Marfan syndrome is a heritable disorder of the connective tissue with an estimated prevalence of 1 in 5000 individuals and no predilection for either sex. The syndrome is inherited as an autosomal dominant trait with complete penetrance but with phenotypic expression that varies considerably, both between and within families. Severe forms such as neonatal Marfan syndrome with <1 year survival usually result from de novo mutations, whereas about 75% of persons with classic Marfan syndrome have a positive family history. Affected individuals develop varying patterns of organ involvement including the cardiovascular, ocular, skeletal, and pulmonary system, the skin, and the dura. In classical Marfan syndrome, many manifestations present during puberty or later and severe complications rarely develop before adulthood. Such complications include severe scoliosis or pectus excavatum, spontaneous pneumothorax, retinal detachment or sight‐threatening glaucoma resulting from dislocated lenses. Before the evolution of open heart surgery, however, Marfan patients usually died from acute aortic dissection or rupture, and thus had an average life‐expectancy of only 32 years.1
Today, management by expert centres extends the life expectancy of Marfan patients to over 60 years of age.1 Such centres usually have a generalist with broad experience with Marfan patients to coordinate an interdisciplinary team comprising cardiologists, heart surgeons, orthopaedic surgeons, ophthalmologists, paediatricians, geneticists and psychologists. The principles for managing cardiovascular manifestations have remained remarkably unchanged over the past 25 years and Marfan patients have been shown to adhere excellently to medication and physical activity guidelines. However, the classical standards have currently been challenged by two major developments. First, with the increasing life expectancy of Marfan patients there is a shift in the spectrum of medical problems. Second, recent molecular, surgical and clinical research has yielded profound new insights into the pathogenesis and treatment options of inherited connective tissue disorders. This article contrasts classical concepts with recent advances to highlight their potential impact on future concepts of patient care.2
MOLECULAR GENETICS AND PATHOPHYSIOLOGY
Fibrillin‐1 and the closely related fibrillin‐2 protein are major components of the 10 nm microfibrils of the extracellular matrix. These fibrillins are extracellular glycoproteins comprised mainly of tandemly repeated epidermal growth factor (EGF)‐like modules, most of which satisfy the consensus for calcium binding (cbEGF‐like motifs). Both proteins contribute to specific physical properties of elastic and non‐elastic tissues. Recent research has challenged classical pathogenetic concepts of Marfan syndrome and may impact future treatment strategies.
Dominant‐negative mechanism versus haploinsufficiency
The classical view of Marfan pathogenesis is that mutant fibrillin‐1 molecules alter microfibril assembly in a dominant‐negative manner. However, transgenic mice with overexpressed mutant fibrillin‐1 transgene and two normal FBN1 alleles did not exhibit vascular changes of Marfan syndrome that were present in mice heterozygous for a comparable missense mutation (C1039G). On the other hand, transgenic addition of a wild‐type allele to mice heterozygous for C1039G prevented aortic pathology. These findings suggest that haploinsufficiency with half‐normal production of normal protein, rather than presence of mutant protein, is required to cause the Marfan phenotype. Accordingly, boosting of fibrillin‐1 expression might be a better therapeutic strategy than reducing the expression of mutant fibrillin‐1, although at the moment there is no practical way to achieve either of these goals.2
Primary failure of elastogenesis versus postnatally acquired elastolysis
