Abstract
Objective
To evaluate the effect of pre‐procedural acute oral administration of trimetazidine (TMZ) on percutaneous coronary intervention (PCI)‐induced myocardial injury.
Design
Single‐centre, prospective, randomised evaluation study.
Setting
Patients with stable angina pectoris and single‐vessel disease undergoing PCI.
Patients
582 patients were prospectively randomised. Patients who underwent more than one inflation during PCI were excluded, resulting in 266 patients randomly assigned to 2 groups.
Interventions
Patients were randomly assigned to receive or not an acute loading dose of 60 mg of TMZ prior to intervention.
Main outcome
The frequency and the increase in the level of cardiac troponin Ic (cTnI) after successful PCI. cTnI levels were measured before and 6, 12, 18 and 24 h after PCI.
Results
136 patients were assigned to the TMZ group and 130 to the control group. Although no statistically significant difference was observed in the frequency of cTnI increase between the two groups, post‐procedural cTnI levels were significantly reduced in the TMZ group at all time points (6 h: mean (SD) 4.2 (0.8) vs 1.7 (0.2), p<0.001; 12 h: 5.5 (1.5) vs 2.3 (0.4), p<0.001; 18 h: 9 (2.3) vs 3 (0.5), p<0.001; and 24 h: 3.2 (1.2) vs 1 (0.5), p<0.001). Moreover, the total amount of cTnI released after PCI, as assessed by the area under the curve of serial measurement, was significantly reduced in the TMZ group (p<0.05).
Conclusion
Pre‐procedural acute oral TMZ administration significantly reduces PCI‐induced myocardial infarction.
Asymptomatic minor post‐procedural myocardial necrosis does have an important prognostic signification after percutaneous coronary intervention (PCI). The magnitude of increase in the level of cardiac troponin Ic (cTnI) directly correlates with irreversible myocardial injury assessed by cardiovascular MRI.1,2
Trimetazidine (TMZ; 1‐[2,3,4‐trimethoxybenzyl] piperazine) is a cellular anti‐ischaemic agent that selectively inhibits the activity of the final enzyme of the fatty acid oxidation pathway, 3‐ketoacylcoenzyme A thiolase. Administration of this drug leads to a switch in preference of the energy substrate, resulting in partial inhibition of fatty acid oxidation and increased glucose oxidation. Clinical studies have shown that TMZ has cardioprotective effects in the setting of myocardial ischaemia including acute myocardial infarction.3,4,5,6,7,8 However, although Kober et al9 have demonstrated that TMZ reduces pre‐procedural myocardial cell ischaemia as assessed by the duration and amplitude of ST elevation during PCI, whether its cytoprotective effects translate into a reduction of myocardial necrosis is unknown. The aim of this study was to evaluate the protective effect of an acute oral loading dose of TMZ (60 mg) on post‐procedural myocardial infarction as assessed by the frequency and level of cTnI release.
Methods
Patient population and study protocol
A single‐centre, prospective, randomised evaluation study was undertaken after approval by the local research ethics committee. The study was performed according to the principles of the Declaration of Helsinki. Between September 2003 and September 2005, informed consent was obtained from 582 consecutive patients undergoing successful single‐vessel stenting for stable angina pectoris in the Cardiology Department, University Hospital Nord, Marseille, France. Exclusion criteria were: multivessel disease, increased pre‐procedural cTnI before PCI, previous treatment with TMZ and contraindication for aspirin or clopidogrel. Because the number of inflations can modify the release of cTnI,10 patients with more than one inflation performed during PCI were secondarily excluded from the study after randomisation.
Successful PCI was defined according to the American Heart Association/American College of Cardiology Task Force recommendations.11 The criteria used were as follows:
no in‐hospital complication;
improvement in luminal narrowing to <30% of a contiguous normal vessel; and
complete resolution of clinical manifestation of ischaemia after PCI.
