Abstract
Objective
To determine whether longitudinal left ventricular systolic function measured by Doppler tissue imaging (DTI) after exercise can identify early left ventricular dysfunction in asymptomatic patients with moderate–severe aortic stenosis.
Design
Case–control study.
Setting
Outpatient cardiology departments.
Patients
20 patients with aortic stenosis, with or without equivocal symptoms, a peak aortic valve velocity ⩾3 m/s, and left ventricular ejection fraction >50% and 15 aged‐matched normal controls.
Interventions
Echocardiogram performed at rest and immediately after treadmill exercise.
Main outcome measures
The peak systolic velocity of the lateral mitral annulus (S') by DTI at rest and immediately after exercise, exercise capacity, exercise systolic blood pressure and the plasma level of B‐type natriuretic peptide (BNP).
Results
For patients with aortic stenosis, mean (SD) aortic valve area was 0.95 (0.3) cm2. At rest, S' was similar for patients with aortic stenosis and controls, respectively (8.5 (1.5) vs 9.1 (1.8) cm/s, p = 0.15). However, after exercise, S' (12.2 (3.2) vs 17 (2.8) cm/s, p<0.001) and the increase in S' between rest and exercise (4 (3) vs 7.9 (1.5) cm/s, p<0.001) were lower in patients with aortic stenosis. In patients with aortic stenosis, a smaller increase in S' after exercise was associated with lower exercise capacity (r = 0.5, p = 0.02), a smaller increase in exercise systolic blood pressure (r = 0.6, p = 0.005) and higher plasma level of BNP (r = 0.66, p = 0.002).
Conclusion
In asymptomatic patients with moderate–severe aortic stenosis a lower than normal increase in peak systolic mitral annular velocity after treadmill exercise is a marker of early left ventricular systolic dysfunction.
Current guidelines recommend aortic valve replacement for severe aortic stenosis after the onset of symptoms and in asymptomatic patients with impairment of left ventricular systolic function, defined as an ejection fraction <50%.1,2 However, reduction in resting left ventricular ejection fraction occurs late in the natural history of aortic stenosis, and decisions on surgery for severe stenosis therefore usually rest on accurate clinical evaluation of symptoms. Non‐specific symptoms such as exertional dyspnoea and fatigue are often difficult to assess and can vary considerably between individuals because of differences in physical fitness, comorbidity and illness perception. An early measure of cardiac decompensation would improve the reliability of the clinical assessment of patients with severe aortic stenosis.
Longitudinal left ventricular contractile function measured by Doppler tissue imaging (DTI) decreases with decrease in left ventricular ejection fraction,3 and predicts prognosis after adjusting for ejection fraction in patients with a range of cardiac diseases.4 In previous studies of aortic stenosis, which included predominantly patients with symptoms, long axis left‐ventricular systolic function was impaired even when left‐ventricular ejection fraction was normal.5,6,7 In this study, we tested the hypothesis that long axis left‐ventricular function measured by DTI after treadmill exercise is more sensitive than resting measurements for early cardiac decompensation in asymptomatic patients with aortic stenosis. We compared resting and post‐exercise DTI measures of longitudinal left‐ventricular function in patients with aortic stenosis and in normal controls, and evaluated associations with known clinical markers of early cardiac decompensation including decreased exercise capacity, abnormal blood pressure response to exercise and raised plasma levels of B‐type natriuretic peptide (BNP).
Methods
Study population
Patients with moderate or severe aortic stenosis, defined as a peak aortic velocity of ⩾3 m/s who were asymptomatic or had equivocal symptoms were invited to participate. Patients were excluded if there was a history of myocardial infarction, angina or heart failure, left‐ventricular impairment (ejection fraction <50%), atrial fibrillation, significant renal disease (creatinine >0.16 mmol/l), respiratory disease or other significant valvular disease. Symptomatic status was assessed by a study cardiologist at enrolment. Dyspnoea was graded according to the New York Heart Association (NYHA) classification. Control subjects had no clinical evidence of cardiovascular or respiratory disease and were age‐matched and sex‐matched to patients with aortic stenosis. The study protocol was approved by the regional ethics committee and all subjects gave written informed consent.