Weakness of the aortic wall was previously believed to result from defects of fibrillin‐1 microfibrils that prevented proper assembly of elastic fibres (elastogenesis). Thus, individuals with Marfan syndrome were thought to have an inborn lack of functional elastic fibres starting from late fetal development. More recently, it has been shown that the structural and functional integrity of the normal vessel wall is maintained by elastic lamina that are anchored to the intima and smooth muscle cells (SMC) through connecting filaments that are composed of fibrillin‐1. Mice homozygous for a targeted hypomorphic allele (mgR) of FBN1 exhibited loss of these connecting filaments as their primary vessel wall abnormality. This loss of filaments subsequently initiated vascular smooth muscle cells to overproduce matrix elements and mediators of elastolysis including matrix metalloproteinases 2 and 9 (MMP), resulting in medial degeneration.3 Moreover, experiments in cell culture have shown that fibrillin‐1 fragments can induce MMP upregulation.4 In addition, experiments with the mgR mouse model demonstrated that elastin‐binding protein ligand, including elastin fragments from the aorta, can act as a chemotactic stimulus for macrophages that may also promote aortic wall degeneration.5 Thus, rather than developing from a prenatal defect, the Marfan vascular phenotype develops gradually throughout life. This view offers improved perspectives for therapeutic intervention.2
Structural versus regulatory role of fibrillin
The classical paradigm regards Marfan syndrome as the result of structural weakness of connective tissue. Recently, microfibrils were found to regulate transforming growth factor β (TGF‐β) activity. TGF‐β represents a group of cytokines that control cellular proliferation, cell cycle arrest, differentiation, apoptosis and matrix deposition. Sequestration of TGF‐β and control of its activation depends on binding of TGF‐β to latent TGF‐β binding proteins (LTBP), which in turn bind to fibrillin‐rich microfibrils. A deficiency of fibrillin‐1 therefore causes excess of active TGF‐β, and studies on fibrillin‐1‐deficient mice documented increased activity of TGF‐β, resulting in both inborn airspace enlargement and myxomatous cardiac valve disease. Moreover, both phenotypes were prevented by application of TGF‐β neutralising antibodies.6,7 In humans, a pathogenetic role of TGF‐β signalling is documented in patients with Loeys‐Dietz aortic aneurysm syndrome. These patients were shown to suffer heterozygous loss‐of‐function mutations in the genes encoding the type I (TGFBR1) or type II TGF‐β receptor (TGFBR2).8 Thus, pharmacological manipulation of TGF‐β signalling may offer an option to treating Marfan syndrome and related disorders.
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
Marfan syndrome cannot be diagnosed by a single molecular test but requires a scoring system that combines various diagnostic items. The so‐called Ghent nosology subdivides diagnostic features into “major criteria”, “minor criteria”, “organ involvement” and manifestations that only in combination with other manifestations constitute a “major” or “minor” criterion (table 1). Individuals without a family history of Marfan syndrome require major criteria in at least two different organ systems and involvement of a third organ system. Individuals carrying an FBN1 mutation known to cause Marfan syndrome or cases with a positive family history require one major criterion and involvement of an additional organ to establish Marfan syndrome.9 The overall threshold for diagnosing Marfan syndrome is comparatively high, and it is also crucial to evaluate the risk for thoracic aortic aneurysm or thoracic aortic dissection (TAAD) in individuals not meeting the criteria of Marfan syndrome.
Table 1 The Ghent nosology for diagnosing Marfan syndrome.
| Major criterion | Minor criterion | Diagnosis | |||
|---|---|---|---|---|---|
| Skeletal system | |||||
| Pectus carinatum | □ | Pectus excavatum not requiring surgery | □ | Major criterion (⩾4 of the major criteria) | □ |
| Pectus excavatum that requires surgeryUpper to lower segment ratio <0.85 or arm‐span to height ratio >1.05Positive wrist and thumb sign | □□□ | Joint hypermobilityHigh‐arched palate with crowding of teethFacial features (⩾2): | □□ | Skeletal involvement (2 of the major criteria or 1 of the major and 2 of the minor criteria) | □ |
| Medial displacement of the medial malleolus causing pes planusScoliosis >20° or spondylolisthesis | □□ | DolichocephalyMalar hypoplasiaEnophthalmos | □ | ||
| Extension at elbows <170°Protrusio acetabuli of any degree | □ | Retrognathia | |||
| Down‐slanting palpebral fissures | |||||
