Abstract
Objective
To assess whether circulating levels of carbohydrate antigen 125 (CA125) predict subsequent 6‐month all‐cause mortality in patients after the index hospitalisation for acute heart failure (HF).
Design and setting
Prospective cohort study at a single teaching centre in Spain.
Methods
529 consecutive patients with acute HF admitted in a single university centre were analysed. In addition to the traditional clinical information, CA125 (U/ml) was measured during the early course of hospitalisation. The independent association between baseline CA125 and mortality was assessed with Cox regression analysis. The follow‐up was limited to 6 months.
Results
349 (66%) patients showed serum levels of CA125 >35 U/ml (established cut‐off point value). At a 6‐month follow‐up, 89 (16.8%) deaths were identified. A positive trend between mortality and CA125 quartiles was observed; 3.8%, 15.2%, 22% and 26.5% of deaths occurred from quartile 1 to 4 of CA125 (p<0.001). Likewise, a monotonic, ascending trend in the risk ratios was estimated from the multivariable Cox model. Compared with the first quartile of CA125, the HRs (95% CI) for the second, third and fourth quartiles were 3.25 (1.20 to 8.79), 4.91 (1.88 to 12.85) and 8.41 (3.24 to 21.79), respectively.
Conclusions
Serum levels of CA125 obtained in patients admitted with a diagnosis of acute HF was shown to be an independent predictor of mortality up to the 6‐month follow‐up.
Heart failure (HF) is increasingly recognised as a major public health problem in the USA and in Western countries.1,2,3 Several biomarkers, including natriuretic peptides4,5 and some inflammatory markers,6,7,8 have been shown to be valuable tools for diagnostic and risk stratification purposes. However, the availability of biomarkers in this field has been limited by an educational lag in the translation of research findings to general practice, increases in healthcare costs and a lack of consensus/guidelines for the use of appropriate biomarkers.
Carbohydrate antigen 125 (CA125) is a glycoprotein synthesised by epithelial serosal cells. It is used clinically for diagnosing and monitoring several neoplasias, especially ovarian cancer.9,10 Recently, CA125 has emerged as a potential biomarker in HF, by showing correlations with clinical, haemodynamic and echocardiographic parameters indicative of the severity of the disease.11,12,13,14,15,16 Interestingly, fluctuations in serum levels of CA125 after treatment were also found, suggesting potential utility in a serial and long‐term therapeutic intervention assessment.11,14 Astoundingly, researchers have also found that increased levels of CA125 were, in a univariate setting, predictive of mortality and rehospitalisation at 6 months since admission.11
The main objective of the present study was to assess the ability of CA125 independently to predict subsequent mortality in patients admitted to hospital with a diagnosis of acute HF. As a secondary aim, we sought to determine the optimal threshold, among the continuum of CA125 values, which best discriminate between high‐ and low‐risk populations for subsequent mortality.
Methods
Study population
Initially, we studied a cohort of 565 patients consecutively admitted to our cardiology department from 1 January 2003 to 1 January 2005, with the diagnosis of acute HF (defined as the rapid onset of symptoms and signs secondary to abnormal cardiac function, and established by trained cardiologists). Patients with a diagnosis of acute coronary syndrome, cancer, pneumonia, sepsis, severe hepatic disease or end‐stage renal disease undergoing dialysis treatment were excluded from this registry (n = 25). Additionally, 11 patients were excluded from the final analysis owing to missing CA125 values, leaving a final study sample of 529 patients. Demographic, medical history, vital signs, 12‐lead ECG and laboratory tests were determined routinely. Parallel to this registry, 181 patients, with heterogeneous cardiac conditions (stable ischaemic heart disease, atrial fibrillation and hypertension), were consecutively selected from an outpatient setting, and were classified as controls. Patients were excluded from this cohort if they had a history of cancer, or when the diagnosis was made during the index hospitalisation, as well as those with evidence of HF.
A comprehensive two‐dimensional echocardiography was performed with commercially available equipment (Agilent Sonos 5500, Phillips). Chamber diameters and wall thickness were measured using two‐dimensionally guided M‐mode echocardiography. Left ventricular ejection fraction (LVEF) was calculated using the Simpson method. Serum levels of CA125 were obtained during hospitalisation (48±12 h) using a commercially available immunoassay kit (Elecsys CA125 II assay, Roche Diagnostics). The established normal range for this assay was 0–35 U/ml. Indications for other diagnostic techniques and/or treatments were individualised following established guidelines.17
Ascertainment of end point
Death from any cause was selected as the main end point and ascertained during hospitalisation, by telephone contact with patient/family or during routine clinic visits. A patient's follow‐up was censored if he or she had had valve replacement surgery or cardiac transplantation.
