1. INTRODUCTION
A 2003 National Survey on Drug Use and Health (NSDUH) estimated the rate of illicit drug use among pregnant women at 4.3%; over 130,000 infants were exposed to illicit drugs in utero each year [1]. Substance abuse remains a major public health problem that affects millions of children and places enormous financial and social burdens on society.
The NSDUH reported that adults who used illicit drugs were more than twice as likely to have a serious mental illness as non-using adults; 26.9% of illicit drug users reported having a serious mental illness in the past year. Major depressive disorder (MDD) is the predominant mental illness reported by women, with a lifetime occurrence of 17% [34]. The first onset of MDD is most likely to occur during the childbearing years [19, 34]. Postpartum depression occurs at a rate of 7.4% to 25%, with the highest rates occurring in lower socioeconomic samples [10, 40, 68, 91]. Thus the probability that a woman who used cocaine during her pregnancy will experience a depressive episode in the postpartum period is at least 1 in 6 (based on an average 15% prevalence)[34, 73]. Yet, little is known about the association between maternal depression and prenatal substance abuse and the effects on infant outcomes.
Prenatal cocaine exposure is often associated with a subtle but statistically significant decrease in infant birthweight, gestational age and head circumference [6, 13, 30, 71, 84, 93]. There is emerging evidence supporting a neurobehavioral profile of the cocaine exposed infant, namely, that prenatally cocaine exposed infants tend to have difficulty with arousal and state regulation, including increased or impaired behavioral and physiological reactivity to stress [12, 58, 59, 61, 64, 81]. In a previous report, we found results consistent with these prior studies. One-month old cocaine exposed infants had lower arousal, poorer quality of movement and self-regulation, as well as increased motor tone and lower auditory brain response measures after controlling for other drug use and maternal caregiving variables [50, 51]. Data conflicting with these findings have also been reported with no differences found in young infants prenatally exposed to cocaine [65, 92]. The collective data on prenatal cocaine exposure effects on infant neurobehavior has been inconclusive and warrants further research.
Maternal depression in the first 30 days postpartum is an integral component of the care giving environment of the infant and may have a significant impact on infant development, particularly in an already vulnerable environment [48]. Consistent evidence documents an association between postpartum depression and less positive mother-infant interactions during play and care-taking routines. In addition, women who are depressed immediately postpartum are more likely to have been depressed during the pregnancy, presenting another potential exposure to the fetus [18, 33]. Infants born to mothers with depression during pregnancy were reported to be more aroused, reactive, harder to console, had poorer self-regulation and sleep and less mature neuromotor development. [3, 39, 94].
Despite the growing evidence that depression is highly prevalent in women using cocaine and that both cocaine exposure and maternal depression is associated with adverse infant outcomes, the impact of postpartum depression on infant neurobehavioral development in cocaine exposed samples has not been previously reported. Therefore, the purpose of this study was to examine the effects of postpartum depression on infant neurobehavior outcomes in infants prenatally exposed to cocaine compared to non-exposed infants.
The infants were originally recruited for the Maternal Lifestyles Study (MLS) [48]. The MLS was developed in the early 1990s as an interagency collaborative effort involving the National Institute of Child Health and Human Development (NICHD); the National Institute on Drug Abuse (NIDA); the Administration on Children, Youth and Families; and the Center for Substance Abuse Treatment and is the largest prospective longitudinal study of acute neonatal events and long-term health and developmental outcomes associated with cocaine use during pregnancy.
We hypothesized that the infants whose mothers were experiencing depression in the early postpartum would perform less optimally on a structured examination of infant neurobehavior, and that infants in the maternal depression condition who were prenatally exposed to cocaine would have the poorest performance, particularly in self-regulation, arousal, and reactivity (more handling needed, more excitability) than infants who were not exposed to cocaine prenatally.