| Ocular system | |||||
| Ectopia lentis of any degree | □ | Flat cornea | □ | Major criterion | □ |
| Increased axial length of the globe (>23.5 mm) | □ | Ocular involvement (⩾2 minor criteria) | □ | ||
| Hypoplastic ciliary muscle causing decreased miosis | □ | ||||
| Cardiovascular system | |||||
| Aneurysm of the ascending aorta involving at least the sinuses of Valsalva | □ | Mitral valve prolapse (irrespective of mitral regurgitation) | □ | Major criterion | □ |
| Dissection of the ascending aorta | □ | Dilatation of the main pulmonary artery <40 years of age (unassociated with pulmonic stenosis) | □ | Cardiovascular involvement (⩾1 minor criterion) | □ |
| Calcification of the mitral annulus <40 years of age | □ | ||||
| Dilatation or dissection of the descending thoracic or abdominal aorta <50 years of age | □ | ||||
| Pulmonary system | |||||
| Spontaneous pneumothorax | □ | Organ involvement (⩾1 minor criteria) | □ | ||
| Apical blebs (radiography) | □ | ||||
| Skin and integument | |||||
| Striae distensae | □ | ||||
| Recurrent or incisional hernia | □ | ||||
| Dura | |||||
| Lumbosacral dural ectasia | □ | Major criterion | □ | ||
| Family and genetic history | |||||
| First‐degree relative who independently meets Marfan criteria | □ | Major criterion (⩾1) | □ | ||
| FBN1 mutation that is likely to be pathogenetic | □ | ||||
| Haplotype around the FBN1 locus inherited by descent and unequivocally associated with diagnosed Marfan in family | □ |
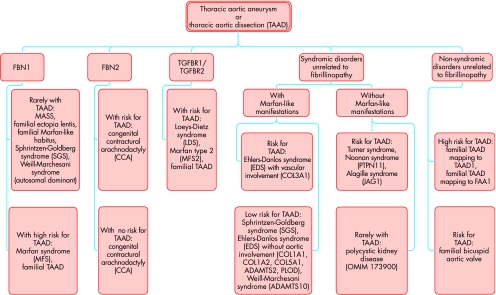
Several disorders may be considered in patients with marfanoid features in their skeletal, aortic, or ophthalmologic system. Since aortic manifestations are most life‐threatening, we currently implement a new diagnostic algorithm at our institution that includes molecular information to stratify patients at risk for aortic complications (fig 1). Much progress has been made in elucidating the molecular basis for the inherited risk for TAAD; thus, the differential diagnosis of TAAD can be based on combined clinical and molecular criteria.10 About 80% of inherited TAAD are caused by fibrillinopathies, among which Marfan syndrome is by far the most frequent disorder. Some alternative disorders that are also caused by mutations in the FBN1 gene tend to be milder and are not at high risk for aortic complications. These comprise the MASS phenotype (myopia, mitral valve prolapse, aortic dilatation, skin and skeletal involvement; OMIM 604308), familial ectopia lentis (OMIM 129600) and familial Marfan‐like habitus (OMIM 154705). Diagnosis of these more benign disorders may not be established in young patients without family history because full‐scale Marfan syndrome usually manifests in an age‐dependent manner; thus, clinical follow‐up is indicated in young individuals with fibrillinopathies not fulfilling criteria of Marfan syndrome.
Figure 1 Differential diagnosis of familial thoracic aortic aneurysms and dissections (TAAD) based on molecular and clinical findings. MASS, myopia, mitral valve prolapse, aortic dilatation, skin and skeletal involvement.
The Weill‐Marchesani syndrome (WMS) is genetically heterogeneous and only its autosomal dominantly inherited variant is caused by FBN1 mutations. Both the autosomal recessively (OMIM 277600) and the autosomal dominantly inherited forms of the syndrome (OMIM 608328) exhibit ectopia lentis and skeletal features such as brachydactyly and stiff joints, but are not associated with TAAD. Similarly, the Shprintzen‐Goldberg syndrome (SGS) is heterogeneous and rarely is caused by FBN1 mutations (OMIM 182212). The syndrome presents with craniosynostosis, hypertelorism, arched palate, learning disability, bone overgrowth, pectus deformity and scoliosis. Only a minority of patients develop aneurysm or dissection of the aortic root. Familial TAAD (OMIM 132900) presents without systemic features of Marfan syndrome but carries a high risk for aortic complications. In some of these cases FBN1 mutations have been documented. Mutations in the FBN2 gene are associated with congenital contractural arachnodactyly (CCA), which exhibits skeletal features of Marfan syndrome, congenital contractures and crumpled ear helices (OMIM 121050). Patients with CCA do not have ophthalmologic manifestations, but may develop aneurysms of the aortic root.