Statistical analysis
Continuous variables were expressed as mean (1 SD) or median (interquartile range) when appropriate. Discrete variables were presented as percentages. Baseline characteristics were compared among categories created by quartiles of CA125 distribution. For continuous normally distributed variables, the omnibus p value for comparison was calculated with analysis of variance; for highly skewed variables, the Kruskal–Wallis rank test was used. A test for trend among ordered categories was also performed. The χ2 test was used for the comparison of discrete variables.
Three goals were pursued:
We compared the mean CA125 between patients assigned to a control group (subjects without clinical evidence of HF) versus patients included in the acute HF registry. Owing to a severe imbalance in the number of samples (181 vs 529), we matched each control with up to four cases in age, gender, hypertension, diabetes, dyslipidaemia, smoking habit, serum creatinine and atrial fibrillation. The nearest‐neighbour matching estimator was implemented as the matching algorithm. The mean for each matched pair was compared with a paired t test.
We tested the predictive ability of CA125 for all‐cause mortality by using survival analysis. Patients were censored under the following circumstances: (1) at the 6‐month follow‐up after index admission; (2) if death occurred during the 6‐month interval; (3) if any surgical procedure had notorious influences on the long‐term hazard, such as cardiac transplantation and valvular replacement surgery (21 patients); or (4) if the patient was lost to follow‐up (12 patients). Cumulative mortality curves were estimated using the Kaplan–Meier method and their differences were tested by the Peto‐Peto–Prentice test. The independent effect of CA125 serum levels on mortality was assessed by Cox regression analysis; crude and adjusted hazard ratios were presented with their respective 95% CIs. Candidate covariates for multivariate Cox analysis were introduced into the model based on previous medical knowledge independent of their p values. From this initial model, a parsimonious, highly predictive model was derived using a backward stepdown selection.18 The proportionality assumption for the hazard function over time was tested by the mean of the Schoenfeld residuals. The functional form of continuous variables in the log hazard scale was examined by means of fractional polynomials.19 The predictive ability of the Cox models was assessed by estimating Harrell's C statistics.20
In the absence of known biological evidence supporting a particular threshold along the continuum of CA125, we used the generalised additive model (GAM) with Cox extension to depict the underlying relationship between CA125 and mortality. GAM uses a non‐parametric algorithm with cubic smoothing splines. Visually depicted by the GAM plot, the threshold represents the value by which CA125 can be stratified into two categories: the threshold above or below which the risk for mortality was deemed higher or lower.21,22 As a sensitivity analysis, we identified, from the receiver operating characteristic curve, the value in the continuum of CA125 that minimises the square of the difference between sensitivity and specificity, as the optimal threshold that should be used when dichotomising this biomarker. Note that the term “optimal” only indicates the value of CA125 yielding the highest combination of sensitivity and specificity. A two‐sided p value of <0.05 was considered to be significant for all analyses. All statistical analyses were performed using STATA V.9.0.
Results
Clinical and demographic characteristics
The mean (SD) age in our sample was 73 (10) years; 52.9% were women and 66% of the sample exhibited serum levels of CA125 >35 U/ml. Table 1 shows the clinical characteristics of the study population according to quartiles of CA125 distribution. Age and gender prevalence were equally distributed among CA125 quartiles. Overall, there was a positive, monotonic association between the parameters of disease severity and increasing levels of CA125. Indeed, a higher proportion of patients in New York Heart Association (NYHA) class III/IV (measured under clinically stable conditions, before the index admission), LVEF ⩽45% and atrial fibrillation were found among quartiles 1–4 of CA125. Likewise, lower systolic and diastolic blood pressure as well as higher uric acid concentrations, arterial pH and left atrial dimension were positively related to increased CA125. Higher levels of CA125 were more often associated with pleural effusion, peripheral oedema, acute decompensated HF and cardiogenic shock. On the contrary, an inverse association was observed in patients with acute pulmonary oedema, hypertensive acute HF, a history of hypertension, dyslipidaemia and arterial carbon dioxide partial pressure concentrations. No other significant differences were observed in other baseline characteristics (table 1).