Randomisation
Patients were screened after coronary angiography had established that they fulfilled the study criteria. Once written informed consent was obtained, patients were randomly assigned to receive or not an acute loading dose (60 mg) of TMZ orally, starting 30 min before recanalisation, after which the operator was allowed to proceed with angioplasty. Previous studies have demonstrated that such an oral loading dose was efficient and safe and that TMZ anti‐ischaemic effects were present within 1 h after administration of a 60 mg oral loading dose.12
Interventional procedure
PCI was performed using a standard technique, through the femoral route using tubular slotted stents only. Procedures using direct stenting were considered to be mandatory, because this approach seems to be safer, reducing the number of devices, fluoroscopy time and contrast administration.10 Routine care was taken before and after the procedure for all patients, including pretreatment with a loading dose of clopidogrel (300 mg initial oral bolus) the day before the procedure, followed by 75 mg/day for 1 month, in addition to lifelong aspirin medication (160 mg/day). Intra‐coronary administration of linsidomine chlorhydrate (NO donor, an active metabolite of molsidomine; 1 mg) was given as required during the procedure. Intravenous bolus of unfractionated heparin (100 IU/kg), with activated coagulation time adjusted (200–300 s with Hemochron devices), was administered at the beginning of the procedure. No additional bolus of heparin or glycoprotein IIb/IIIa inhibitors was needed during or after the procedure. As recommended recently for coronary intervention when stent placement is expected, ioxaglate was used as the x ray contrast media.13 The sheath was removed immediately after the end of the procedure. The operator was not blind to the treatment.
ECG monitoring
A 12‐lead ECG was recorded before, 1 h after PCI and the following day. During the procedure, three ECG leads were constantly monitored. Occurrence, severity and duration of chest pain, acute ST elevation or depression (0.1 mV) and/or T‐wave abnormalities were recorded. Peri‐procedural variables such as length and diameter of the stent and duration of the inflation were recorded. Patients were monitored for at least 24 h. ST‐segment or T‐wave changes and Q waves that were clearly new compared with pre‐angioplasty baseline data were considered as clinical events if they persisted until hospital discharge. New Q waves were defined as those of at least 30 ms width and deeper than 25% of the correlating R amplitude, in at least two of the three diaphragmatic leads (II, III, aVF), in at least two of the four anteroseptal leads (V1–V4) or in at least two of the lateral leads (I, Vl, V5, V6)
Angiographic analysis
Classification of coronary artery morphology based on the report of the American Heart Association/American College of Cardiology Task Force.11 was used.
The cineangiograms were reviewed by two experienced angiographers who coded lesion‐related morphological variables and were blind to the results of biochemical assays. Intimal major or minor dissection, thrombus, abrupt closure in a previously patent vessel, no reflow, spasm and side‐branch occlusion were assessed. No reflow was defined as impaired or missed flow in the presence of an apparently open coronary vessel. Left ventricular function was assessed by angiography in all patients.
Blood sampling
Venous blood samples for measurement of cTnI were obtained from all patients before PCI and at 6, 12, 18 and 24 h after the procedure. The samples were drawn into tubes without anticoagulant and were kept at room temperature for 20 min to allow clotting. The samples were centrifuged at 3000 g for 10 min, and the serum was stored in aliquots at a temperature of −70°C until analysis.
Analytical method
Biochemical analysis was performed by a biochemist unaware of the patients' outcome. Serum samples were analysed for cTnI using the Dimension RxL/HM analyser (Dade Behring, Glasgow, Delaware, USA). The analytical sensitivity for cTnI was 0.2 ng/ml. Total imprecision expressed as coefficient of variation ranged between 8.6% and 9.5%.
End points
End points were collected by a blinded investigator who was not aware of the treatment status and clinical characteristics of the patients. The primary end point was the level of peak cTnI. Secondary end points were the frequency of cTnI release in the two groups and the total amount of cTnI release after the procedure.
Power calculation
We postulated that the average difference in cTnI release at peak would be 2 (4) ng/ml between the two groups. Therefore, for a 90% of power and an α risk of 1%, we estimated that 121 patients with one inflation during the procedure had to be included in each group. In our practice, 2.4 times more patients were undergoing multiple inflations than patients undergoing one inflation during PCI. Therefore, a total of 291 patients was necessary in each group.
Statistical analysis
The statistical analysis was performed using SPSS V.10.1 software. Significance was considered to be achieved for rounded two‐tailed p values <0.05. Results were expressed as mean (SD). Comparisons between the two groups were performed using Student's t tests or the Mann–Whitney U test for continuous variables as appropriate. χ2 Tests or Fisher's exact tests were used for categorical variables. Areas under the curve (AUC) of cTnI measurements were compared between the two studied groups (statistical means of summarising information from a series of measurements on one individual).