Familial TAAD may be part of syndromic disorders that are not related to abnormalities in the fibrillin genes. Among these, left ventricular obstruction syndromes including Turner syndrome and Noonan syndrome (OMIM 163950) and the Alagille syndrome (OMIM 118450) are associated with a risk for TAAD but do not exhibit Marfan‐like systemic features. TAAD occurs rarely in persons with polycystic kidney disease. Conversely, the Loeys‐Dietz syndrome (LDS; OMIM 609192) has some systemic manifestations of Marfan syndrome but exhibits unique features including cleft palate, bifid uvula, blue sclerae, translucent skin, easy bruising, craniosynostosis, cleft palate, Chiari type I malformation of the brain, learning disability, patent ductus arteriosus, atrial septal defect, bicommissural aortic valve and clubfoot deformity. Aneurysms and dissections tend to be diffuse, and can occur at almost normal vascular diameters with lethal outcome already in young childhood. Mutations in the TGFBR2 gene may also underlie familial TAAD without systemic manifestations, and may even be present in patients fulfilling Marfan criteria but exhibiting only mild ocular involvement (called Marfan syndrome type II by some authors, although the occurrence of LDS‐features may remain to be assessed in this syndrome).11,12 Ehlers–Danlos syndromes may be associated with marfanoid habitus, joint hypermobility and kyphoscoliosis. However, only the vascular type (OMIM 130050) is associated with aortic aneurysm, rupture, and dissection that are localised in the smaller arteries rather than in the aortic vessel, leading to death at a medium age of 48 years. The condition can result from mutations in the COL3A1 gene.10
Non‐syndromic disorders that are unrelated to fibrillinopathy result either from a congenitally bicuspid aortic valve or from monogenic disorders with isolated or predominant aortic manifestations. The latter represent familial TAAD (OMIM 132900) that are usually inherited in an autosomal dominant fashion. These aneurysms exhibit reduced penetrance with pronounced variability in their age of onset. In rare cases, FBN1 mutations have been identified in affected individuals, but the majority of cases are due to mutations in genes mapped to 5q13–15 (TAAD1), 11q23.2–q24 (FAA1) and, most recently, to 3p24–25 (TAAD2, corresponding to the TGFBR2‐locus). In some families a bicuspid aortic valve is associated with ascending aortic aneurysm that usually forms beyond the sinotubular junction. These forms of TAAD are inherited in an autosomal dominant fashion, and some of the causative genes remain to be identified.10
MEDICAL AND SURGICAL MANAGEMENT OF CARDIOVASCULAR MANIFESTATIONS
Classical standards
The classical standards imply: (1) counselling on lifestyle modification including moderate restriction of physical activity; (2) endocarditis prophylaxis; (3) serial imaging of the aorta; (4) β‐blocker medication for aortic protection; and (5) prophylactic replacement of the aortic root (table 2).13,14 In brief, established guidelines for adults recommend reducing emotional stress and restricting physical activity to isotonic, low‐impact exercise such as swimming, biking, or jogging, with aerobic level of work (50% of capacity) and a pulse rate <110 beats/min, or <100 beats/min if on β‐blockers. Any sports of competitive or isometric nature, contact sports and activities with rapid acceleration and deceleration such as martial arts, football, basketball, soccer, skiing or sprinting should be avoided. Children should be channelled away from competitive athletics and contact sports, but they do not require outright restrictions. There has been a single trial that has demonstrated the efficacy of β‐blockers for aortic protection.15 This study was conducted in a prospective randomised fashion and compared the outcome of 32 Marfan patients (mean age 15.4 years) treated with high dosages of propranolol (212 (68) mg per day) to 38 controls with Marfan syndrome (mean age 14.5 years) over a 10 year interval. This study showed that β‐blockers lowered the rate of aortic dilatation and cardiovascular events and improved survival. Other retrospective studies indicated a heterogeneous response of adults to β‐blockers. Calcium antagonists were also shown to retard aortic growth in children and adolescents and are an alternative in individuals with β‐blocker intolerance.
Table 2 Classical standards for managing cardiovascular manifestations of Marfan syndrome.