Table 1 Baseline characteristics of the study population.
| Variables | CA125 (U/ml) | Omnibus p value | p Value for trend | |||
|---|---|---|---|---|---|---|
| Q1 (4.6–25.1) (n = 133) | Q2 (25.3–60.3) (n = 132) | Q3 (61.2–141.5) (n = 132) | Q4 (141.7–1169.5) (n = 132) | |||
| Demographic and medical history | ||||||
| Age, years | 73 (10) | 75 (10) | 73 (10) | 72 (11) | 0.123 | 0.250 |
| Women, % | 63 (47.4) | 52 (39.4) | 66 (50.0) | 68 (51.5) | 0.201 | 0.239 |
| Hypertension, % | 104 (78.2) | 106 (80.3) | 97 (73.5) | 83 (62.9) | 0.006 | 0.002 |
| Dyslipidaemia, % | 46 (34.6) | 51 (38.6) | 38 (28.8) | 29 (22.0) | 0.021 | 0.008 |
| Current smoker, % | 16 (12.1) | 11 (8.3) | 13 (9.9) | 13 (9.9) | 0.784 | 0.651 |
| Diabetes mellitus, % | 42 (31.6) | 58 (43.9) | 49 (37.1) | 55 (41.7) | 0.172 | 0.214 |
| Ischaemic heart disease, % | 45 (33.8) | 57 (43.2) | 51 (38.6) | 49 (37.1) | 0.469 | 0.775 |
| Valvular heart disease, % | 38 (28.6) | 26 (19.7) | 41 (31.1) | 35 (26.5) | 0.184 | 0.765 |
| Previous hospitalisation for AHF, % | 63 (47.4) | 52 (39.7) | 55 (41.7) | 57 (43.2) | 0.633 | 0.579 |
| ADHF, % | 76 (57.1) | 85 (64.3) | 97 (73.4) | 105 (79) | <0.001 | <0.001 |
| Cardiogenic shock, % | 3 (2.3) | 5 (3.8) | 7 (5.3) | 10 (7.5) | 0.291 | 0.049 |
| Acute pulmonary oedema, % | 35 (26.3) | 29 (22) | 18 (13.6) | 9 (6.8) | <0.001 | <0.001 |
| Hypertensive AHF, % | 18 (13.5) | 12 (9.1) | 9 (6.8) | 8 (6.1) | 0.135 | 0.026 |
| NYHA class III/IV, %* | 18 (13.5) | 24 (18.3) | 30 (22.7) | 46 (34.9) | <0.001 | <0.001 |
| COPD, % | 29 (21.8) | 22 (16.7) | 18 (13.6) | 27 (20.5) | 0.299 | 0.634 |
| Stroke, % | 10 (7.5) | 10 (7.6) | 19 (14.4) | 15 (11.4) | 0.190 | 0.120 |
| PAD, % | 13 (9.8) | 11 (8.3) | 10 (7.6) | 9 (6.8) | 0.838 | 0.365 |
| Radiological pleural effusion, % | 19 (14.3) | 45 (34.4) | 67 (50.8) | 92 (69.7) | <0.001 | <0.001 |
| Peripheral oedema, % | 51 (38.4) | 62 (47.3) | 84 (63.6) | 90 (68.2) | <0.001 | <0.001 |
| Previous use of diuretics, % | 84 (65.1) | 85 (65.9) | 88 (66.7) | 95 (72.0) | 0.631 | 0.244 |
| Vital signs | ||||||
| Heart rate, bpm† | 106 (29) | 100 (29) | 103 (28) | 98 (28) | 0.163 | 0.104 |
| Systolic blood pressure, mm Hg† | 159 (38) | 156 (34) | 149 (35) | 144 (32) | 0.002 | <0.001 |
| Diastolic blood pressure, mm Hg† | 86 (21) | 88 (20) | 81 (18) | 81 (18) | 0.003 | 0.004 |
| ECG | ||||||
| Atrial fibrillation, % | 43 (32.3) | 50 (37.9) | 62 (47.3) | 66 (50.0) | 0.012 | 0.001 |
| QRS>120 ms, % | 34 (28.6) | 36 (32.1) | 24 (21.1) | 29 (25.4) | 0.278 | 0.271 |
| Laboratory | ||||||
| Haemoglobin, g/dl | 13.2 (1.8) | 12.6 (1.9) | 12.3 (1.7) | 13.1 (1.8) | <0.001 | 0.487 |
| Serum creatinine, mg/dl† | 1.2 (0.4) | 1.2 (0.5) | 1.2 (0.5) | 1.3 (0.6) | 0.117 | 0.123 |
| Uric acid, mg/dl | 7.5 (2.2) | 7.6 (2.5) | 8.1 (2.4) | 8.8 (2.9) | <0.001 | <0.001 |
| Sodium, meq/l | 140 (4) | 139 (5) | 139 (4) | 139 (5) | 0.467 | 0.202 |
| Troponin I >0.2 ng/ml, % | 42 (32.8) | 39 (31.0) | 35 (28.0) | 35 (27.8) | 0.784 | 0.321 |
| pH† | 7.38 (0.14) | 7.40 (0.12) | 7.42 (0.10) | 7.43 (0.06) | <0.001 | <0.001 |
| pO2, mm Hg† | 57 (20) | 59 (14) | 59 (20) | 60 (14) | 0.352 | 0.103 |
| pCO2, mm Hg† | 43 (13) | 39 (14) | 38 (12) | 38 (10) | <0.001 | <0.001 |
| Echocardiography | ||||||
| LVEF⩽45% | 45 (33.8) | 49 (37.1) | 49 (37.1) | 67 (50.8) | 0.025 | 0.008 |
| LAD, mm | 42 (8) | 43 (8) | 46 (9) | 47 (7) | <0.001 | <0.001 |
| LVDD, mm | 57 (10) | 56 (11) | 55 (9) | 56 (10) | 0.717 | 0.927 |
ADHF, acute decompensated heart failure; AHF, acute heart failure; CA125, carbohydrate antigen 125; COPD, chronic obstructive pulmonary disease; LAD, left atrial diameter; LVDD, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PAD, peripheral artery disease; pO2, arterial oxygen partial pressure; pCO2, arterial carbon dioxide partial pressure.
