang Q, Adams J Y, Tooley A J, Bi M, Fife B T, Serra P.et al Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol 2006783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin‐2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol 2004342480–2488. [DOI] [PubMed] [Google Scholar]
- 36.Huehn J, Siegmund K, Lehmann J C, Siewert C, Haubold U, Feuerer M.et al Developmental stage, phenotype, and migration distinguish naive‐ and effector/memory‐like CD4+ regulatory T cells. J Exp Med 2004199303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suffia I, Reckling S K, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol 20051745444–5455. [DOI] [PubMed] [Google Scholar]
- 38.Bruhl H, Cihak J, Schneider M A, Plachy J, Rupp T, Wenzel I.et al Dual role of CCR2 during initiation and progression of collagen‐induced arthritis: evidence for regulatory activity of CCR2+ T cells. J Immunol 2004172890–898. [DOI] [PubMed] [Google Scholar]
- 39.Lee I, Wang L, Wells A D, Dorf M E, Ozkaynak E, Hancock W W. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med 20052011037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wysocki C A, Jiang Q, Panoskaltsis‐Mortari A, Taylor P A, McKinnon K P, Su L.et al Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft‐versus‐host disease. Blood 20051063300–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K. CCR6 expression defines regulatory effector/memory‐like cells within the CD25(+)CD4+ T cell subset. Blood 20051052877–2886. [DOI] [PubMed] [Google Scholar]
- 42.Firan M, Dhillon S, Estess P, Siegelman M H. Suppressor activity and potency among regulatory T cells is discriminated by functionally active CD44. Blood 2006107619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stassen M, Fondel S, Bopp T, Richter C, Muller C, Kubach J.et al Human CD25+ regulatory T cells: two subsets defined by the integrins alpha 4 beta 7 or alpha 4 beta 1 confer distinct suppressive properties upon CD4+ T helper cells. Eur J Immunol 2004341303–1311. [DOI] [PubMed] [Google Scholar]