Exercise testing
All subjects underwent a symptom‐limited exercise treadmill test. A modification of the standard Bruce protocol was used in which the speed and gradient increased in equal increments.8 At the end of each 3 min stage, the work performed was equivalent to the standard Bruce protocol. Exercise was stopped for marked dyspnoea, fatigue, chest discomfort, a decrease in systolic blood pressure of ⩾10 mm Hg, ⩾5 mm ST depression, significant arrhythmia and slowing of heart rate, on patient request, or on completion of the treadmill protocol after 15 min or 17.2 metabolic equivalents. An abnormal blood pressure response was defined as <20 mm Hg increase or fall in systolic blood pressure during exercise.1
Resting and stress echocardiogram
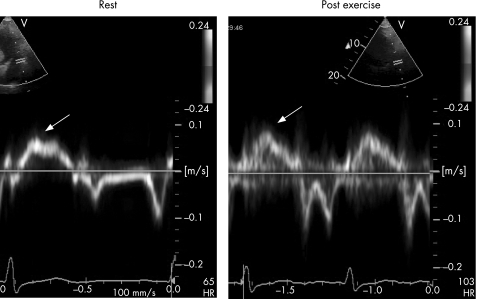
Before exercise, an echocardiogram was performed documenting severity of aortic stenosis (by the continuity equation using peak velocity), left‐ventricular volumes and left‐ventricular mass (area‐length) using standard two dimensional and Doppler techniques.9,10 Ejection fraction was measured from a single plane measurement in the apical four‐chamber view using the modified Simpson's method.9 Early (E) and late (A) mitral inflow velocities were measured by pulsed Doppler ultrasound at the mitral leaflet tips. DTI was performed at the lateral mitral annulus from the apical four‐chamber view.11 The E/E' ratio was used to assess left‐ventricular diastolic function.11,12 Peak S' velocity and the time velocity integral (TVI) for the S' wave were measured before and after exercise (fig 1). When there was more than one peak in the S' wave, the highest velocity was used for analysis. The initial upward deflection of the S' wave associated with isovolumetric contraction was included in TVI measurement.13 The mean between observer difference for measurement of S' at rest was 8%, and S' after exercise was 12%. An intravenous injection of agitated saline was used to enhance the tricuspid regurgitant jet for estimation of right ventricular systolic pressure using continuous wave Doppler both at rest and after exercise.
Figure 1 Doppler tissue imaging of the lateral mitral annulus at rest and after exercise in an asymptomatic patient with aortic stenosis. The peak systolic velocity S' (indicated by the arrows) was 7 cm/s at rest and increased to 9 cm/s after exercise. In most normal subjects, S' increased by >5 cm/s after exercise.
Immediately after peak exercise, ECG images were obtained in the following sequence; mitral inflow velocity, DTI of the lateral mitral annulus, tricuspid regurgitant jet velocity, left‐ventricular volumes and aortic valve velocity. In most patients, data was obtained within 1 min of peak exercise. Images were stored digitally, and analysed later off‐line using a commercial analysis system (EchoPac PC V.3) by an investigator who was blinded to the clinical, exercise and natriuretic peptide data.
Measurement of natriuretic peptides
A venous blood sample was collected from an indwelling intravenous catheter with the subject supine before exercise. Samples were collected in an EDTA tube and immediately placed on ice and centrifuged within 2 h at −4°C. Plasma was stored at −80°C before being assayed for BNP using an established radioimmunoassay.14 The normal range for BNP is 3–12 pmol/l and the within‐assay coefficient of variation is 5.2%. To convert BNP measured in pmol/l to pg/ml, divide by 0.289.
Statistical analysis
Group data are presented as mean (SD). Two sample t test or the Mann–Whitney U test was used for comparing continuous variables between patients with aortic stenosis and controls. χ2 test or Fisher's exact test were used for categorical variables. Pearson or Spearman correlation coefficients were reported for pairwise associations among exercise variables, post‐exercise ECG variables and natriuretic peptide levels. Multiple regression analysis with stepwise selection was used to investigate the relationship between Doppler tissue measures and exercise capacity, exercise blood pressure and BNP adjusting for age, sex, body mass index, ejection fraction and aortic valve area. Analysis of covariance was performed to adjust the group comparison for significant confounders. Natriuretic peptide levels underwent natural logarithmic transformation in the analysis due to its right skewed distribution. SAS release V.8 software and R 2.1.1 were used for analysis. A p value of <0.05 was considered significant.
Results
Comparison of patients with aortic stenosis with normal controls
Table 1 summarises the baseline clinical characteristics and resting ECG measures for patients with aortic stenosis and controls. Patients with aortic stenosis were of similar age to controls and most were male. Twelve patients had NYHA class 1 and eight NYHA class 2 symptoms. For subjects with symptoms, the patient's cardiologist was not certain whether the symptoms were due to aortic stenosis. Resting heart rate was lower in patients with aortic stenosis, six of whom were taking a β‐blocker. Eight patients with aortic stenosis had a history of hypertension, but resting systolic blood pressure was similar for the two groups.