Values are presented as mean (SD), unless otherwise specified; categorical variables are presented as percentages.
*NYHA functional class measured under clinicaly stable conditions, before the index admission.
†Variable presented as the median (interquartile range).
Serum CA125 levels in patients with and without HF
We found significant differences between patients with and without HF. Patients with acute HF exhibited a sevenfold increase in the mean CA125 serum levels compared with controls (105.2 (SD 139) vs 14.9 (22) U/ml, respectively p<0.001).
Relationship of CA125 to 6‐month mortality
Time‐to‐event analyses
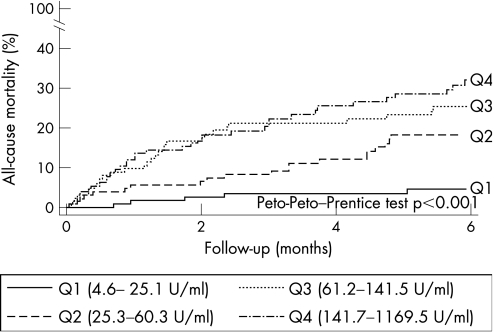
At the 6‐month follow‐up (median 5.94, interquartile range 1.80–5.94) a total of 89 deaths were identified: 37 during the index hospitalisation and 52 at follow‐up; their distribution among quartiles of CA125 shows a positive, monotonic increase in the proportion of deaths by moving from quartiles 1 to 4 (p<0.001; table 2). Figure 1 shows, by means of the Kaplan–Meier method, significant differences in mortality among quartiles of CA125 (Peto‐Peto–Prentice test p<0.001). In multivariate analysis, CA125 was a predictor of mortality independently of age (years), gender, diabetes and their interaction (gender×diabetes), NYHA class III/IV, valvular heart disease aetiology for HF, systolic blood pressure (mm Hg), serum creatinine (mg/dl) and haemoglobin (g/dl). Variables that were excluded from the final model owing to lack of statistical significance were LVEF (continuous as well as categorised at 45%), aetiology of ischaemic heart failure and type of acute HF presentation. The adjusted hazard ratios (HRs (95% CI)) progressively increased from quartile 1 to 4 of CA125 Q2 = 3.25 (1.20 to 8.79); Q3 = 4.91 (1.88 to 12.85); and Q4 = 8.41 (3.24 to 21.79). Because the functional form of CA125 within the Cox model was identified as having a parabolic shape (fig 2A), the HR, when the variable was included in its continuous form, was expressed by comparing the change between the 25th and the 75th centiles. The resulting HRs expressing a change between the 25th and 75th centiles among the variable distribution were similar when tested under univariate or multivariate settings (2.76 and 2.86, respectively). Varying the threshold level when dichotomising the variable did not change the previous conclusions. CA125 was independently associated with mortality when dichotomised at values 35 and 60 U/ml, which are the cut‐off points that represent the upper normal limit accepted for this biomarker, and the prognostic‐driven cut‐off point estimated in our sample, respectively. No interactions were found between CA125 and any of the covariates included in the final model. Harrell's C statistics were calculated for each of the Cox models with results ranging from 0.596 to 0.659 for univariate models and from 0.803 to 0.816 for multivariate models (table 3).
Table 2 Mortality across quartiles of carbohydrate antigen 125 serum levels.
| Variable | Q1 (U/ml) 4.6–25.1 (n = 133) | Q2 (U/ml) 25.3–60.3 (n = 132) | Q3 (U/ml) 61.2–141.5 (n = 132) | Q4 (U/ml) 141.7–1169.5 (n = 132) | All (n = 529) |
|---|---|---|---|---|---|
| Event rates | |||||
| P‐M | 623.93 | 589.00 | 533.29 | 500.70 | 2246.91 |
| Events, n % | 5 (3.8) | 20 (15.2) | 29 (22.0) | 35 (26.5) | 89 (16.8) |
| Crude rates (×100 P‐M) | 0.80 | 3.40 | 5.44 | 6.99 | 3.96 |
| 95% CI | 0.34 to 2.38 | 2.21 to 5.44 | 3.73 to 8.14 | 4.98 to 9.99 | 3.21 to 4.93 |
P‐M, person‐months; Q, quartile.