2. METHODS
2.1 Study Design
The Maternal Lifestyles Study (MLS) was conducted at four NICHD Neonatal Research Network sites (Brown University, University of Miami, Wayne State University, and the University of Tennessee at Memphis). Mother-infant dyads were screened and recruited immediately after delivery (N=11,811 consented to participate). A trained nurse or social worker conducted a semi-structured interview with the mother to determine past and current drug use and sociodemographic information. A physical examination of the infant was conducted, and the infant’s meconium collected. Before mothers and infants were discharged from the hospital, their charts were abstracted to collect selected medical data. Procedures used to determine exposure status have been described previously[24, 49]. Briefly, meconium samples were collected in the nursery and analyzed in a central laboratory. The study definition of “exposure” was maternal admission of cocaine or opiate use during this pregnancy based on the hospital interview or positive gas chromatography/mass spectroscopy (GC/MS) confirmation of cocaine metabolites. “Unexposed” was defined as denial of cocaine or opiate use during this pregnancy and a negative enzyme-multiplied immunoassay technique screen for cocaine and opiate metabolites. Once exposure status was confirmed by GC/MS analyses, the eligible participants were invited to come into the center for follow-up visit.
2.2 Participants
The infant’s first follow-up visit took place between 42 and 44 weeks post-menstrual age. An approximate time of one-month post delivery was determined to be a period that would allow for exposure status confirmation and a time when potential birth effects of labor and delivery stress and substance withdrawal are less likely to impact infant neurobehavior. Mothers were eligible to participate in the study if they were at least 18 years old, had no identified psychosis, were not institutionalized for mental retardation or mental illness, had no language barriers, and planned to stay in the catchment area. Their singleton infants were eligible if they were at least 501g and less than 42 weeks gestational age at birth without chromosomal abnormalities, fatal illnesses or TORCH infection. All eligible exposed infants who attended the one month visit were matched with an unexposed infant on race, gender, and gestational age [51]. At the one month visit, 1388 mother-infant dyads (658 in the exposed group and 730 in the comparison group) were enrolled in the MLS longitudinal study.
The analyses conducted for the present study were limited to those infants still residing with their biological mothers at the follow-up visit to control for a consistent physiological and care giving environment from prenatal to postnatal life (N=1260). Only dyads without prenatal opiate exposure were included in these analyses to limit potential substance exposure confounds (N=1165). 1,053 mother-infant dyads met these criteria and accurately completed the semi-structured interview of psychiatric symptoms. The methods used to determine the final sample of 1,053 are detailed in the maternal measures section (2.3.1).
2.3 Measures
The one month visit included neurobehavioral, medical and physical status measures of the infant; social and demographic questionnaires; The Maternal Interview of Substance Use (MISU), and the Addiction Severity Index (ASI).
2.3.1 Maternal Measures
The MISU was developed for the MLS and is a structured interview that provides information about the frequency and quantity of substance use for each trimester during this pregnancy for cocaine, alcohol, marijuana, and nicotine.
The ASI is a structured clinical interview that evaluates treatment outcomes of alcohol and drug problems [42, 78]. The respondents are asked about current and lifetime substance use, psychiatric symptoms, diagnoses and hospitalizations. In addition, the interview elicits severity and duration for each psychiatric symptom present in the past 30 days to produce a summary score for psychiatric status [42, 78].
The ASI was used to determine participants who “experienced serious depression” in the past 30 days and those that experienced “a lifetime history of depression”. Although this measure is not diagnostic, the ASI as an indicator of depression has been correlated with diagnoses of depression attained via semi-structured interview and has been a strong predictor of functional outcome in opiate and cocaine abusing cohorts [5, 78 ]. In comparison to semi-structured interview diagnoses of depression, the ASI was found to separate depressed from non-depressed addicts with a sensitivity of .83 and a specificity of .55 [78]. The ASI has been used successfully as a screening tool for depression in pregnant and postpartum women [60].
The presence or absence of a reported serious depression in the past 30 days (lasting at least 2 weeks), coupled with the symptom severity scores on the ASI “psychological problems” subscale (0-5) was used to determine study depression groups (Table 1):
Depressed group: experienced serious depression in the past 30 days AND psychiatric symptom score of 3 or more.
Non-Depressed group: denied serious depression in the past 30 days AND psychiatric symptom score less than 3, regardless of psychiatric history.
TABLE 1.
