Abstract
Aim
To investigate the role of pulmonary arterial hypertension (PAH) in adult patients born with a cardiac septal defect, by assessing its prevalence and its relation with patient characteristics and outcome.
Methods and results
From the database of the Euro Heart Survey on adult congenital heart disease (a retrospective cohort study with a 5‐year follow‐up), the relevant data on all 1877 patients with an atrial septal defect (ASD), a ventricular septal defect (VSD), or a cyanotic defect were analysed. Most patients (83%) attended a specialised centre. There were 896 patients with an ASD (377 closed, 504 open without and 15 with Eisenmenger's syndrome), 710 with a VSD (275, 352 and 83, respectively), 133 with Eisenmenger's syndrome owing to another defect and 138 remaining patients with cyanosis. PAH was present in 531 (28%) patients, or in 34% of patients with an open ASD and 28% of patients with an open VSD, and 12% and 13% of patients with a closed defect, respectively. Mortality was highest in patients with Eisenmenger's syndrome (20.6%). In case of an open defect, PAH entailed an eightfold increased probability of functional limitations (New York Heart Association class >1), with a further sixfold increase when Eisenmenger's syndrome was present. Also, in patients with persisting PAH despite defect closure, functional limitations were more common. In patients with ASD, the prevalence of right ventricular dysfunction increased with systolic pulmonary artery pressure (OR = 1.073 per mm Hg; p<0.001). Major bleeding events were more prevalent in patients with cyanosis with than without Eisenmenger's syndrome (17% vs 3%; p<0.001).
Conclusion
In this selected population of adults with congenital heart disease, PAH was common and predisposed to more symptoms and further clinical deterioration, even among patients with previous defect closure and patients who had not developed Eisenmenger's physiology.
In heart defects with incomplete physical separation of the systemic and pulmonary circulation, such as septal defect, shunting of blood from left to right may lead to increased flow and pressure in the pulmonary circulation. When this, in turn, induces irreversible changes of the medium‐sized and small arteries, the pressure in the pulmonary artery may reach the height of systemic pressure, with consequent reversal of the shunt and cyanosis, a condition known as Eisenmenger's syndrome. Prevention of this sequence of events is an important target in the management of septal defects, and may, in particular, motivate closure of the defect. However, the clinical course of patients with septal defects is variable. In particular, it is not known how frequently pulmonary arterial hypertension (PAH) develops since closure of defects has taken place, and which levels of PAH lead to symptoms and irreversible changes.
The recently completed Euro Heart Survey on congenital heart disease in adults included a considerable number of patients with septal defects. We used this relatively large multicentre database to examine the potential role of PAH in adults born with a heart septal defect, including patients with Eisenmenger's syndrome.
Methods
All patients were selected from the database of the Euro Heart Survey on adult congenital heart disease who had a type II atrial septal defect (ASD) as the primary diagnosis, or a ventricular septal defect (VSD), Eisenmenger's syndrome, or another cyanotic defect. The methods that were used to collect the data for the Euro Heart Survey have been described previously.1,2 Briefly, consecutive patients with one of eight congenital cardiac defects visiting an outpatient clinic of one of the participating centres in 1998 were identified, and their clinical course was documented in retrospect until April 2004. Data on medical history, results of diagnostic procedures and interventions were transcribed from patient records into an electronic case record file. Participating centres included both specialised (tertiary referral) and non‐specialised centres. A specialised centre was defined as a centre fulfilling the following three criteria: (1) paediatric cardiology or congenital cardiac surgery available; (2) at least one cardiologist dedicated to adults with congenital heart disease; (3) a minimum of 200 congenital outpatient visits per year.
For this analysis, the following data collected in the survey for the above categories of patients were used: general patient information (age and sex), medical history (arrhythmias and major bleeding events in patients with cyanosis), thromboembolic events and interventions, in particular, closure of the defect. Major bleeding events were defined as bleeding requiring hospital admission and were further categorised as “haemoptysis”, “intracranial haemorrhage” or “other”. Baseline clinical characteristics were: systolic pulmonary artery pressure (sPAP), ventricular function, and New York Heart Association (NYHA) functional class. Changes during follow‐up were: death, interventions pertaining to the septal defect and change in functional status (NYHA class).