Table 1 Clinical characteristics, Echo parameters measured at rest, and B‐type natriuretic peptide in patients with aortic stenosis and normal controls.
| Aortic stenosis (n = 20) | Normal controls (n = 15) | p Value* | |
|---|---|---|---|
| Age, years | 67 (9) | 68 (4) | 0.6 |
| Male, n (%) | 19 (95%) | 13 (87%) | 0.3 |
| Body mass index, kg/m2 | 28.3 (4.6) | 25.8 (3.1) | 0.1 |
| β‐blockers, n | 6 | 0 | 0.02 |
| ACE inhibitors, n | 1 | 0 | 0.3 |
| Resting heart rate, beats/min | 66 (9) | 73 (9) | 0.03† |
| Resting systolic blood pressure, mm Hg | 142 (20) | 137 (21) | 0.4 |
| ST depression on resting ECG ⩾0.5 mm, n | 6 | 3 | 0.2 |
| ECG parameters | |||
| Aortic valve area, cm2 | 0.95 (0.3) | — | — |
| Aortic peak velocity, m/s | 3.9 (0.8) | — | — |
| Ejection fraction, % | 69 (7) | 62 (5) | 0.01† |
| Left‐ventricular mass index, g/m2 | 119 (41) | 107 (24) | 0.2† |
| Right ventricular systolic pressure, mm Hg | 21 (6) | 19 (4) | 0.3 |
| Mitral inflow velocity, E, m/s | 0.8 (0.2) | 0.6 (0.2) | 0.02† |
| Lateral mitral annular velocity, E', cm/s | 7.9 (2.1) | 9.5 (2.6) | 0.09 |
| E/E' ratio | 10.5 (2.8) | 6.7 (1.8) | 0.001† |
| Doppler tissue imaging, S', cm/s | 8.5 (1.5) | 9.1 (1.8) | 0.15 |
| Time velocity integral, S', cm | 1.58 (0.4) | 1.85 (0.4) | 0.04 |
| Natriuretic peptide | |||
| B‐type natriuretic peptide, pmol/l | 9.2 (5.8) | 7.4 (4) | 0.3† |
Results are means (SD) unless otherwise indicated.
*For continuous variables, two sample t test was used. For categorical variables, χ2 test or fisher exact test was used where appropriate.
†Mann–Whitney U test used where variables not normally distributed.
For patients with aortic stenosis the mean aortic valve area (AVA) was 0.95 (0.3) cm2 and mean aortic valve gradient was 34 (15) mm Hg. Aortic stenosis was due to a bicuspid valve in five and degenerative calcification in 15 patients. Compared with normal subjects, patients with aortic stenosis had a higher ejection fraction and a higher mitral E velocity, lower E' velocity and higher E/E' on the resting echo (table 1). Left‐ventricular mass index was also higher in patients with aortic stenosis, but this difference was not statistically significant. There was a trend for S' velocity and the VTI of S' wave to be lower and for the plasma BNP to be higher in patients with aortic stenosis compared with controls, but these differences were not statistically significant (table 1).
Exercise testing
In patients with aortic stenosis, the primary reasons for stopping treadmill exercise were dyspnoea (n = 14), leg discomfort (n = 1), frequent ventricular ectopics (n = 1), and an abnormal increase in systolic blood pressure (n = 4). Additional outcomes that were not the primary reason for stopping treadmill exercise were dyspnoea (n = 2), fatigue (n = 8), self‐limiting atrial fibrillation during recovery (n = 1) and an abnormal blood pressure response (n = 4). No patients had ST depression ⩾5 mm, angina, dizziness or syncope during exercise and there were no adverse outcomes. Table 2 summarises the outcomes of exercise treadmill. Patients with aortic stenosis had reduced exercise capacity compared with controls. Two control subjects reached the end of the exercise protocol and the other 13 subjects stopped because of dyspnoea and/or fatigue. All control subjects had an increase in systolic blood pressure of ⩾20 mm Hg during exercise. The increase in systolic blood pressure with exercise, and heart rate at both peak and 1 min after exercise were lower in patients with aortic stenosis than in controls (table 2).
Table 2 Exercise treadmill outcomes for patients with aortic stenosis and controls.
| Aortic stenosis (n = 20) | Normal controls (n = 15) | p Value* | |
|---|---|---|---|
| Treadmill exercise results | |||
| Exercise time, min | 8.9 (3.2) | 11 (2.6) | 0.1 |
| Exercise capacity, METS | 9.7 (3.5) | 12.3 (3.1) | 0.09 |
| Peak heart rate, beats/min | 134 (20) | 154 (12) | 0.002 |
| Heart rate 1 min after exercise, beats/min | 109 (18) | 123 (13) | 0.1 |
| Peak systolic blood pressure, mm Hg | 158 (26) | 184 (16) | 0.04 |
| Increase in systolic blood pressure with exercise, mm Hg | 17 (26) | 49 (16) | 0.001 |
| ST depression ⩾2 mm, n | 6 | 2 | 0.2 |
| Angina, n | 0 | 0 | — |
METS, metabolic equivalents.
Results are means (SD).