Number of Participants in the Depression Groups by Cocaine Exposure
| Cocaine Exposed | Non Cocaine Exposed | ||
|---|---|---|---|
| ( COC ) | (NonCOC) | ||
| N (%) | N (%) | Total in Depression Groups | |
| Depressed (DEP) | 76 (19.3) | 104 (15.5) | 180 |
| Non-Depressed (NonDEP) | 309 (80.7) | 564 (84.6) | 873 |
|
| |||
| Total in Cocaine Groups | 385 | 668 | 1053 |
Participants that did not meet these criteria were eliminated from the analyses, as their psychiatric status could not be determined from their ASI data (N=112).
2.3.2 Infant Measures
In addition to medical and demographic characteristics of the sample, the NICU Network Neurobehavioral Scale (NNNS) was used to examine infant neurobehavior at 42-44 weeks post-menstrual age. The NNNS was specifically designed for the MLS [52] and used in studies of intrauterine exposure to cocaine, opiates, and nicotine [25, 38, 44, 46, 47, 64]. The NNNS is composed of three parts: neurological, behavioral, and stress/abstinence. The neurological component includes active and passive tone, primitive reflexes, and items that reflect the integrity of the central nervous system and maturity of the infant. The behavioral component is based on items from the Neonatal Behavioral Assessment Scale (NBAS) modified to be sensitive to putative drug effects [8]. The stress/abstinence component is a checklist of signs observed throughout the examination organized by organ system based primarily on the work of Finnegan that are indicative of stress, hyper-stimulation, or nervous system instability [29]. The NNNS follows a relatively invariant sequence of administration that starts with a pre-examination observation, followed by the neurological and behavioral components. The individual NNNS items are aggregated into summary scales (see Table 2). The summary scores were formed from a conceptual and statistical approach and have psychometric properties with coefficient α ranging from 0.56 to 0.85 [53]. The habituation data were not used, as infants are required to be asleep for this part of the assessment and most infants were awake at the beginning of the examination. The actual sequence of administration and the means used by the examiner to maintain an infant’s participation in the examination were recorded. All infants were examined between 42 and 44 weeks post-menstrual age by certified psychometrists at each site who were masked to infant exposure status. The certification process for the NNNS ensures initial reliability with certified trainers. A gold-standard NNNS trainer visited each site for the training and certification process to ensure reliability across sites.
TABLE 2.
Summary Scores of the NICU Network Neurobehavioral Scale (NNNS)
| Attention | Indicates an infant’s ability to attend and respond to auditory and visual stimulation; high scores on this scale show good response and sustained alertness |
| Handling | Indicates the number and type of maneuvers necessary to keep the infant in the appropriate state to administer items; high scores indicate infants who need substantial input from the examiner in order to elicit attention and response to stimuli |
| Quality Movement | A measure of motor control including smoothness, maturity, modulation of movement of the arms and legs as well as startles and tremors; high scores indicate good quality of movement |
| Self Regulation | Indicates how the infant copes with the demands of the exam, with higher scores indicating better regulation |
| Non-Optimal Refl. | The number of non-optimal reflex responses |
| Stress/Abstinence | The number of stress/abstinence signs displayed by the infant across five organ systems |
| Arousal | Indicates the infant’s overall level of arousal and associated motor activity during the exam; high scores indicate high arousal, activity, and fussing and crying during the exam |
| Hypertonia | Indicates increased muscle tone in the arms, legs, and trunk; a high score describes an infant whose overall tone is consistently hypertonic |
| Hypotonia | Indicates decreased muscle tone in the arms, legs, and trunk; a high score on this scale describes an infant who was consistently hypotonic |
| Asymmetrical Refl. | A count of the number of times a reflex on one side of the body is stronger or weaker than on the other side |
| Excitability | A measure of high levels of motor, state and physiological reactivity; high scores indicate high levels of irritability even with attempts to soothe |
| Lethargy | A measure of low levels of motor, state and physiological reactivity; high scores indicate extreme under-arousal despite the necessary handling during the exam |
2.4 Ethics
The procedures followed for this study were approved by the Internal Review Board of each institution involved. All participants signed an IRB-approved consent form prior to participating in the study. A NIDA Certificate of Confidentiality was obtained by each site that assured confidentiality of information regarding the subjects’ drug use. The certificate superseded the mandatory reporting of illegal substance use that was in effect in the Florida and Rhode Island sites. The certificate was explained to the mother during the recruitment and informed consent procedure, including the condition that the certificate did not exclude reporting evidence of child abuse or neglect.