| General measures for all adults with Marfan syndrome |
| • Moderate restriction of physical activity |
| • Prophylaxis of endocarditis |
| • Echocardiography at annual intervals |
| • β‐blocking treatment |
| Counselling for pregnancy |
| • 50% risk for transmitting a pathogenic mutation to offspring |
| • High risk pregnancy with aortic root diameter ⩾40 mm or previous cardiovascular surgery or severe heart disease |
| • Prepartum aortic root replacement with diameters ⩾40 mm |
| • Serial echocardiography until 3 months postpartum |
| Indications for prophylactic surgery of the aortic root in adults (at least one criterion) |
| • Aortic root diameter >55 mm (50 mm according to other authors)• Aortic root diameter >50 mm (45–50 mm according to other authors) in patients with high risk for aortic complications |
| ‐ family history for aortic dissection |
| ‐ growth of the aortic root >10 mm/year |
| ‐ dilatation of the aortic sinus involving the ascending aorta |
| ‐ more than mild aortic regurgitation |
| ‐ severe mitral regurgitation |
| ‐ before major non‐cardiovascular surgery |
| ‐ women planning pregnancy |
| • Aortic ratio >1.5 |
| • Ratio of the diameters of the aortic root and the descending aorta >2 |
| Indications for prophylactic surgery of the aortic root in children |
| • If possible, surgery should be delayed until adolescence |
| • Aortic root diameters with similar thresholds as in adults |
| • Aortic root diameters outside the upper confidence interval deviate upward from the perused centile on follow‐up echocardiograms |
| Indications for mitral valve surgery |
| • Follow the general recommendations of the American Heart Association |
Immediate surgical intervention is the single, life‐saving measure to rescue patients with acute dissection or intramural haemorrhage of the ascending aorta (Stanford type A). However, it may only be 20% of individuals with acute aortic syndromes who make it into the operating room, and of those who get operated upon more than 10% do not survive acute intervention. Moreover, survivors of emergency surgery frequently experience complications from the dissected aortic flap that persists downstream from the ascending aorta. Conversely, when the aortic root is replaced before complications occur, both early and late survival improves dramatically. A classical study with retrospective review of outcomes from 10 centres has set the standard for elective prophylactic aortic root replacement in Marfan syndrome. The vast majority of patients were treated with a composite‐graft replacement according to Bentall and De Bono or a modification of that technique. The study documented an early mortality of 1.5% and an actuarial survival rate of 84% at 5 years, 75% at 10 years and 59% at 20 years.13 Table 2 lists classical standards for managing Marfan patients.13
New directions
Current molecular and clinical research has yielded new insights and it may be possible today to outline their impact on future concepts for managing cardiovascular manifestations for Marfan syndrome.
Marfan syndrome update: key points
New results from molecular research document: (1) fibrillin‐1 haploinsufficiency, rather than a purely dominant‐negative mechanism, plays a role in the pathogenesis of Marfan syndrome; (2) lack of functionally competent microfibrils is related to postnatally acquired elastolysis rather than primary failure of elastogenesis; (3) besides the structural role of fibrillin there is increasing evidence for a regulatory role involving TGF‐β activity. Insight into these mechanisms offers new opportunities for medical intervention.
The majority of cases of thoracic aortic aneurysm or thoracic aortic dissection (TAAD) are caused by Marfan syndrome or related fibrillinopathies. However, TAAD may be caused by various other genetic disorders and molecular analysis may be instrumental to tailor management strategies to the underlying genetic diseases.
The standard for managing cardiovascular manifestations of Marfan syndrome implies: (1) counselling on lifestyle modification including moderate restriction of physical activity; (2) endocarditis prophylaxis; (3) serial imaging of the aorta; (4) β‐blocker medication for aortic protection; and (5) prophylactic replacement of the aortic root.
Emerging challenges for managing strategies result from the increasing life expectancy of Marfan patients. Marfan syndrome currently evolves from aortic root disease to full‐scale cardiovascular disease comprising valvular disease, myocardial dysfunction including arrhythmia, and distal aortic disease.
New treatment options comprise both medication strategies for aortic protection including ACE inhibitors, AT‐blockers and selective MMP inhibitors, and new surgical concepts that preserve rather than replace the heart valves.
Cystic medial necrosis as a typical manifestation of Marfan syndrome versus a non‐specific manifestation of heterogeneous diseases
The inborn weakness of the aortic wall associates with macroscopic and histologic changes that were identified with the Marfan syndrome or its “forme fruste”, which was macroscopically described as “annulo‐aortic ectasia”. Today cystic medial necrosis is considered an aortic phenotype that is associated with numerous syndromic or non‐syndromic genetic diseases that exhibit different natural courses and require different strategies of management. It is increasingly becoming possible to base diagnosis of various aortic diseases on molecular analysis of different causative genes or gene loci (fig 1).