Figure 1 Kaplan–Meier method: differences in mortality among carbohydrate antigen 125 quartiles (Q1–Q4).
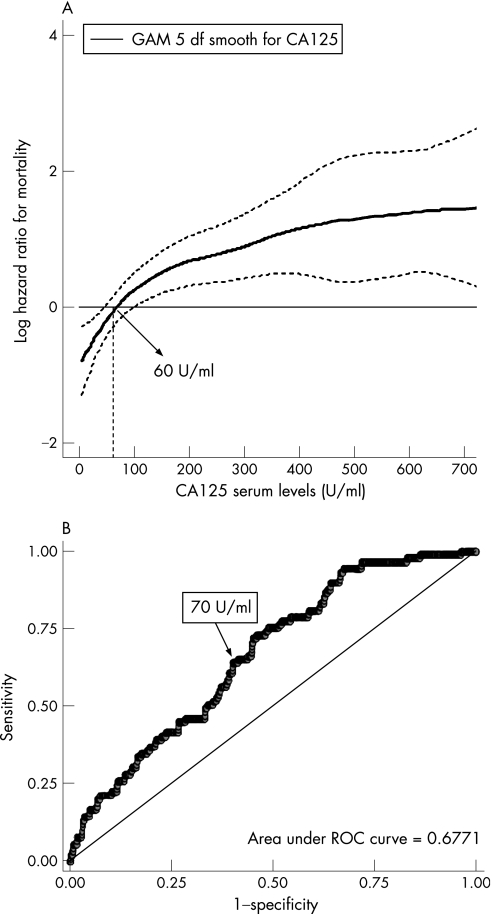
Figure 2 Optimal carbohydrate antigen 125 (CA125) threshold suggested by the generalised additive model (GAM) plot. (A) The functional form in the association between CA125 and the hazard rate for all‐cause mortality. This association is adjusted simultaneously by age, gender, diabetes and their interaction (gender×diabetes), stable New York Heart Association class III/IV, aetiology of valvular heart disease, systolic blood pressure, serum creatinine and haemoglobin. The dotted curves indicate approximate 95% CIs for the smoothed hazard. The arrow indicates the point in the continuum of CA125 that crosses the threshold between high and low risk for mortality (around 60 U/ml). The CA125 distribution was truncated at values >700 U/ml. (B) The receiver operating characteristic (ROC) curve showing the sensitivity and specificity corresponding to each CA125 value, with all‐cause mortality, evaluated up to the 6‐month follow‐up, used as the reference test. The optimal cut‐off point was estimated at 70 U/ml, with an associated sensibility and specificity of 63 and 61, respectively. df, degrees of freedom.
Table 3 Hazard ratios for all‐cause mortality according to quartiles of carbohydrate antigen 125 serum levels.
| Cox regression models | HR (95% CI) | p Value | Harrell's C statistics |
|---|---|---|---|
| Unadjusted HRs | |||
| CA125, U/ml*† | 2.73 (1.81 to 4.10) | <0.001 | 0.656 |
| CA125 quartiles (min and max values, U/ml) | 0.659 | ||
| Q1 (4.6–25.1) | 1 | ||
| Q2 (25.3–60.3) | 4.17 (1.57 to 11.12) | 0.004 | |
| Q3 (61.2–141.5) | 6.61 (2.56 to 17.07) | <0.001 | |
| Q4 (141.7–1169.5) | 8.27 (3.24 to 21.11) | <0.001 | |
| CA125 >35 U/ml | 2.79 (1.60 to 4.86) | <0.001 | 0.596 |
| CA125 >60 U/ml | 2.91 (1.83 to 4.62) | <0.001 | 0.630 |
| Adjusted HRs‡ | |||
| CA125, U/ml* | 2.83 (1.81 to 4.42) | <0.001 | 0.816 |
| CA125 quartiles (min and max values, U/ml) | 0.815 | ||
| Q1 (4.6–25.1) | 1 | ||
| Q2 (25.3–60.3) | 3.25 (1.20 to 8.79) | 0.020 | |
| Q3 (61.2–141.5) | 4.91 (1.88 to 12.85) | 0.001 | |
| Q4 (141.7–1169.5) | 8.41 (3.24 to 21.79) | <0.001 | |
| CA125 >35 U/ml | 2.41 (1.37 to 4.24) | 0.002 | 0.803 |
| CA125 >60 U/ml | 2.86 (1.77 to 4.62) | <0.001 | 0.810 |
CA125, carbohydrate antigen 125; HRs, hazard ratios; max, maximum; min, minimum; Q, quartile.