2.5 Statistics
Analysis of variance (ANOVA) and Chi-square were used to determine group differences on infant and maternal characteristics. The neurobehavioral dependent measures were tested for main effects and interactions using a series of ANOVA with Type III sums of squares: 1). A 2 (Cocaine: exposed/non-exposed) x 2 (Depression: depressed/non-depressed) factorial ANOVA was conducted with each NNNS summary score variable. 2). An ANCOVA with the 2 X 2 design with covariates selected because they met the following statistical criteria: the variable was correlated with the independent variables (cocaine exposure or depression group) or with the NNNS subscales (p < .05) and not correlated with other covariates (Pearson r < 0.70) [37, 43, 45, 76]. The covariates that were used in the adjusted analysis included Index of Social Position Score from the Hollingshead scale of socioeconomic status (SES), birthweight, maternal age, research site, nicotine, marijuana, and alcohol use throughout the pregnancy. 3.) An ANCOVA design using a three cocaine exposure groups: no exposure, some exposure, and heavy exposure by the 2 depression groups was conducted to determine if depression effects would differ based on amount of cocaine exposure. 4.) An ANCOVA design comparing depression effects based on trimester of exposure, no exposure, first trimester only, and second or third trimester. Post-hoc analyses were conducted on the significant variables using Tukey HSD tests.
3. RESULTS
3.1 Prevalence of reported depression
The number of participants excluded from the analyses due to lack of consistent ASI data was significantly greater in the cocaine-exposed (COC) group (N=59;13.29%) than the non cocaine-exposed (nonCOC) groups (N=53; 7.35%) (X2/Yates = 10.48, p<.002). The 112 participants excluded from further analyses were not different on demographic characteristics or infant outcome measures than the participants that were included in the remaining analyses.
Based on the ASI, there was not a significant difference in the number of women meeting criteria for depression in the past 30 days in the COC group than the nonCOC group (X2/Yates =2.71, p=.09) (Table 2). Only 14 of the 172 depressed (DEP) mothers reported taking psychotropic medication in the past 30 days; 3 of them were cocaine users.
3.2 Maternal Variables
3.2.1 Demographics
The cocaine groups were originally matched on demographic variables. Therefore, we analyzed demographic variables using Chi-square for DEP differences within the COC groups. Table 3 presents the maternal characteristics of the DEP and non-depressed (nonDEP) groups in both the COC and nonCOC groups. Only one significant difference was found for DEP within COC groups: more women in the nonCOC/DEP group were less than 27 years of age (X2=8.24, df=2, p<.02) than women in the nonCOC/nonDEP group.
TABLE 3.