Marfan syndrome as a disease that predominantly affects the aortic root versus a syndrome that develops full‐scale cardiovascular disease
Surgery of the aortic root removes the weakest spot in the cardiovascular system of Marfan patients. However, with increasing life expectancy weaknesses of the heart valves, the myocardium and distal aorta get time to evolve. Currently, about one quarter of Marfan patients requiring surgery undergo mitral valve surgery, another quarter undergo reintervention at distal sites of the aorta, 6% have tricuspid valve surgery, and 3% require heart transplantation for dilated cardiomyopathy.16 Moreover, 21% of adult Marfan patients develop ventricular arrhythmia with lethal outcome in 3% of cases.17 We believe that future strategies need to consider these potential complications.14
β‐blockers as the exclusive medical option for Marfan patients versus an evolving spectrum of medical treatment strategies
New insights into the molecular mechanisms of aortic wall degeneration open perspectives for innovative therapeutic approaches. For instance, tissue expression of angiotensin II (AT) receptor type 2 (2R), and tissue angiotensin II concentration is increased in Marfan aortas and both angiotensin‐converting enzyme (ACE) inhibitors and angiotensin II type 2 receptor (AT2R) blockers (but not angiotensin II type 1 receptor (AT1R) blockers) inhibited vascular smooth muscle cell apoptosis.18 Accordingly, a recent study of 58 Marfan patients comparing propranolol or atenolol with enalapril suggests better aortic protection with ACE inhibitors than with β‐blockers.19 However, this uncontrolled study has considerable weaknesses and requires confirmation. Despite conflicting experimental findings, other researchers suggest that AT1R‐blockers such as losartan may lead to clinically relevant attenuation of TGF‐β signalling and thus may be more effective than β‐blockade.2,20 Another, as yet untested, approach is based on the use of MMP inhibitors to prevent medial wall degeneration.
Valve replacement versus valve repair
In Marfan patients, the surgical standard for both aortic and mitral valvular disease is to implant an artificial valve prosthesis. However, restorative operations are appealing since these approaches permit maintenance of some of the functional crosstalk between the aortic, mitral and tricuspid valves that link the fibrous skeleton and the myocardium of the heart to achieve optimal valve function.21 Despite pronounced aortic root dilatation, aortic valve leaflets may remain functionally and morphologically normal; thus, aortic root replacement with preservation of the native aortic valve may avoid thromboembolism, haemodynamic mismatch, hydraulic dysfunction and bacterial endocarditis. There are two distinct approaches to valve‐sparing aortic root replacement, both of which require reimplantation of the coronaries. The so‐called “remodelling technique” initially suggested by Sarsam and Yacoub radically excises the aortic tissue down to the annulus and sews a tripartite crown‐shaped aortic tube graft to the insertion line of the aortic cusps, thus preserving the distensibility of the aortic root. Alternatively, David and Feindel created the “reimplantation technique” that also resects aortic tissue down to the annulus but entirely includes the semilunar valves into the aortic tube graft that gets fixed both to the fibrous base of the aorto‐ventricular junction and to the scalloped valvular annulus that is sewn into the tube. Both techniques have been modified, but most surgeons prefer variants of the reimplantation technique. Whereas early and late survival is excellent with valve‐sparing procedures, it remains to be established whether these techniques are capable of maintaining aortic valve competence over a long period of time.
Surgical options for reconstructing the mitral valve have been established in large cohorts, although currently there are no data for Marfan patients. At our institution, we perform reconstruction of the mitral valve whenever possible, and our early and midterm results in Marfan patients do not differ from those in myxomatous mitral valve disease unrelated to Marfan syndrome.
INTERACTIVE MULTIPLE CHOICE QUESTIONS
This Education in Heart article has an accompanying series of six EBAC accredited multiple choice questions (MCQs).
To access the questions, click on BMJ Learning: Take this module on BMJ Learning from the content box at the top right and bottom left of the online article. For more information please go to: http://heart.bmj.com/misc/education.dtl
Please note: The MCQs are hosted on BMJ Learning—the best available learning website for medical professionals from the BMJ Group.
If prompted, subscribers must sign into Heart with their journal's username and password. All users must also complete a one‐time registration on BMJ Learning and subsequently log in (with a BMJ Learning username and password) on every visit.