*For continuous variable, two‐sample t test used and for categorical variable, χ2 test or fisher exact test where appropriate.
†Mann–Whitney U test used where variables not normally distributed.
Echo after exercise
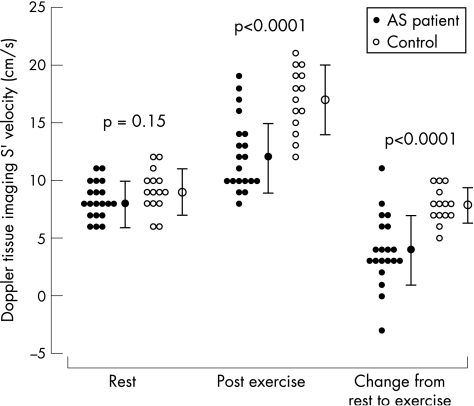
Table 3 summarises the Echo measures of left‐ventricular function at rest, after exercise and the change from rest to post‐exercise for patients with aortic stenosis and controls. S' could be measured in all subjects both at rest and after exercise, but the recording was not saved in one control subject. At rest, S' was similar for patients with aortic stenosis and controls, but after exercise S' was significantly lower in patients with aortic stenosis (table 3). This was because the increase in S' after exercise was lower in patients with aortic stenosis than controls. Figure 2 illustrates S' at rest and after exercise for all study participants. The difference in post‐exercise S' and change from rest to exercise S' between patients with aortic stenosis and controls remained significant after adjusting for age, sex, body mass index, exercise time and heart rate (p = 0.0002 and p = 0.0005, respectively). After excluding the six patients with aortic stenosis who were taking a β‐blocker, the post exercise S' and change in S' after exercise were still significantly lower in patients with aortic stenosis than in normal controls (table 3). The VTI of the S' wave was also lower in patients with aortic stenosis than in controls both at rest and after exercise (table 3).
Table 3 Echocardiographic measurements at rest, after exercise and the change between rest and exercise for patients with aortic stenosis and controls.
| ECG | Rest | After exercise | Difference between rest and exercise | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Aortic stenosis | Controls | p Value | Aortic stenosis | Controls | p Value | Aortic stenosis | Controls | p Value | |
| Ejection fraction, % | 69 (7) | 62 (5) | 0.01† | 72 (9) | 73 (9) | 0.6* | 4 (7) | 11 (8) | 0.02 |
| End systolic volume, ml | 37 (19) | 48 (14) | 0.08 | 23 (12) | 23 (13) | 0.8* | −13 (19) | −25 (10) | 0.03* |
| End diastolic volume, ml | 94 (33) | 119 (33) | 0.04 | 83 (25) | 81 (23) | 1.0* | −12 (32) | −38 (24) | 0.01 |
| Peak systolic mitral annular velocity, S', cm/s | 8.5 (1.5) | 9.1 (1.8) | 0.15 | 12.2 (3.2) | 17 (2.8) | <0.001† | 4 (3) | 7.9 (1.5) | <0.001† |
| Velocity time integral of S', cm | 1.58 (0.4) | 1.85 (0.4) | 0.04 | 1.66 (0.51) | 2.24 (0.21) | 0.0003 | 0.13 (0.38) | 0.41 (0.47) | 0.04 |
| Right ventricular systolic pressure, mm Hg‡ | 21 (6) | 19 (4) | 0.3 | 47 (15) | 35 (9) | 0.03* | 26 (14) | 17 (9) | 0.05* |
*Mann–Whitney U test used where variables are not normally distributed.
†For post‐exercise, S' and difference between rest and exercise of S' p values were 0.0002 and 0.0005, respectively after adjusting for age, sex, body mass index, heart rat and exercise time with analysis of covariance. After excluding the six patients with aortic stenosis taking a β‐blocker, p values were 0.01 and 0.006, respectively.
‡RSVP could be measured after exercise in 16 patients with aortic stenosis and 14 controls.
Figure 2 The peak S' velocity measured by Doppler tissue imaging (DTI) at rest and after exercise, and the difference in S' between rest and exercise for patients with aortic stenosis and controls. AS, aortic stenosis.
After peak exercise, patients with aortic stenosis had a smaller decrease in left‐ventricular end‐diastolic and end‐systolic volumes and hence smaller increase in ejection fraction compared with controls. E/E' was higher in patients with aortic stenosis than in controls after exercise. However, the change in E/E' from rest to post exercise was similar for patients with aortic stenosis and controls (data not shown). The analysis of the diastolic DTI signals after exercise was complicated by E'/A' merging (three patients with aortic stenosis and five controls) and by poor‐quality images (five patients with aortic stenosis and two controls). The increase in right ventricular systolic pressure after exercise was greater in patients with aortic stenosis.