Characteristics of Women in the Depression Groups by Cocaine Exposure
| COC | NonCOC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dep | NonDep | Dep | NonDep | |||||||||
| N=76 | N=309 | N=104 | N=564 | |||||||||
| N | % | N | % | N | % | N | % | |||||
| Age | 18-25 | 18 | 23.68 | 57 | 18.45 | n.s. | 67 | 66.67 | 284 | 49.35 * | ||
| 26-49 | 58 | 76.32 | 252 | 81.55 | 37 | 29.89 | 280 | 42.62 | ||||
| Marital Status | Married | 8 | 10.53 | 35 | 11.33 | n.s. | 18 | 17.31 | 154 | 27.30 | ns | |
| Single | 68 | 89.47 | 274 | 88.67 | 86 | 82.69 | 410 | 72.70 | ||||
| Parity | First Born | 6 | 7.89 | 31 | 10.03 | n.s. | 27 | 25.96 | 175 | 31.08 | ns | |
| 2-3 Children | 30 | 39.47 | 128 | 41.42 | 44 | 42.31 | 269 | 47.78 | ||||
| 4 or More | 40 | 52.63 | 150 | 48.54 | 33 | 31.73 | 119 | 21.14 | ||||
| Race | Black | 62 | 81.58 | 253 | 81.88 | n.s. | 88 | 84.62 | 439 | 77.84 | n.s. | |
| White | 10 | 13.16 | 34 | 11.00 | 12 | 11.54 | 84 | 14.89 | ||||
| Other | 4 | 5.26 | 22 | 7.12 | 4 | 3.85 | 41 | 7.27 | ||||
| SES | % low | 22 | 28.95 | 86 | 27.83 | n.s. | 21 | 29.81 | 103 | 18.26 | n.s. | |
| Insurance | % Medicaid | 67 | 88.16 | 276 | 89.32 | n.s. | 89 | 85.58 | 434 | 76.95 | n.s. | |
Within group percentages presented. n.s.: p>.05, *p<.05
3.2.2 Maternal Drug Use during Pregnancy
The amount of cocaine use throughout pregnancy and in the first month postpartum for the DEP and nonDEP cocaine groups is shown in Figure 1. Both groups decreased their cocaine use throughout the pregnancy and into the first month postpartum (F=59.27, p<.0001). Although the DEP group reported consistently more cocaine use than the nonDEP, the difference was only marginally significant (F=3.15, p<.07). Significantly more prenatal cocaine users reported using cocaine in the first 30 days postpartum if they were in the DEP group (26.3%) than those in the nonDEP group (14.3%), X2=6.35, p<.02.
Figure 1. Amount of cocaine exposure during pregnancy reported for the DEP and nonDEP groups.
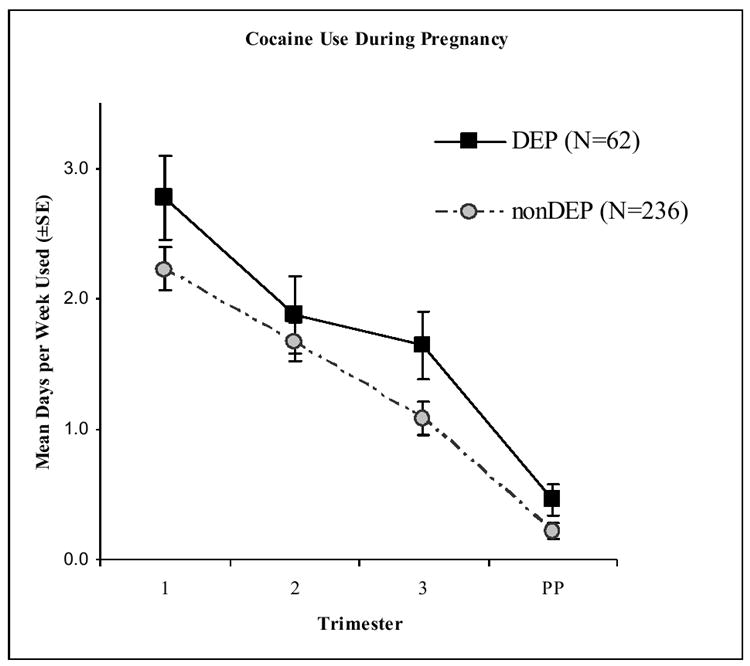
Maternal report of the mean number of days using cocaine per week.
PP=first 30 days postpartum
Table 4 shows the mean amount of other reported drug use throughout the pregnancy. The COC and nonCOC groups differed in their use of other drugs. Women who used cocaine during the pregnancy also drank more alcohol (F(1, 1039)=50.56, p<.0001), smoked more marijuana (F(1, 1039)=8.22, p<.01), and smoked more cigarettes (F(1, 1039)=103.83, p<.0001) during the pregnancy than those who did not use cocaine. There was a significant depression effect for nicotine use during pregnancy; women in the DEP group smoked more cigarettes throughout the pregnancy than the nonDEP (F (1, 1039) =11.67, p<.001).
TABLE 4.