Subgroups of patients
Patients were divided into subgroups for the purpose of analysis according to type and closure status (open or closed) of the defect, presence or absence of cyanosis, and presence or absence of Eisenmenger's physiology. Patients with Eisenmenger's syndrome were further distinguished according to underlying defect.
Definition of pulmonary arterial hypertension
PAH is usually defined as a sPAP of >25 mm Hg, when measured invasively, or >35 mm Hg when estimated echocardiographically on the basis of measurement of the velocity of the tricuspid regurgitation jet.3,4 For the purposes of this study, PAH was assumed to exist when a value of ⩾40 mm Hg had been entered in the patient record, or when sPAP was qualified as “abnormal”.
Statistical analysis
Categorical baseline characteristics were expressed as percentages and compared among the relevant groups with Pearson's χ2 test. Continuous variables were expressed as mean (SD) when normally distributed and compared with the two‐tailed t test for independent samples, or, when not normally distributed, as medians (1st–3rd quartiles) and compared with the Mann–Whitney U test. Normality was assessed with the Kolmogorov–Smirnov test. Two‐sided p values of <0.05 were considered as significant.
The prevalence of PAH and the relationship with age and sex
For each subgroup, the prevalence of PAH was expressed as a percentage calculated from the number of patients fulfilling the criteria for PAH at baseline, and the total number of patients included in that subgroup. To explore the relationship with age and sex, age and sex distribution were compared between patients with and without PAH. The association of prevalence with age was further assessed using a multivariate analysis taking the presence of PAH as the dependent variable, and with the following independent variables included in the model: age, type of defect (VSD or ASD), closure status (defect closed or open at inclusion in the study) and sex.
PAH, sPAP and NYHA functional status
In a first exploratory analysis, values for sPAP were compared between patients grouped into three categories according to their baseline functional status: NYHA class I, NYHA class II or NYHA class III/IV. This was done separately for patients with an open ASD and those with an open VSD, in both cases excluding the patients with Eisenmenger's physiology. The association between sPAP and NYHA class was analysed with the Kruskal–Wallis test, the Mann–Whitney U test for pairwise comparisons, and the Jonckheere–Terpstra test for a trend.
This exploratory analysis was followed by logistic regression to describe the relationship between PAH and functional status adjusting for potential confounders, with the presence of symptoms (NYHA class >1) as the dependent variable. Independent variables included in the model were PAH status, presence or absence of Eisenmenger's physiology, type of defect, age and sex.
The association between PAH and outcome
Standard techniques of survival analysis were employed to investigate the relationship between PAH and mortality. Kaplan–Meier curves were generated comparing the subgroups. The following subgroups were compared with the log rank test: (1) among patients with cyanosis those with and those without PAH; (2) among patients with cyanosis, those with Eisenmenger's syndrome and those with cyanotic defect; (3) among patients with Eisenmenger's syndrome those with different underlying defects. Patients with open defects at baseline whose defect was closed during follow‐up were considered censored at the date of closure.
Right ventricular function and pulmonary artery pressure
Right ventricular function was evaluated according to three categories: good (ejection fraction >50%, or qualified as “good” in the patient record), moderate (30–50%, or “moderate”) or poor (<30% or “poor”). Right ventricular dysfunction was then defined as moderate or poor right ventricular function. Presence of right ventricular dysfunction was taken as the dependent variable in a logistic regression analysis to investigate the relationship between sPAP and ventricular function.
Cyanosis versus PAH in Eisenmenger's syndrome
To differentiate the effects of cyanosis from those that can be ascribed to PAH itself, baseline characteristics were compared between the patients with cyanosis with Eisenmenger's syndrome and those without. To further investigate the significance of one variable that was identified as distinguishing between the groups (a history of major bleeding events), logistic regression was used in order to adjust for the degree of cyanosis (resting oxygen saturation) and general potential confounders (age and sex).