Results
From the 582 patients with single‐vessel disease undergoing PCI for stable coronary disease randomised, 155 patients were excluded from the TMZ group because they had more than one inflation performed. For the same reason, 161 patients were excluded from the control group. Finally, 266 patients were enrolled in this study. The studied population was composed of 136 patients in the TMZ group and 130 patients in the control group.
Pretreatment with a loading dose of TMZ was well tolerated and there were no instances of serious adverse events during the inhospital follow‐up.
Despite randomisation and because of the secondary exclusion criteria (more than one inflation during the angioplasty procedure), there were significant differences between the two groups for demographic and angiographic data.
Patients' demographic characteristics and baseline medication
Patients in the TMZ group were older (68 (12) vs 53 (13) years; p<0.001) and had a higher prevalence of risks factors for coronary artery disease including diabetes mellitus and high body mass index (59 vs 23 patients, p<0.001 and 27.2 (2.3) vs 21.6 (2.9); p<0.001, respectively). They also belonged to a higher angina class according to the Canadian classification (types 3 and 4: 47 vs 20 patients, p<0.001 (table 1)). According to these differences, the TMZ group was at higher clinical risk for complication. There was no difference in baseline characteristics for the of use medications between the two groups.
Table 1 Clinical demographics.
| Variables | TMZ group (n = 136) | Control group (n = 130) | p Value |
|---|---|---|---|
| Mean (SD) age (years) | 68 (12) | 53 (13) | <0.001 |
| Sex, male, n (%) | 98 (72) | 92 (71) | 0.8 |
| Renal insufficiency, n (%) | 23 (13) | 19 (15) | 0.6 |
| Angina class,* n (%) | |||
| 1 + 2 | 53 (39) + 36 (27) | 68 (52) + 42 (32) | <0.01 |
| 3 + 4 | 22 (16) + 25 (18) | 12 (9) + 8 (6) | <0.001 |
| Risk factors, n (%) | |||
| Smoking (past or current) | 79 (58) | 72 (55) | 0.65 |
| Diabetes mellitus | 59 (43) | 23 (17) | <0.001 |
| Hypercholesterolaemia | 56 (41) | 52 (40) | 0.84 |
| Hypertension | 55 (40) | 42 (31) | 0.13 |
| Family history | 14 (10) | 16 (12) | 0.6 |
| Mean (SD) BMI (kg/m2) | 27.2 (2.3) | 21.6 (2.9) | <0.001 |
| Medication, n (%) | |||
| β Blockers | 39 (28) | 41 (32) | 0.44 |
| Calcium antagonist | 42 (31) | 43 (33) | 0.7 |
| Aspirin | 76 (56) | 75 (58) | 0.76 |
| Clopidogrel | 24 (18) | 25 (19) | 0.74 |
| Statin | 42 (31) | 39 (30) | 0.88 |
| Insulin | 23 (17) | 29 (22) | 0.27 |
| ACE inhibitors | 43 (31) | 36 (28) | 0.57 |
BMI, body mass index; TMZ, trimetazidine.
*According to the Canadian classification.
Angiographic characteristics
The TMZ group exhibited significantly more B2 and C type lesions (American Heart Association/American College of Cardiology class) than the control group (59 vs 30 patients, p<0.001). TMZ group was at higher angiographic risk for complications (table 2).
Table 2 Angiographic characteristics.
| Variable, n (%) | TMZ group (n = 136) | Control group (n = 130) | p Value |
|---|---|---|---|
| Lesion class* | |||
| A + B1 | 77 (56) | 100(77) | <0.001 |
| B2 + C | 59 (44) | 30 (23) | <0.001 |
| Artery involved | |||
| Left main artery | 0 | 0 | 1 |
| Left anterior descending artery | 62 (46) | 65 (50) | 0.55 |
| Left circumflex artery | 27 (20) | 39 (30) | 0.06 |
| Right coronary artery | 33 (24) | 21 (16) | 0.1 |
| Diagonal | 4 (3) | 3 (2) | 0.95 |
| Saphenous venous graft | 10 (7) | 2 (2) | 0.56 |
| Bifurcation lesion | 22 (16) | 10 (8) | 0.03 |
| Mean (SD) LVEF (%) | 65 (8) | 62 (10) | 0.7 |
LVEF, left ventricular ejection fraction; TMZ, trimetazidine.
*According to the American Heart Association/American College of Cardiology classification.