Associations with exercise capacity, exercise blood pressure and BNP
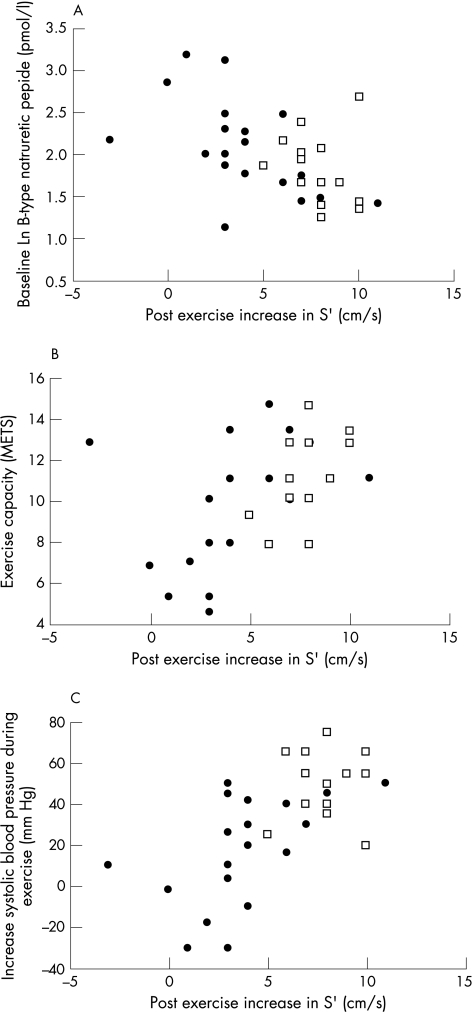
For patients with aortic stenosis, increase in peak S' velocity after exercise was associated with a lower plasma level of BNP (r = −0.66, p = 0.002), a higher exercise capacity (r = 0.50, p = 0.02) and greater increase in systolic blood pressure with exercise (r = 0.60, p = 0.005). Figure 3 illustrates these associations. Associations were similar, but weaker between post exercise S' and BNP (r = −0.50, p<0.01), increase in systolic blood pressure (r = 0.56, p<0.01) and exercise capacity (r = 0.51, p<0.05). When analysis was repeated excluding the six patients with aortic stenosis who were taking a β‐blocker, the association between change in S' after exercise and BNP (r = 0.49, p = 0.01), increase in systolic blood pressure (r = 0.56, p = 0.04) and exercise capacity (r = 0.51, p = 0.07) were similar. The association between increase in S' after exercise and aortic valve area indexed to body surface area was not significant (r = 0.11, p = 0.64). Compared with increase in S' after exercise all other ECG parameters measured at rest or after exercise were more weakly associated with exercise capacity, exercise increase in blood pressure and the plasma level of BNP.
Figure 3 Associations between increase in S' velocity from rest to after exercise, and the natural logarithm (Ln) of the plasma level of B‐type natriuretic peptide (A), exercise capacity (B), and exercise increase in systolic blood pressure (C) in patients with aortic stenosis (•) and controls (□). For patients with aortic stenosis correlation coefficients were for Ln BNP (r = −0.66, p = 0.0015), exercise capacity (r = 0.50, p = 0.02) and increase in systolic blood pressure with exercise (r = 0.60, p = 0.005). METS, metabolic equivalents.
In stepwise multivariate analysis that included increase in S', age, sex, weight, aortic valve area and ejection fraction, the post‐exercise change in S' was the strongest predictor of both the plasma level of BNP and the exercise increase in systolic blood pressure in patients with aortic stenosis. An increase in peak S' velocity of 1 cm/s was associated with a 10% decrease in the plasma level of BNP (95% CI 3% to 16%) and a 4.4 (1.1 to 7.7) mm Hg increase in systolic blood pressure during exercise. In patients with aortic stenosis, older age was associated with both a smaller increase in S' after exercise (r = 0.54, p = 0.01) and a lower exercise capacity (r = 0.43, p = 0.06). In multivariate analysis, the association between increase in S' and exercise capacity was not significant.
An abnormal post exercise increase in S' was defined as >2 SD below the mean increase for control subjects (⩽5 cm/s). For the 14 patients with aortic stenosis with an increase in peak S' of ⩽5 cm/s, compared with the six patients with aortic stenosis with an increase in S' velocity of >5 cm/s and normal controls, respectively; exercise capacity was lower (8.7 (3.5) vs 12.2 (1.7), p = 0.03, controls 12.3 (3.1) metabolic equivalents), increase in systolic blood pressure with exercise was less (11 (27) vs 35 (12), p = 0.05, controls 49 (16) mm Hg), and resting plasma BNP higher (10.6 (6.3) vs 5.9 (2.9), p = 0.04, controls 7.1 (4) pmol/l). Differences between patients with aortic stenosis with an increase in S' of >5 cm/s and normal controls were not significant. However, aortic valve area was similar for patients with aortic stenosis who had a peak change in S' after exercise ⩽5 cm/s compared with those >5 cm/s (0.9 (0.3) vs 0.9 (0.2) cm2, respectively, p = 0.5).