All statistical analyses were performed using the SPSS package, V.12.01.
Results
The database contained data on 1877 patients who had one of the relevant defects. These patients were included in a total of 76 centres; 1553 (83%) of the patients were treated at a specialised centre. Table 1 shows the distribution of these patients in the various subgroups, and displays their baseline characteristics. Patients with cyanosis without Eisenmenger's syndrome had the following underlying defects: pulmonary atresia (n = 36), abnormal pulmonary venous drainage (n = 5), double inlet left ventricle (n = 23), truncus arteriosus (n = 4), transposition of the great arteries (n = 10), Ebstein abnormality (n = 6) and complex/other/unspecified (n = 54).
Table 1 Baseline characteristics per subgroup of a total number of 1877 patients.
| Closed ASD | Open ASD | Closed VSD | Open VSD | Cyan non‐Eis | Eis other | |||
|---|---|---|---|---|---|---|---|---|
| Eisenmenger's syndrome | Eisenmenger's syndrome | |||||||
| No | Yes | No | Yes | |||||
| n | 377 | 504 | 15 | 275 | 352 | 83 | 138 | 133 |
| Median (Q1–Q3) age (years) | 38 (24–50) | 40 (26–54) | 44 (34–57) | 27 (21–35) | 27 (20–36) | 30 (24–39) | 28 (22–35) | 28 (23–36) |
| Females (%) | 65 | 68 | 80 | 53 | 53 | 55 | 59 | 68 |
| PAH, n (%) | 45 (12%) | 164 (33%) | 15 (100%) | 35 (13%) | 39 (11%) | 83 (100%) | 17 (12%) | 133 (100%) |
| sPAP (in PAH), median (Q1–Q3)* | 45 (42–57) | 51.5 (42–62) | 110 (92–126) | 54 (41–75) | 60 (48–88) | 106 (96–119) | 49.5 (42–59) | 104 (90–120) |
ASD, atrial septal defect; Cyan non‐Eis, Cyanotic defect without Eisenmenger syndrome; Eis other, Eisenmenger's sydrome with an underlying defect other than a simple ASD or VSD; PAH, pulmonary arterial hypertension; Q1–Q3, 1st quartile–3rd quartile; sPAP, systolic pulmonary artery pressure; VSD, ventricular septal defect.
*Median systolic pulmonary artery pressures for the patients with PAH.
Baseline patient characteristics
In all groups, there were more females, especially among patients with ASD and in the group “Eis other” (Eisenmenger's syndrome with an underlying defect other than a simple ASD or VSD). Patients with VSD were younger than patients with ASD, and patients with Eisenmenger's syndrome were older than those without.
Prevalence of PAH
A total of 531 patients (28% of all patients) were identified as having PAH. Table 1 displays the percentages of patients with PAH. Among all patients with open defects, including the patients with Eisenmenger's syndrome, 34% (179/519) of patients with ASD and 28% (122/435) of patients with VSD had PAH; in patients with a closed defect, the corresponding figures were 12% (45/377) and 13% (35/275), respectively.
The relation with age and sex
In patients with an ASD, median age was 15 years higher in patients with PAH compared to those without (51 years vs 36 years; p<0.001). In patients with a VSD, this difference was 30 years versus 26 years (p = 0.001). In line with these age differences, the prevalence of PAH was significantly higher among patients aged ⩾40 years: among patients with an open ASD (including Eisenmenger's syndrome), 49.4% versus 18.9% (p<0.001), and among those with an open VSD, 40.2% versus 25.0% (p = 0.007).
Multivariate analysis, adjusting for type of defect, closure status and sex, showed that the probability of PAH increased with a factor of 1.041 (p<0.001) for each extra year of age.
In none of the subgroups was there a significant relation between sex and the prevalence of PAH.