Procedural characteristics
There was no stent loss or imprecise stent placement in either group (table 3). No “no reflow” or coronary spasm phenomenon was observed in the TMZ group. There was a trend towards more angioplasty complications such as side‐branch occlusion in the TMZ group (13 vs 6 patients, p = 0.12). ST‐segment changes during inflation tend to occur more often in the control group. However, this difference was not significant (29 vs 36 patients, p = 0.23). The frequency of procedural complication was not different between the two groups (20% in both groups).
Table 3 Procedural characteristics and complications.
| TMZ group (n = 136) | Control group (n = 130) | p Value | |
|---|---|---|---|
| Stent length (mm) | 12.3 (3.2) | 11.8(3.2) | 0.2 |
| Stent diameter (mm) | 3.13 (0.42) | 3.06 (0.46) | 0.2 |
| Total inflation time (s) | 30 (5) | 30 (5) | 1 |
| Inflation maximal pressure (atm) | 13.3 (0.2) | 13.3 (0.4) | 1 |
| TIMI flow grade ⩽2, n (%) | 0 (0) | 3 (2) | 0.22 |
| TIMI flow grade = 3, n (%) | 136 (100) | 127 (98) | 0.23 |
| ST change during inflation, n (%) | 29 (21) | 36 (28) | 0.23 |
| Procedural complication, n (%) | |||
| Side‐branch occlusion | 13 (10) | 6 (5) | 0.12 |
| Coronary dissection | 12 (9) | 13 (10) | 0.74 |
| No reflow | 0 | 3 (2) | 0.26 |
| Coronary spasm | 0 | 3 (2) | 0.26 |
| Coronary embolisation | 1 | 4 (3) | 0.36 |
| Increase in cTnI, n (%) | 30 (22) | 26 (20) | 0.68 |
cTnI, cardiac troponin Ic; TIMI, Thrombolysis In Myocardial Infarction Trial; TMZ, trimetazidine.
Values are represented as mean (SD) unless otherwise specified.
Serial change of cTnI after PCI
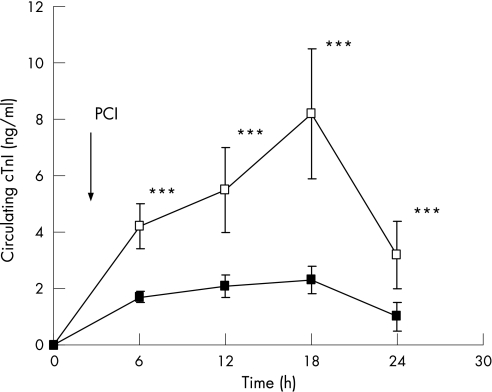
Table 3 shows the serial change of cTnI concentration after PCI. The frequency of patients with an increase in cTnI above the upper limit of the control range was not different between the two groups (30 vs 26, p = 0.7). However, post‐procedural cTnI was significantly higher in the control group than in the TMZ group after PCI at each time (6 h: 4.2 (0.8) vs 1.7 (0.2), p<0.001; 12 h: 5.5 (1.5) vs 2.3 (0.4), p<0.001; 18 h: 9 (2.3) vs 3 (0.5), p<0.001); and 24 h: 3.2 (1.2) vs 1 (0.5), p<0.001). To compare more accurately the total amount of cTnI release after PCI between the two groups, we calculated the AUC of post‐procedural cTnI release in each group (fig 1). The AUC of cTnI was significantly higher in the control group than in the TMZ group (p<0.05).
Figure 1 Time course of cardiac troponin Ic (cTnI) release. cTnI levels were measured in blood samples collected from patients before (T0) and 6, 12, 18 and 24 h after percutaneous coronary (PCI). Values are mean (SD) of values obtained for 130 (control, open symbols) and 136 (trimetazidine (TMZ), filled symbols) patients, respectively. The arrow indicates the time of PCI. ***p<0.001.
Discussion
This study demonstrates that pretreatment with a 60 mg acute oral loading dose of TMZ before elective PCI limits myocardial damage, as shown by a lower total amount of cTnI release after coronary angioplasty. This protective effect was found despite the fact that patients in the TMZ group were at higher risk for complications considering both clinical and angiographic data.
This result is consistent with a previous report by Kober et al9, who observed that TMZ administration before PCI reduces per‐procedural myocardial cells ischaemia, and further demonstrates that TMZ effect does translate into less myocardial necrosis assessed by cTnI measurement.