Discussion
Previous studies have reported decreased systolic mitral annular excursion by M‐mode ECG,7 and decreased peak systolic velocity of the mitral annulus by DTI5,6 in predominantly symptomatic patients with severe aortic stenosis. In the current study, both the velocity time integral and the peak S' velocity at rest were slightly lower in asymptomatic patients or patients with mildly symptomatic aortic stenosis compared with normal controls, but overlap between these patient groups was large. However, after exercise, the increase in S' was significantly lower in patients with aortic stenosis compared with normal controls. In addition, patients with aortic stenosis and a smaller increase in peak S' after exercise were more likely to have other evidence of early left‐ventricular dysfunction. These observations suggest that measurement of left‐ventricular longitudinal function by tissue Doppler imaging after exercise allows detection of latent left‐ventricular dysfunction while other conventional measures of resting left‐ventricular systolic function are still normal.
Longitudinally oriented fibres are predominantly located in the subendocardium and may be at greater risk from ischaemia than circumferential fibres, which are located in intermediate layers of the myocardium.15 The peak systolic mitral annular velocity is a sensitive measure of left‐ventricular function from various causes.16,17,18 In addition, abnormal longitudinal function is an important predictor of adverse outcomes in a range of cardiac diseases.4 Exercise stress testing has been used to identify patients with mitral regurgitation who have decreased left‐ventricular contractile reserve and are more likely to have impaired left‐ventricular function after mitral valve surgery.18,19 The current study applies the same principle to patients with aortic stenosis using a simpler and more sensitive measure of left‐ventricular contractile function than change in left‐ventricular volumes or ejection fraction.
A smaller increase in ejection fraction after exercise in patients with aortic stenosis has also been reported in previous studies.20 However, in the current study, increase in ejection fraction after exercise less reliably distinguished patients with aortic stenosis from controls, and was not consistently associated with exercise capacity or the plasma level of BNP. Measurement of change in ejection fraction after exercise with echo requires good endocardial definition, is technically demanding and in some patients cannot be achieved. By contrast, mitral annular velocities are easier to measure and adequate images can be obtained in most patients.3 In this study, the TVI of the S' wave, which is a measure of the distance descended by the mitral annulus during systole, was lower in patients with aortic stenosis than in controls both at rest and after exercise. However, this measure was less discriminating than peak S' after exercise, probably because it is more difficult to record and measure accurately. The variability in TDI measures of long axis function was higher after exercise than at rest because of respiratory movement and tachycardia. Measurement error will decrease the strength of associations with exercise outcomes and BNP, but should not bias the study findings. Fractional shortening, which measures circumferential systolic function, was not measured after exercise in this study. However, ejection fraction and end systolic volume index after exercise were similar in patients with aortic stenosis and normal controls. In previous studies of predominantly symptomatic patients with aortic stenosis, fractional shortening was normal when longitudinal function measured by DTI at rest was impaired.5,7
The plasma level of BNP increases with left‐ventricular dysfunction from any cause, and is a powerful predictor of outcome even when levels are within the “normal” range in the general population.21 Plasma levels of BNP are higher in symptomatic than in asymptomatic patients with aortic stenosis;22 and in asymptomatic patients, a higher plasma level of BNP or N‐terminal pro‐BNP predicts symptomatic deterioration during follow‐up.23,24 In the current study, patients with aortic stenosis had no or mild symptoms and the plasma level of BNP was within the normal range for most participants. Despite this, the increase in peak S' with exercise was lower in patients with aortic stenosis than in controls and was associated with higher plasma levels of BNP. These observations suggest peak S' after exercise is an early marker of left‐ventricular dysfunction, which can be abnormal while the plasma level of BNP is still within the normal range.