Association between sPAP, PAH and NYHA functional class
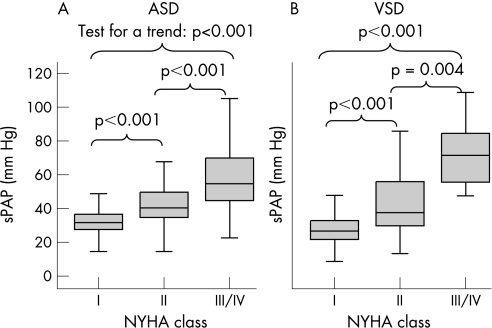
It is shown in fig 1 that among patients who had an open septal defect without Eisenmenger's physiology, sPAP increased with NYHA class, both in patients with ASD (fig 1A) and in patients with VSD (fig 1B; p<0.001 for all comparisons).
Figure 1 Distribution of systolic pulmonary artery pressure (sPAP) according to New York Heart Association (NYHA) functional class for patients with an open septal defect but without Eisenmenger's physiology. (A) the results for atrial septal defect (ASD); (B) results for ventricular septal defect (VSD). The thick black lines indicate the median, the boxes the interval between the 25th and 75th percentiles, and the outer lines the extreme values.
Table 2 displays the results of the subsequent logistic regression analysis for patients with an open defect, with or without Eisenmenger's physiology. After adjustment for age, sex and type of defect, the presence of PAH was found to entail an almost eightfold increased probability of functional limitations. The presence of Eisenmenger's syndrome was associated with an additional sixfold increase in probability.
Table 2 Determinants of baseline functional limitations (New York Heart Association class >1).
| Variable | OR (95% CI) | p Value |
|---|---|---|
| PAH present | 7.7 (5.1 to 11.8) | <0.001 |
| Eisenmenger's syndrome present | 6.3 (2.8 to 14.2) | <0.001 |
| VSD versus ASD | 0.37 (0.26 to 13.4) | <0.001 |
| Age >40 years | 2.4 (1.7 to 3.4) | <0.001 |
| Female sex | 1.1 (0.79 to 1.5) | 0.571 |
ASD, atrial septal defect, PAH, pulmonary arterial hypertension; VSD, ventricular septal defect.
The relation between PAH and exercise capacity was also explored in patients with a closed defect. In these patients, after adjustment for age and sex, PAH was associated with a sevenfold increased probability (odds ratio (OR) 7.1, 95% CI 3.5 to 15.1; p<0.001) in the case of an ASD, and a 12‐fold increased probability (OR 11.8, 95% CI 5.1 to 27.5; p<0.001) in the case of a VSD.
PAH and outcome
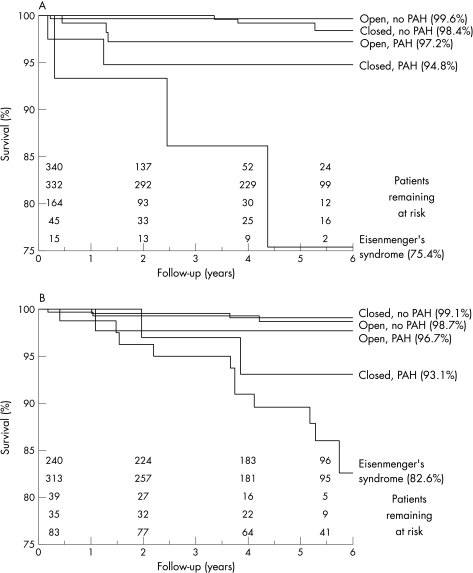
Figure 2A,B displays Kaplan–Meier curves. For each of the two defects, there were differences in survival according to subgroup (p<0.001), but the significance of this result was largely due to the high mortality in patients with Eisenmenger's syndrome as compared to the other subgroups. The presence of PAH seemed to be associated with increased mortality more strongly in the group of patients with closed defects. In patients with a closed ASD, (Kaplan–Meier) estimated survival for those with versus those without PAH was 94.8% and 98.4%, respectively (p = 0.036; log rank test, not correcting for multiple testing). In patients with an open ASD at baseline, this difference was 97.2% versus 99.6% (p = 0.118). In patients with a closed VSD, estimated survival for those with versus those without PAH was 93.1% and 99.1%, respectively (p = 0.021; log rank test, not correcting for multiple testing). In patients with an open VSD at baseline, this difference was 96.7% versus 98.7% (p = 0.299). Median (range) estimated mortality for the patients with Eisenmenger's syndrome was 20.6% (14.5–26.7%), and for other patients with cyanosis was 16.6% (2.7–30.5%). After adjusting for age and sex, this was not significantly different. In patients with Eisenmenger's syndrome, mortality was not significantly different according to underlying defect (ASD, VSD or other).