*Per 25–75th centile change.
†Area under the curve (95% CI) calculated by receiver operating characteristic curve analysis = 0.667 (0.61 to 0.72)
‡Cox model adjusted by age, gender, diabetes and their interaction (gender×diabetes), New York Heart Association class III/IV, aetiology of valvular heart disease, systolic blood pressure, serum creatinine and haemoglobin.
Proposed cut‐off points
The GAM plot depicted in fig 2A shows that mortality risk increased markedly as CA125 increases up to 60 U/ml, levelling off afterwards. The curve also identified a value around 60 U/ml to be the value of CA125 where the increased hazard rate for mortality begins (the point that the GAM curve crossed a hazard rate of 0). This threshold value was further supported by the finding that the best operating point derived from the receiver operating characteristic curve pointed towards a similar value (fig 2B).
Discussion
To our knowledge, this study is the first to demonstrate that serum levels of CA125 obtained in patients hospitalised for acute HF were independently associated with increased mortality. We observed an almost threefold increase in risk for all‐cause mortality for a change between the 25th and 75th centiles across the continuum of its distribution. Similar conclusions were reached by including CA125 dichotomised by its median (60 U/ml), by using the standard cut‐off point accepted for this biomarker in trials on cancer (35 U/ml). The same result was found when the entire distribution was grouped by its quartiles. Regardless of how this biomarker is presented, it is consistently shown to be a powerful predictor for mortality in a population previously admitted for acute HF (table 3). This association persists even after adjustments for variables traditionally accepted to be powerful predictors for mortality.
Rationale for the study
HF is associated with an excessive and increasing morbidity and mortality, generating an extreme burden on our healthcare system.23,24,25,26,27,28 In the USA, HF is the leading cause of hospital admission in the Medicare population.1,24 In Spain, there are 80 000 new hospitalisations for HF per year in patients aged ⩾65 years, being the third most common cause of cardiovascular mortality.26 Despite the high mortality after 1 year following an episode of decompensation,3,28 risk stratification is not performed routinely, owing to the limited availability of biomarkers. Therefore, searching for the optimal biomarkers constitutes a source of permanent research in the HF field, to determine diagnostic and therapeutic measures tailored to the level of risk.
To date, increases in serum levels of CA125 have been described predominantly in women with ovarian cancer, particularly in those cases with peritoneal involvement,10,29 and used as a tool for monitoring treatment.9 CA125 has also been reported to be increased, although less frequently, in other types of cancer (lung, uterine, breast and gastrointestinal tract), and in some non‐malignant diseases characterised by serosal effusions (nephrotic syndrome and hepatic cirrhosis). However, the role of CA125 as a cardiac biomarker has only emerged in recent observational studies11,12,13,14,15,16 that have found CA125 (1) to be increased in patients with HF; (2) to be associated with clinical and echocardiographic parameters indicative of disease severity; and (3) to have a potential role as a prognostic marker.
Previous studies
CA125 has been found to be increased in patients with left ventricular systolic dysfunction, showing a close relationship with the NYHA class, and with haemodynamic parameters (pulmonary artery wedge pressure and right atrial pressure), echocardiographic abnormalities (deceleration time of early filling on transmitral Doppler)11 and levels of brain natriuretic peptide.30 D'Aloia et al11 reported a positive (although unadjusted) association between CA125 and short‐term combined end point (total mortality and hospitalisation for worsening HF) in a cohort of 240 patients with left ventricular systolic dysfunction. In addition, recent studies have reported that serum levels of CA125 fluctuate according to clinical improvement due to medical treatment, underlying a potential role for monitoring the response to treatment.11,12,14,15,30
CA125 has been found to be increased in patients with pericardial, pleural and peritoneal effusions.31,32,33 Epiney et al31 reported that mesothelial cells from peritoneum and pleura are able to synthesise CA125. We showed an important association between the presence of radiological pleural effusion and CA125 serum concentrations at baseline (table 1). This finding can be interpreted as indirect evidence supporting the claim that the main source of CA125 increases in HF is from serosal effusions, especially from the pleura. Similar findings were recently reported by Turk et al,16 by comparing mean values of CA125 in patients with HF with and without pleural effusion and a control group. These authors showed higher CA125 values in patients with pleural effusion, intermediate values in those with no pleural effusion and lower values in the control group, with differences reported to be statistically significant. On the basis of this preliminary evidence, we assume that the increase in CA125 that occurred in patients with HF is a reliable surrogate of the amount of serosal effusions (pleura, pericardium and peritoneum) as a consequence of the increase in systemic and pulmonary venous pressure. Other authors have speculated that mesothelial cells are able to synthesise CA125, even in the absence of fluid accumulation, and are triggered by cytokine stimulation, as occurs in ovarian cancer and lymphoma.34,35,36 However, the link between inflammatory biomarkers such as tumour necrosis factor and interleukin 6 cytokines, often found to be increased in HF, and CA125 remains unknown.