In the present study, we observed a trend towards a decrease in the frequency of ST‐segment change during inflation, although this difference was not significant (p = 0.22). However, it must be underlined that angioplasty procedures with systematic direct‐stent implantation reduce ischaemia duration compared with balloon angioplasty without stent implantation, which was used by Kober et al. In addition, the lack of statistical difference may be related to the higher risk profile of patients in the TMZ group. Moreover, we have studied a low‐risk population treated by direct stenting angioplasty, which may also explain the low frequency of ST change during angioplasty in our study.
Several characteristics of the present study are worthy of additional consideration and reinforce the significance of the TMZ protection detected.
First, because the number of inflations during PCI can affect troponin release, we chose to exclude patients with more than one inflation performed to avoid a potential confounding factor.10 This post‐randomisation exclusion resulted in significant differences between the two groups.
Specifically, according to these differences in age, angina class, diabetes and lesion class, the TMZ group was at higher risk. Interestingly, this suggests that the protective effect of TMZ was observed despite the fact that the post‐randomisation exclusion process worked against the detection by selecting a population at higher risk in the TMZ group. In addition, by selecting patients with a single‐vessel disease treated with direct stenting, we have recruited an overall low‐risk population for troponin increase, and this may also have precluded the evaluation of the maximal TMZ effects. Importantly, the frequency of procedural complication was not different between the two groups, which suggests the absence of procedural‐related confounding factors. Finally, given the pharmacodynamics of TMZ, it is possible that an earlier administration could enhance the detected effect of the drug on troponin release.12,14,15
Mechanistically, the effect of TMZ on myocardial necrosis could be explained by both its metabolic and biological effects. TMZ has been shown to act as a cellular anti‐ischaemic agent without any haemodynamic effects.16 It acts by improving cardiac energy metabolism by switching ATP production from lipid to glucose oxidation, thus enhancing intramitochondrial coupling and favouring a more efficient mode of ATP production per mole of oxygen.3 Moreover, TMZ reduces intracellular acidosis and protects against oxygen free radical‐induced toxicity. The drug therefore directly protects myocyte structure and function, and increases cell resistance to hypoxic stress.17,18,19 Those effects might be highly relevant in the setting of PCI.
TMZ is also beneficial in preventing ischaemia‐reperfusion injury. In fact, a recent animal experiment demonstrated that TMZ could limit lethal ischaemia‐reperfusion injury by inhibiting mitochondrial permeability transition pore opening, which represents a crucial event in cardiomyocyte death following myocardial ischaemia‐reperfusion.20,21 Altogether, these effects could explain the reduction of cardiac myonecrosis in patients pretreated with TMZ before angioplasty.
Conclusion
The results of this single‐centre, prospective, open randomised study support the effectiveness of pretreatment with a loading dose of TMZ on limiting peri‐procedural myocardial injury, without reducing the frequency of minor infarction, in patients undergoing elective PCI. The question of whether the observed beneficial effects of TMZ could translate into an improved post‐procedural outcome needs further investigation. Clearly, the results presented here warrant large‐scale longitudinal studies to investigate the effects of pre‐procedural oral TMZ treatment on late outcome in patients undergoing elective PCI.
Abbreviations
AUC - area under the curve
cTnI - cardiac troponin Ic
PCI - percutaneous coronary intervention
TMZ - trimetazidine
Footnotes
Competing interests: None.