Figure 2 Kaplan–Meier curves for five subgroups of patients. (A) Patients with an atrial septal defect. (B) Patients with a ventricular septal defect. PAH, pulmonary artery hypertension.
Right ventricular function and pulmonary artery pressure
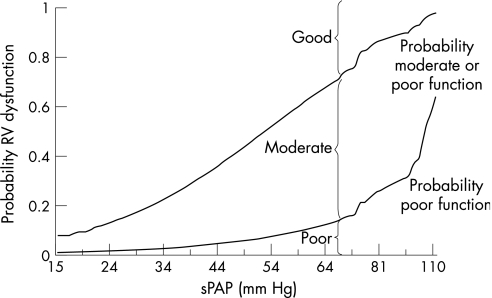
Among patients with an unclosed ASD, increased sPAP was associated with an increased prevalence of right ventricular dysfunction (patients with Eisenmenger's syndrome excluded; fig 3). Logistic regression showed that, after adjusting for age and sex, each increase in sPAP of 1 mm Hg, increased the probability of right ventricular dysfunction by a factor of 1.073 (95% CI 1.045 to 1.102; p<0.001).
Figure 3 Right ventricular (RV) dysfunction versus systolic pulmonary artery pressure (sPAP) in atrial septal defect (patients with Eisenmenger's syndrome not included). The lines depict the predicted probabilities (the fitted values in a logistic regression model) for RV function as function of pulmonary artery pressure. The upper line represents the probability of having a moderate or poor RV function; the lower line that of having a poor function. Thus, at each level of sPAP, the vertical distances above, in between and below the two lines represent the probabilities of having good, moderate and poor RV function, respectively.
Cyanosis with and without Eisenmenger's syndrome
In table 3, baseline characteristics of patients having cyanosis with and without Eisenmenger's syndrome are compared. The prevalence of a history of major bleeding events was much higher in patients with Eisenmenger's syndrome. The multivariate logistic regression model, which included as variables resting oxygen saturation, age and sex, revealed that patients with Eisenmenger's syndrome had a 22‐fold increased probability of having a history of major bleeding events compared to patients with another cyanotic defect (OR 22.4, 95% CI 3.0 to 167.6, p = 0.002).
Table 3 Patients with cyanosis: Eisenmenger's syndrome versus non‐Eisenmenger's syndrome.
| non‐Eis | Eis | p Value | |
|---|---|---|---|
| n | 138 | 231 | |
| Median (Q1–Q3) age | 28 (22–35) | 30 (24–38) | 0.075 |
| Females, n (%) | 81 (59%) | 148 (64%) | 0.179 |
| Oxygen saturation at rest, median (Q1–Q3) | 84 (79–88) | 82 (78–87) | 0.095 |
| History of major bleeding, n (%) | 4 (3%) | 37 (17%) | <0.001 |
| Haemoptysis, n | 3 | 28 | |
| Intracranial haemorrhage, n | 1 | 0 | |
| Others, n | 0 | 9 | |
| History of thromboembolism, n (%) | 11 (8%) | 29 (13%) | 0.224 |
Eis, Eisenmenger's syndrome; Q1–Q3, 1st–3rd quartiles.