In several studies, an abnormal treadmill exercise test in patients with aortic stenosis has been associated with a higher risk of symptomatic deterioration during follow‐up.25,26,27 Patients with a normal exercise test at a high workload have a good prognosis, whereas a low exercise capacity with cardiac symptoms or a fall in systolic blood pressure is usually an indication for surgery in severe aortic stenosis.1,2 However, in many patients, interpreting the outcome of an exercise test is difficult because of an intermediate exercise capacity, non‐specific symptoms, or ST depression without angina. In these patients, measurement of left‐ventricular systolic function after exercise with DTI may be valuable. Although higher peak aortic jet velocity predicts adverse outcomes in aortic stenosis, previous research suggests that an increase in the aortic valve gradient with exercise does not add additional prognostic information.28
Study limitations
Although the study population was small, the difference in S' after exercise between aortic stenosis patients and controls was highly statistically significant, and consistent associations were observed with independent measures of cardiac status known to be associated with adverse outcomes. We considered other factors that may influence S' after exercise. Subendocardial myocardial ischaemia is likely to contribute to impairment of left‐ventricular long axis function during exercise in aortic stenosis. However, coronary angiography was not performed for most of the participants, so the importance of unrecognised coronary artery disease could not be evaluated. Six patients with aortic stenosis were taking β‐blockers, which could influence the heart rate, blood pressure and plasma levels of BNP.29 However, results were similar when these patients were excluded from the analysis. Exercise duration and heart rate during the post‐exercise ECG were lower in patients with aortic stenosis than in controls. The explanation for lower peak exercise heart rates in patients with aortic stenosis is uncertain, but statistical adjustment for heart rate and exercise duration did not significantly change study findings. Peak S' may be influenced by afterload,11 but in this study there was no statistically significant association between post‐exercise S' and aortic valve area, suggesting that S' provides information on left‐ventricular function independent of the severity of aortic stenosis. The additional effect of systemic arterial compliance during exercise was not evaluated in the current study.30
Higher E/E' at rest suggests a higher left‐ventricular end diastolic pressure in patients with aortic stenosis compared with controls.5,12 In previous studies, lower E' and higher E/E' measured at rest were associated with reduced exercise capacity in patients without heart valve disease.31,32 However, in the current study there was no statistically significant association between E', E/E' or S' measured at rest and exercise capacity, increase in systolic blood pressure during exercise or the plasma level of BNP. These observations suggest that post‐exercise S' is a more sensitive marker of early left‐ventricular dysfunction than are resting DTI measures of long axis function in aortic stenosis. In this study, DTI was performed at the lateral mitral annulus, which moves more rapidly than the medial annulus, so normal values for E' and S' are higher.13 It is currently uncertain whether medial or lateral mitral annular measurements have greater diagnostic value.
Further studies that include assessment of clinical outcomes during follow‐up are needed to guide the clinical application of exercise DTI for management of asymptomatic patients with moderate to severe aortic stenosis, and to compare its value with other methods of assessing risk, including raised plasma level of BNP and standard outcomes on treadmill exercise testing.
In conclusion, in patients with moderate to severe aortic stenosis failure to increase the peak systolic mitral annular velocity after treadmill exercise is an early marker of left‐ventricular systolic dysfunction.
Acknowledgements
The study was supported by a grant from the National Heart Foundation of New Zealand. Dr NCVP received salary support from the Middlemore Cardiac Trust and Dr RAHS from the Green Lane Research and Education Trust. GAW holds the Senior Fellowship of the National Heart Foundation of New Zealand. We thank Jenny White for assistance with study management.
Abbreviations
BNP - B‐type natriuretic peptide
DTI - Doppler tissue imaging
NYHA - New York Heart Association classification
TVI - time velocity integral
Footnotes
Competing interests: None declared.
References
- 1.Bonow R O, Carabello B, de Leon A C., Jret al ACC/AHA guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). J Am Coll Cardiol 1998321486–1588. [DOI] [PubMed] [Google Scholar]
- 2.Lung B, Gohlke‐Barwolf C, Tornos P.et al Recommendations on the management of the asymptomatic patient with valvular heart disease. Eur Heart J 2002231253–1266. [DOI] [PubMed] [Google Scholar]
- 3.Gulati V K, Katz W E, Follansbee W P.et al Mitral annular descent velocity by tissue Doppler echocardiography as an index of global left ventricular function. Am J Cardiol 199677979–984. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Yip G W, Wang A Y.et al Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value. J Am Coll Cardiol 200341820–826. [DOI] [PubMed] [Google Scholar]