References
- 1.Selvanayagam J B, Porto I, Chanon K.et al Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury. Circulation 20051111027–1032. [DOI] [PubMed] [Google Scholar]
- 2.Herrmann J, Von Birgelen C, Haude M.et al Prognostic implication of cardiac troponin T increase following stent implantation. Heart 200287549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopaschuk G D. Treating ischemic heart disease by pharmacologically improving cardiac energy metabolism. Am J Cardiol 19988214K–17K. [DOI] [PubMed] [Google Scholar]
- 4.McClellan K J, Plosker G L. Trimetazidine: a review of its use in stable angina pectoris and others conditions. Drugs 199958143–157. [DOI] [PubMed] [Google Scholar]
- 5.Steg P G, Grollier G, Gallay P.et al A randomized double‐blind trial of intravenous trimetazidine as adjunctive therapy to primary angioplasty for acute myocardial infarction. Int J Cardiol 200177263–273. [DOI] [PubMed] [Google Scholar]
- 6.Padadopoulos C L, Kanonidis I E, Kotridis P S.et al The effect of trimetazidine on reperfusion arrhythmia in acute myocardial infarction. Int J Cardiol 199655137–142. [DOI] [PubMed] [Google Scholar]
- 7.The EMIP–FR Group (European Myocardial Infarction Project‐Free Radicals) Effect on 48‐h intravenous trimetazidine on short and long‐term outcomes of patients with acute myocardial infarction, with and without thrombolytic therapy; a double‐blind, placebo‐controlled, randomized trial. Eur Heart J 2000211537–1546. [DOI] [PubMed] [Google Scholar]
- 8.Di Pasquale P, Lo verso P, Bucca V.et al Effects of trimetazidine administration before thrombolysis in patients with anterior myocardial infarction: short‐term and long term results. Cardiovasc Drug Ther 199913423–428. [DOI] [PubMed] [Google Scholar]
- 9.Kober G, Buck T, Sievert H.et al Myocardial protection during percutaneous transluminal angioplasty: effects of trimetazidine. Eur Heart J 1992131109–1115. [DOI] [PubMed] [Google Scholar]
- 10.Kovar L I, Monrad E S, Sherman W.et al A randomized trial of stenting with or without balloon predilatation for the treatment of coronary artery disease. Am Heart J 2001142e9. [DOI] [PubMed] [Google Scholar]
- 11.Smith S C, Jr, Dove J T, Jacobs A K.et al Guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines)—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on assessment of diagnostic and therapeutic cardiovascular procedures (Committee on percutaneous transluminal coronary angioplasty). J Am Coll Cardiol 2001372215–2239. [DOI] [PubMed] [Google Scholar]
- 12.Sellier P. The effects of trimetazidine on ergometric parameters in exercise‐induced angina. Controlled multicenter double blind versus placebo study. Arch Mal Cœur Vaiss 1986791331–1336. [PubMed] [Google Scholar]
- 13.Scheller B, Hennen B, Pohl A.et al Acute and subacute stent occlusion; risk reduction by ionic contrast media. Eur Heart J 200122385–391. [DOI] [PubMed] [Google Scholar]
- 14.Genissel P, Chodjania Y, Demolis J L.et al Assessment of the sustained release properties of a new oral formulation of trimetazidine in pigs and dogs and confirmation in healthy human volunteers. Eur J Drug Metab Pharmacokinet 20042961–68. [DOI] [PubMed] [Google Scholar]
- 15.Stanley W C, Marzilli M. Metabolic therapy in the treatment of ischaemic heart disease: the pharmacology of trimetazidine. Fundam Clin Pharmacol 200317133–145. [DOI] [PubMed] [Google Scholar]
- 16.Pornin M, Harpey C, Allal J.et al Lack of effects of trimetazidine on systemic hemodynamics in patients with coronary artery disease: a placebo‐controlled study. Clin Trials Metaanal 19942949–56. [PubMed] [Google Scholar]
- 17.Kantor P F, Lucien A, Kozak R.et al The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid‐oxidation to glucose oxidation by inhibiting mitochondrial long‐chain 3‐ketoacyl coenzyme thiolase. Circ Res 200086580–588. [DOI] [PubMed] [Google Scholar]
- 18.Lopaschuk G D, Rick B, Panakkezhum T.et al Beneficial effects of trimetazidine in ex vivo working ischemic hearts are due to a stimulation of glucose oxidation secondary to inhibition of long‐chain 3‐ketoacyl coenzyme A thiolase. Circ Res 200393e33–e37. [DOI] [PubMed] [Google Scholar]
- 19.Morin D, Elimadi A, Sapena R.et al Evidence for the existence of [3H]‐trimetazidine binding sites involved in the regulation of the mitochondrial permeability transition pore. Br J Pharmacol 19981231385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss J N, Korge P, Honda H M.et al Role of the mitochondrial permeability transition in myocardial disease. Circ Res 200393292–301. [DOI] [PubMed] [Google Scholar]
- 21.Argaud L, Gomez L, Gateau‐Roesch O.et al Trimetazidine inhibits mitochondrial permeability transition pore opening and prevents lethal ischemia‐reperfusion injury. J Mol Cell Cardiol 200539893–899. [DOI] [PubMed] [Google Scholar]