Discussion
The data bank of the Euro Heart Survey on congenital heart disease in adults allowed us to study the epidemiology of PAH in association with a cardiac septal defect in a large cohort. PAH appeared to be present in an unexpectedly high number of patients, the majority of them being seen at specialised centres. Among the patients with an open defect, PAH was associated with an eightfold increased probability of functional limitations. Similarly, functional limitations were present among patients with persisting PAH despite defect closure. Mortality was particularly high in patients with Eisenmenger's syndrome: one in five of these patients died during the over 5‐year follow‐up period of the study. Furthermore, patients with Eisenmenger's syndrome had an increased frequency of major bleeding events compared to the remainder of the patients with cyanosis. Finally, PAH was associated with right ventricular dysfunction.
Considering the importance of prevention of PAH in the management of patients with a septal defect, the high prevalence of PAH among the patients of this study is remarkable. In almost one out of every three patients with an unclosed septal defect PAH was present, although the sPAP was also increased in one out of every eight patients with a closed defect. It should be emphasised, however, that most of the patients were treated at referral centres. Our estimates are, therefore, not representative of the general population of patients with septal defects and higher than what has been reported by others. In a recent study of a general population of adult patients with congenital heart disease, it was found that 6.2% of patients with septal defects had PAH.5
In addition, it should be realised that our cohort largely reflects past medical practice. Patients aged >40 years lived through their youth before operative closure of septal defects had become a relatively safe procedure. Since then, diagnosis, screening and treatment have continued to improve. It might, therefore, be expected that PAH will become increasingly rare. However, it cannot be excluded that the increase in prevalence with age is related to the process of aging in itself. In that case, a strong decline in PAH prevalence will be unlikely, given the fact that patients are getting older.
The presence of PAH was associated with clinical manifestations, even before the stage of Eisenmenger's physiology. There was a clear relation with reduced exercise capacity, both in patients with a closed and in those with an open defect. In the latter group of patients, we found a strong correlation between NYHA functional class and pulmonary artery pressure: the higher the sPAP, the more the functional limitations.
The haemodynamic mechanisms leading to PAH are different in patients with an ASD compared with those with a VSD. In a non‐restrictive VSD, the pulmonary circulation is exposed to systemic pressure in systole directly after birth.6 In the case of an ASD, the pathogenic condition is hypercirculation and “volume overload” in the pulmonary circulation, which induces PAH after a much more protracted course.7,8 Our findings do seem to confirm that an ASD leads to problems relatively later in life, but we found no indication that PAH has a less unfavourable impact on outcome in patients with one defect than in those with the other.
An important haemodynamic consequence of PAH is its negative effect on right ventricular function,9 as was apparent in the patients with ASD in our study: the probability of right ventricular dysfunction increased with higher sPAP values.
Once pulmonary artery pressure has reached systemic levels and Eisenmenger's physiology with shunt reversal has developed, mortality is high, as has been found in all studies that have followed‐up patients with Eisenmenger's syndrome.9,10,11,12,13 The poor outcome is largely due to the effects of cyanosis and the compensatory mechanisms it induces, in particular erythrocytosis. We tried to assess the roles of cyanosis on the one hand, and severe pulmonary hypertension and vascular pathology on the other. The finding that major bleeding events were substantially more frequent in patients with Eisenmenger's syndrome than in the remaining patients with cyanosis is probably related to the fragile and dilated pulmonary vessels in patients with Eisenmenger's syndrome.14,15
Limitations
Apart from the general limitations inherent in a large retrospective multicentre study, it should be emphasised that the cohort of this study consisted of a selected group of patients, with an over‐representation of patients treated at tertiary referral centres. Further, the survey was not designed to specifically study PAH, and the data set was restricted to a limited number of variables, which precluded adjusting for all possible confounding factors. In particular, we had no information on the age at closure for patients with defects that had been closed before study entry. Thus, we were not able to evaluate whether the high prevalence of PAH among patients with closed defects could be related to the timing of the intervention. Also, we were not able to reliably estimate the haemodynamic “size” of the shunt, and thus could not analyse the relation of the size of the shunt and the occurrence of PAH. Finally, it should be noted that the group of patients with cyanosis was a heterogeneous one with many different underlying defects.