Strengths and limitations
This study has several strengths. To the best of our knowledge, this is the first time that a study has shown the independent effect of CA125 on mortality after an episode of acute HF. Secondly, the patients in our registry have been followed prospectively using careful and consistent methods, including clinical chart reviews performed by specialised medical personnel and a dedicated database software for storage. Finally, owing to an observational nature, our results can be extrapolated to patients commonly seen in routine clinical practice, adding a “real‐world” picture to what is already published in the medical literature.
Likewise, some limitations need to be addressed. First, the pathophysiological basis underlying the association observed between an increase in CA125 and mortality in HF is not yet clear. Increases in CA125 could merely reflect a surrogate for the progression of the disease, or may play an active role in worsening cardiac function. Secondly, the lack of availability of the measurements of brain natriuretic peptide in our registry does not permit a comparison of diagnostic accuracy between these two biomarkers.
Conclusions
To our knowledge, this is the first study demonstrating the independent prognostic value of CA125 for increased mortality after an episode of acute HF. The low cost, wide availability and easy determination of this biomarker make it a potential tool for risk stratification in this population. However, our preliminary results need to be supported by further studies looking at the ability of CA125 to predict cardiovascular outcomes. Furthermore, our findings need to be extended by studies comparing the diagnostic accuracy between CA125 and the natriuretic peptides biomarker family. Only then, and by means of cost‐effective analyses, will the true role of CA125 in risk stratification and patient management be established.
Abbreviations
CA125 - carbohydrate antigen 125
GAM - generalised additive model
HF - heart failure
LVEF - left ventricular ejection fraction
NYHA - New York Heart Association
Footnotes
Competing interests: None declared.
References
- 1.Thom T, Haase N, Rosamond W.et al Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006113e85–151. [DOI] [PubMed] [Google Scholar]
- 2.Stewart S, MacIntyre K, Capewell S.et al Heart failure and the aging population: an increasing burden in the 21st century? Heart 20038949–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonarow G C, Adams K F, Jr, Abraham W T.et al Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005293572–580. [DOI] [PubMed] [Google Scholar]
- 4.Doust J A, Pietrzak E, Dobson A.et al How well does B‐type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ 2005330625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamada Y, Tanaka N, Murata K.et al Significance of predischarge BNP on one‐year outcome in decompensated heart failure—comparative study with echo‐Doppler indexes. J Card Fail 20051143–49. [DOI] [PubMed] [Google Scholar]
- 6.Iaccarino G, Barbato E, Cipolletta E.et al Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur Heart J 2005261752–1758. [DOI] [PubMed] [Google Scholar]
- 7.MacGowan G A, Mann D L, Kormos R L.et al Circulating interleukin‐6 in severe heart failure. Am J Cardiol 1997791128–1131. [DOI] [PubMed] [Google Scholar]
- 8.Orus J, Roig E, Perez‐Villa F.et al Prognostic value of serum cytokines in patients with congestive heart failure. J Heart Lung Transplant 200019419–425. [DOI] [PubMed] [Google Scholar]
- 9.Bates S E. Clinical applications of serum tumor markers. Ann Intern Med 1991115623–638. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien T J, Tanimoto H, Konishi I.et al More than 15 years of CA 125: what is known about the antigen, its structure and its function. Int J Biol Markers 199813188–195. [DOI] [PubMed] [Google Scholar]
- 11.D'Aloia A, Faggiano P, Aurigemma G.et al Serum levels of carbohydrate antigen 125 in patients with chronic heart failure: relation to clinical severity, hemodynamic and Doppler echocardiographic abnormalities, and short‐term prognosis. J Am Coll Cardiol 2003411805–1811. [DOI] [PubMed] [Google Scholar]
- 12.Kouris N T, Zacharos I D, Kontogianni D D.et al The significance of CA125 levels in patients with chronic congestive heart failure. Correlation with clinical and echocardiographic parameters. Eur J Heart Fail 20057199–203. [DOI] [PubMed] [Google Scholar]