Other Substance Use for Women in the Depression Groups by Cocaine Exposur
| COC | NonCOC | ||||
|---|---|---|---|---|---|
| DEP | NonDEP | DEP | NonDEP | ||
| (N=74) | (N=285) | (N=98) | (N=546) | ||
| M±SE | M±SE | M±SE | M±SE | ||
| Alcohol (std.drinks/day) | 0.65±.09 | 0.59±.05 | 0.22±.08 | 0.07±.03 | ***COC |
| Marijuana (joints/day) | 0.09±.04 | 0.17±.02 | 0.07±.03 | 0.03±.01 | ** COC |
| Nicotine (Cigs/day) | 12.03±.96 | 9.17±.49 | 4.72±.83 | 2.57±.35 | ***COC, ***DEP |
p<.01,
p<.001
COC, cocaine main effect; DEP, depression main effect
3.2.3 Maternal Mental Health Treatment in the first 30 Days Postpartum
There was a significant COC effect for the mean number of days women reported receiving outpatient mental health treatment in the first 30 days postpartum (Mean (SE)): 4.46 (0.33) COC/nonDEP; 4.75 (0.67) COC/DEP; 0.05 (0.03) nonCOC/nonDEP; 0.07 (0.57) nonCOC/DEP, (F (1, 1,043) = 88.85, p<.0001). More women in the COC groups were in mental health treatment versus nonCOC groups; 26.5% of women in the COC group received outpatient treatment in the postpartum period compared to only 2.3% of women in the nonCOC group.
There was not a significant difference in psychological symptom severity score derived from the ASI between COC (mean (SE) =0.834 (0.07)) and nonCOC (mean (SE)=0.692 (.05)) groups, F(1,1049)=2.68, n.s.
3.3 Infant Variables
3.3.1 Demographics
Table 5 presents the characteristics of the neonates at birth in the depressed (DEP) and non-depressed (nonDEP) groups by cocaine exposure. There were no significant cocaine effects on infant characteristics at birth. There was a significant interaction effect for the variables of birthweight (F(1,1043)=6.93, p<.01), length (F(1,1045)=6.13, p<.02), and head circumference (F(1,1045)=5.40, p<.03). The nonCOC/DEP infants had significantly lower birthweights (F(1,1045)=6.50, p<.01) and were shorter in length than the nonCOC/nonDEP infants (F(1,1045)=6.31p<.01). There were no significant DEP differences.
TABLE 5.
Neonate Characteristics in the Depression Groups by Cocaine Exposure
| COC | NonCOC | ||||
|---|---|---|---|---|---|
| Dep | Non-Dep | Dep | Non-Dep | ||
| (N=76) | (N=306) | (N=104) | (N=560) | ||
| Mean±SE | Mean±SE | Mean±SE | Mean±SE | ||
| GAa | 36.46±0.46 | 36.12±0.23 | 35.61±0.40 | 36.46±0.17 | |
| BirthWt. (kg) | 2.66±0.10 | 2.56±0.05 | 2.47±0.08 | 2.73±0.04 | **Int |
| Length (cm) | 46.91±0.58 | 46.44±0.29 | 45.70±0.50 | 47.27±0.21 | *Int |
| Head Circ. (cm) | 32.41±0.35 | 31.96±0.18 | 31.64±0.31 | 32.34±0.13 | *Int |
| Apgar 1 (median) | 7.70±0.22 | 7.58±0.11 | 7.36±0.19 | 7.45±0.10 | |
| Apgar 5 (median) | 8.75±0.12 | 8.61±0.06 | 8.55±0.10 | 8.53±0.04 | |
| Male (%) | 41.27 | 49.46 | 52.87 | 44.86 | |
p<.05,
p<.01;
Best obstetrical estimate
Int, Interaction effect
3.3.2 Neurobehavioral Scores on the NNNS
There were no significant differences on any of the NNNS measures within the DEP groups for those taking psychotropic medications or receiving psychiatric treatment in the past 30 days. Therefore, psychotropic medication use prescribed by a physician and psychiatric treatment were not used in the following analyses. Infants of women with a lifetime history of depression and not currently depressed did not differ from the infants born to women that were never depressed. Therefore, women with a lifetime history of depression with the absence of a current depression were included in the nonDEP group.