Conclusion
The Euro Heart Survey on adult congenital heart disease shows that PAH remains a risk factor in the long‐term clinical course of congenital heart septal defects. In this selected group of patients, mostly attending specialised centres, PAH was unexpectedly common and associated with worse functional status, more rapid clinical deterioration and enhanced risk of death among patients with Eisenmenger's syndrome. Whether the prevalence of PAH will decline due to improved diagnostics and earlier treatment of CHD remains speculative.
Abbreviations
ASD - atrial septal defect
NYHA - New York Heart Association
PAH - pulmonary arterial hypertension
sPAP - systolic pulmonary artery pressure
VSD - ventricular septal defect
Footnotes
Competing interests: None declared.
References
- 1.Engelfriet P, Boersma E, Oechslin E.et al The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow‐up period: the Euro Heart Survey on adult congenital heart disease. Eur Heart J 2005262325–2333. [DOI] [PubMed] [Google Scholar]
- 2.Engelfriet P, Tijssen J, Kaemmerer H.et al Adherence to guidelines in the clinical care for adults with congenital heart disease: the Euro Heart Survey on adult congenital heart disease. Eur Heart J 200627737–745. [DOI] [PubMed] [Google Scholar]
- 3.McQuillan B M, Picard M H, Leavitt M.et al Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 20011042797–2802. [DOI] [PubMed] [Google Scholar]
- 4.Barst R J, McGoon M, Torbicki A.et al Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 20044340S–47S. [DOI] [PubMed] [Google Scholar]
- 5.Duffels M G J, Engelfriet P M, Berger R M F.et al Pulmonary arterial hypertension in congenital heart disease: an epidemiologic perspective from a Dutch registry. Int J Cardio December 18 2006 [Epub ahead print] [DOI] [PubMed]
- 6.Hoffman J I E, Rudolph A M. The natural history of ventricular septal defects in infancy. Am J Cardiol 196516634–653. [DOI] [PubMed] [Google Scholar]
- 7.Wood P. The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. BMJ 19582701–9, 75562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell M. Natural history of atrial septal defect. Br Heart J 197032820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein P D, Sabbah H N, Anbe D T, Marzilli M. Performance of the failing and nonfailing right ventricle of patients with pulmonary hypertension. Am J Cardiol 1979441050–1055. [DOI] [PubMed] [Google Scholar]
- 10.Daliento L, Somerville J, Presbitero P.et al Eisenmenger syndrome. Factors relating to deterioration and death. Eur Heart J 1998191845–1855. [DOI] [PubMed] [Google Scholar]
- 11.Niwa K, Perloff J K, Kaplan S.et al Eisenmenger syndrome in adults: ventricular septal defect, truncus arteriosus, univentricular heart. J Am Coll Cardiol 199934223–232. [DOI] [PubMed] [Google Scholar]
- 12.Cantor W J, Harrison D A, Moussadji J S.et al Determinants of survival and length of survival in adults with Eisenmenger syndrome. Am J Cardiol 199984677–681. [DOI] [PubMed] [Google Scholar]
- 13.Saha A, Balakrishnan K G, Jaiswal P K.et al Prognosis for patients with Eisenmenger syndrome of various aetiology. Int J Cardiol 199445199–207. [DOI] [PubMed] [Google Scholar]
- 14.Edwards J E. Functional pathology of the pulmonary vascular tree in congenital cardiac disease (the Lewis A. Conner memorial lecture). Circulation 195715164–196. [DOI] [PubMed] [Google Scholar]
- 15.Wagenvoort C A, Nauta J, van der Schaar P J.et al Effect of flow and pressure on pulmonary vessels: a semiquantitative study base on lung biopsies. Circulation 1967351028–1037. [DOI] [PubMed] [Google Scholar]