- 13.Nagele H, Bahlo M, Klapdor R.et al Tumor marker determination after orthotopic heart transplantation. J Heart Lung Transplant 199918957–962. [DOI] [PubMed] [Google Scholar]
- 14.Nagele H, Bahlo M, Klapdor R.et al Fluctuations of tumor markers in heart failure patients pre and post heart transplantation. Anticancer Res 1999192531–2534. [PubMed] [Google Scholar]
- 15.Nagele H, Bahlo M, Klapdor R.et al CA 125 and its relation to cardiac function. Am Heart J 19991371044–1049. [DOI] [PubMed] [Google Scholar]
- 16.Turk H M, Pekdemir H, Buyukberber S.et al Serum CA 125 levels in patients with chronic heart failure and accompanying pleural fluid. Tumour Biol 200324172–175. [DOI] [PubMed] [Google Scholar]
- 17.Nieminen M S, Bohm M, Cowie M R.et al Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J 200526384–416. [DOI] [PubMed] [Google Scholar]
- 18.Ambler G, Brady A R, Royston P. Simplifying a prognostic model: a simulation study based on clinical data. Stat Med 2002213803–3822. [DOI] [PubMed] [Google Scholar]
- 19.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 199928964–974. [DOI] [PubMed] [Google Scholar]
- 20.Harrell F E, Jr, Margolis P A, Gove S.et al Development of a clinical prediction model for an ordinal outcome: the World Health Organization multicentre study of clinical signs and etiological agents of pneumonia, sepsis and meningitis in young infants. WHO/ARI Young Infant Multicentre Study Group. Stat Med 199817909–944. [DOI] [PubMed] [Google Scholar]
- 21.Royston P, Sauerbrei W. A new measure of prognostic separation in survival data. Stat Med 200423723–748. [DOI] [PubMed] [Google Scholar]
- 22.Royston P, Altman D G, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 200625127–141. [DOI] [PubMed] [Google Scholar]
- 23.Berry C, Murdoch D R, McMurray J J. Economics of chronic heart failure. Eur J Heart Fail 20013283–291. [DOI] [PubMed] [Google Scholar]
- 24.Ghali J K, Cooper R, Ford E. Trends in hospitalization rates for heart failure in the United States, 1973–1986. Evidence for increasing population prevalence. Arch Intern Med 1990150769–773. [PubMed] [Google Scholar]
- 25.McMurray J, McDonagh T, Morrison C E.et al Trends in hospitalization for heart failure in Scotland 1980–1990. Eur Heart J 1993141158–1162. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez‐Artalejo F, Banegas B, Jr, Guallar‐Castillon P. Epidemiology of heart failure. Rev Esp Cardiol 200457163–170. [PubMed] [Google Scholar]
- 27.Stewart S, MacIntyre K, MacLeod M M.et al Trends in hospitalization for heart failure in Scotland, 1990–1996. An epidemic that has reached its peak? Eur Heart J 200122209–217. [DOI] [PubMed] [Google Scholar]
- 28.Lee D S, Austin P C, Rouleau J L.et al Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA 20032902581–2587. [DOI] [PubMed] [Google Scholar]
- 29.Bast R C, Jr, Klug T L, St John E.et al A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983309883–887. [DOI] [PubMed] [Google Scholar]
- 30.Faggiano P, D'Aloia A, Brentana L.et al Serum levels of different tumour markers in patients with chronic heart failure. Eur J Heart Fail 2005757–61. [DOI] [PubMed] [Google Scholar]
- 31.Epiney M, Bertossa C, Weil A.et al CA125 production by the peritoneum: in‐vitro and in‐vivo studies. Hum Reprod 2000151261–1265. [DOI] [PubMed] [Google Scholar]
- 32.Seo T, Ikeda Y, Onaka H.et al Usefulness of serum CA125 measurement for monitoring pericardial effusion. Jpn Circ J 199357489–494. [DOI] [PubMed] [Google Scholar]
- 33.Sevinc A, Buyukberber S, Sari R.et al Elevated serum CA‐125 levels in hemodialysis patients with peritoneal, pleural, or pericardial fluids. Gynecol Oncol 200077254–257. [DOI] [PubMed] [Google Scholar]
- 34.Kubonishi I, Bandobashi K, Murata N.et al High serum levels of CA125 and interleukin‐6 in a patient with Ki‐1 lymphoma. Br J Haematol 199798450–452. [DOI] [PubMed] [Google Scholar]
- 35.Marth C, Zeimet A G, Widschwendter M.et al Regulation of CA 125 expression in cultured human carcinoma cells. Int J Biol Markers 199813207–209. [PubMed] [Google Scholar]
- 36.Zeimet A G, Offner F A, Marth C.et al Modulation of CA‐125 release by inflammatory cytokines in human peritoneal mesothelial and ovarian cancer cells. Anticancer Res 1997173129–3131. [PubMed] [Google Scholar]