- 5.Bruch C, Stypmann J, Grude M.et al Tissue Doppler imaging in patients with moderate to severe aortic valve stenosis: clinical usefulness and diagnostic accuracy. Am Heart J 2004148696–702. [DOI] [PubMed] [Google Scholar]
- 6.Giorgi D, Di B, V, Talini E.et al Myocardial function in severe aortic stenosis before and after aortic valve replacement: a Doppler tissue imaging study. J Am Soc Echocardiogr 2005188–14. [DOI] [PubMed] [Google Scholar]
- 7.Takeda S, Rimington H, Smeeton N.et al Long axis excursion in aortic stenosis. Heart 20018652–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart R A H, Kittelson J, Kay I P. Statistical methods to improve the precision of the treadmill exercise test. J Am Coll Cardiol 2000361274–1279. [DOI] [PubMed] [Google Scholar]
- 9.Lang R M, Bierig M, Devereux R B.et al Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005181440–1463. [DOI] [PubMed] [Google Scholar]
- 10.Quinones M A, Otto C M, Stoddard M.et al Recommendations for quantification of doppler echocardiography: a report from the doppler quantification task force of the nomenclature and standards committee of the American Society of Echocardiography. J Am Soc Echocardiogr 200215167–184. [DOI] [PubMed] [Google Scholar]
- 11.Oki T, Fukuda K, Mishiro Y.et al Pulsed tissue Doppler imaging of left ventricular systolic and diastolic wall motion velocities to evaluate differences between long and short axes in healthy subjects. J Am Soc Echocardiogr 199912308–313. [DOI] [PubMed] [Google Scholar]
- 12.Nagueh S, Middleton K, Koplen H.et al Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997301527–1533. [DOI] [PubMed] [Google Scholar]
- 13.Pai R G, Gill K S. Amplitudes, durations, and timings of apically directed left ventricular myocardial velocities: I. Their normal pattern and coupling to ventricular filling and ejection. J Am Soc Echocardiogr 199811105–111. [DOI] [PubMed] [Google Scholar]
- 14.Yandle T G, Richards A M, Gilbert A.et al Assay of brain natriuretic peptide (BNP) in human plasma: evidence for high molecular weight BNP as a major plasma component in heart failure. J Clin Endocrinol Metab 199376832–838. [DOI] [PubMed] [Google Scholar]
- 15.Henein M Y, Gibson D G. Normal long axis function. Heart 199981111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorcsan J, III, Deswal A, Mankad S.et al Quantification of the myocardial response to low‐dose dobutamine using tissue Doppler echocardiographic measures of velocity and velocity gradient. Am J Cardiol 199881615–623. [DOI] [PubMed] [Google Scholar]
- 17.Agricola E, Galderisi M, Oppizzi M.et al Pulsed tissue Doppler imaging detects early myocardial dysfunction in asymptomatic patients with severe mitral regurgitation. Heart 200490406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haluska B A, Short L, Marwick T H. Relationship of ventricular longitudinal function to contractile reserve in patients with mitral regurgitation. Am Heart J 2003146183–188. [DOI] [PubMed] [Google Scholar]
- 19.Leung D Y, Griffin B P, Stewart W J.et al Left ventricular function after valve repair for chronic mitral regurgitation: predictive value of preoperative assessment of contractile reserve by exercise echocardiography. J Am Coll Cardiol 1996281198–1205. [DOI] [PubMed] [Google Scholar]
- 20.Clyne C A, Arrighi J A, Maron B J.et al Systemic and left ventricular responses to exercise stress in asymptomatic patients with valvular aortic stenosis. Am J Cardiol 1991681469–1476. [DOI] [PubMed] [Google Scholar]
- 21.Wang T J, Larson M G, Levy D.et al Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004350655–663. [DOI] [PubMed] [Google Scholar]
- 22.Gerber I L, Stewart R A H, Legget M E.et al Increased plasma natriuretic peptide levels reflect symptom onset in aortic stenosis. Circulation 20031071884–1890. [DOI] [PubMed] [Google Scholar]
- 23.Bergler‐Klein J, Klaar U, Heger M.et al Natriuretic peptides predict symptom‐free survival and postoperative outcome in severe aortic stenosis. Circulation 20041092302–2308. [DOI] [PubMed] [Google Scholar]
- 24.Gerber I L, Legget M E, West T M.et al Usefulness of serial measurement of N‐terminal brain pro‐natriuretic peptide plasma levels in asymptomatic patients with aortic stenosis to predict symptomatic deterioration. Am J Cardiol 200595898–901. [DOI] [PubMed] [Google Scholar]
- 25.Alborino D, Hoffmann J L, Fournet P C.et al Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis 200211204–209. [PubMed] [Google Scholar]
- 26.Amato M C, Moffa P J, Werner K E.et al Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 200186381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005261309–1313. [DOI] [PubMed] [Google Scholar]
- 28.Otto C M, Burwash I G, Legget M E.et al Prospective study of asymptomatic valvular aortic stenosis: clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997952262–2270. [DOI] [PubMed] [Google Scholar]
- 29.Davis M E, Richards A M, Nicholls M G.et al Introduction of metoprolol increases plasma B‐type cardiac natriuretic peptides in mild, stable heart failure. Circulation 2006113977–985. [DOI] [PubMed] [Google Scholar]
- 30.Briand M, Dumesnil J G, Kadem L.et al Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol 200546291–298. [DOI] [PubMed] [Google Scholar]
- 31.Skaluba S, Litwin S. Mechanisms of exercise tolerance. Insights from tissue Doppler imaging. Circulation 2004109972–977. [DOI] [PubMed] [Google Scholar]
- 32.Hadano Y, Murata K, Yamamoto T.et al Usefulness of mitral annular velocity in predicting exercise tolerance in patients with impaired left ventricular systolic function. Am J Cardiol 2006971025–1028. [DOI] [PubMed] [Google Scholar]