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
References
- 1.Silverman D I, Burton K J, Gray J.et al Life expectancy in the Marfan syndrome. Am J Cardiol 199575157–160.Study that documents improved survival of individuals with Marfan syndrome. [DOI] [PubMed] [Google Scholar]
- 2.Judge D P, Dietz H C. Marfan's syndrome. Lancet 20053661965–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunton T E, Biery N J, Myers L.et al Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ Res 20018837–43. [DOI] [PubMed] [Google Scholar]
- 4.Booms P, Ney A, Barthel F.et al A fibrillin‐1‐fragment containing the elastin‐binding‐protein GxxPG consensus sequence upregulates matrix metalloproteinase‐1: biochemical and computational analysis. J Mol Cell Cardiol 200640234–246. [DOI] [PubMed] [Google Scholar]
- 5.Guo G, Booms P, Halushka M.et al Induction of macrophage chemotaxis by aortic extracts of the mgR Marfan mouse model and a GxxPG‐containing fibrillin‐1 fragment. Circulation 20061141855–1862. [DOI] [PubMed] [Google Scholar]
- 6.Neptune E R, Frischmeyer P A, Arking D E.et al Dysregulation of TGFβ activation contributes to pathogenesis in Marfan syndrome. Nat Genet 200333407–411.Study that documents a new pathogenetic mechanism in Marfan syndrome [DOI] [PubMed] [Google Scholar]
- 7.Ng C M, Cheng A, Myers L.et al TGF‐β dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest 20041141586–1592.Study that documents a pathogenetic role of TGF‐β in mitral valve prolapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loeys B L, Chen J, Neptune E R.et al A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 200537275–281.This study describes a new aortic syndrome. [DOI] [PubMed] [Google Scholar]
- 9.De Paepe A, Devereux R B, Dietz H C.et al Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet 199662417–426.Current nosology for diagnosing Marfan syndrome. [DOI] [PubMed] [Google Scholar]
- 10.Hasham S N, Guo D C, Milewicz D M. Genetic basis of thoracic aortic aneurysms and dissections. Curr Opin Cardiol 200217677–683. [DOI] [PubMed] [Google Scholar]
- 11.Pannu H, Fadulu V T, Chang J.et al Mutations in transforming growth factor‐beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation 2005112513–520.This study identifies a new gene related to thoracic aortic syndrome. [DOI] [PubMed] [Google Scholar]
- 12.Mizuguchi T, Collod‐Beroud G, Akiyama T.et al Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet 200436790–792.Study documenting a new gene involved in Marfan syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gott V L, Greene P S, Alejo D E.et al Replacement of the aortic root in patients with Marfan syndrome. N Engl J Med 19993401307–1313.Study that sets the standard for surgical treatment of Marfan syndrome. [DOI] [PubMed] [Google Scholar]
- 14.von Kodolitsch Y, Rybczynski M. Cardiovascular aspects of the Marfan syndrome – a systematic review. In: Robinson PN, Godfrey M, eds. Marfan syndrome: a primer for clinicians and scientists Eurekah.com and Kluwer Academic/Plenum Publishers 200445–69.
- 15.Shores J, Berger K R, Murphy E A.et al Progression of aortic dilatation and benefit of long‐term β‐adrenergic blockade in Marfan's syndrome. N Engl J Med 19943301335–1341.Study that sets the standard for medical treatment of Marfan syndrome. [DOI] [PubMed] [Google Scholar]
- 16.Hetzer R, Pregla R, Barthel F. Surgery for cardiovascular disorders in Marfan syndrome. In: Robinson PN, Godfrey M, eds. Marfan syndrome: a primer for clinicians and scientists Eurekah.com and Kluwer Academic/Plenum Publishers 200481–92.
- 17.Yetman A T, Bornemeier R A, McCrindle B W. Long‐term outcome in patients with Marfan syndrome: is aortic dissection the only cause of sudden death? J Am Coll Cardiol 200341329–332. [DOI] [PubMed] [Google Scholar]
- 18.Nagashima H, Sakomura Y, Aoka Y.et al Angiotensin II type 2 receptor mediates vascular smooth muscle cell apoptosis in cystic medial degeneration associated with Marfan syndrome. Circulation 2001104(suppl I)I282–I287.Study identifies a new pathogenetic mechanism of aortic wall degeneration. [DOI] [PubMed] [Google Scholar]
- 19.Yetman A T, Bornemeier R A, McCrindle B W. Usefulness of enalapril versus propranolol or atenolol for prevention of aortic dilation in patients with the Marfan syndrome. Am J Cardiol 2005951125–1127. [DOI] [PubMed] [Google Scholar]
- 20.Dietz H C, Loeys B, Carta L.et al Recent progress towards a molecular understanding of Marfan syndrome. Am J Med Genet C Semin Med Genet 20051394–9. [DOI] [PubMed] [Google Scholar]
- 21.Yacoub M H, Cohn L H. Novel approaches to cardiac valve repair: from structure to function: part I. Circulation 2004109942–950. [DOI] [PubMed] [Google Scholar]