Analyses were conducted in two steps. First, ANCOVA analyses were run using the factors of DEP (yes/no) and COC (yes/no) groups. All NNNS variables that were significant for a main or interaction effect were further analyzed for the trimester of cocaine exposure and the amount of cocaine use reported.
In the initial analyses there were significant main effects for depression after covariate adjustment for Self-Regulation (F(1, 996)=4.08, p<.05), Excitability (F(1, 991)=4.79, p<.03), and Hypotonia (F(1,986)=4.05, p<.05) with infants in the DEP groups having lower self-regulation, higher excitability, and more hypotonia than infants in the nonDEP groups. There were no significant cocaine main effects after covariate adjustment. There were three significant interaction effects after covariate adjustment: Self-Regulation (F(1, 996)=7.45, p<.012), Stress (F(1, 991)=4.01, p<.05), and Arousal (F(1, 980)=5.93, p<.02).
The pattern of results was the same regardless of the trimester of cocaine use; only the nonCOC groups had a significant DEP effect. Therefore, analyses examining the amount of cocaine exposure included infants who were exposed to cocaine in any trimester.
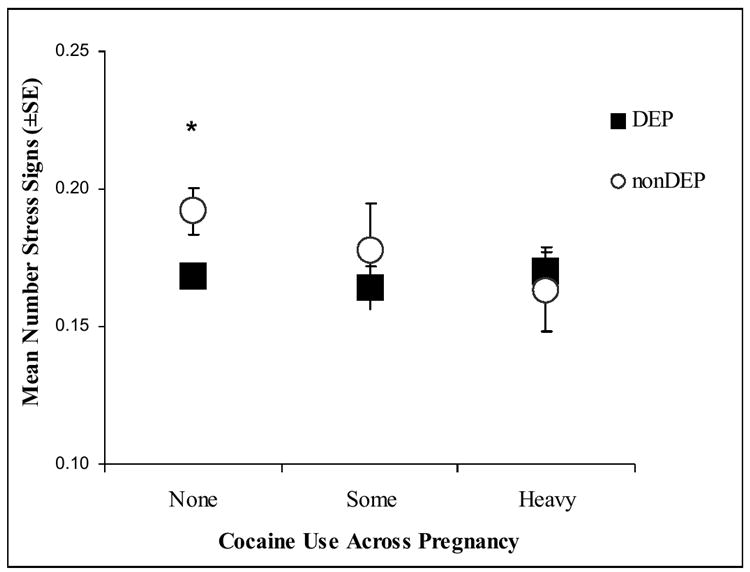
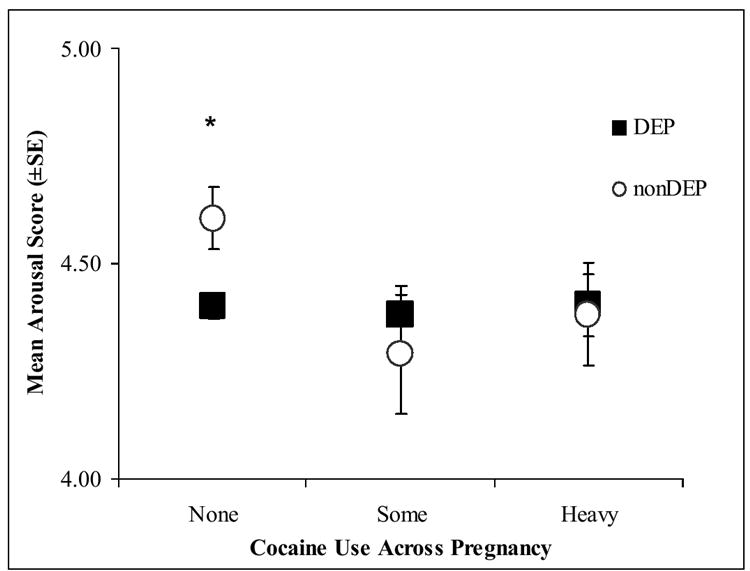
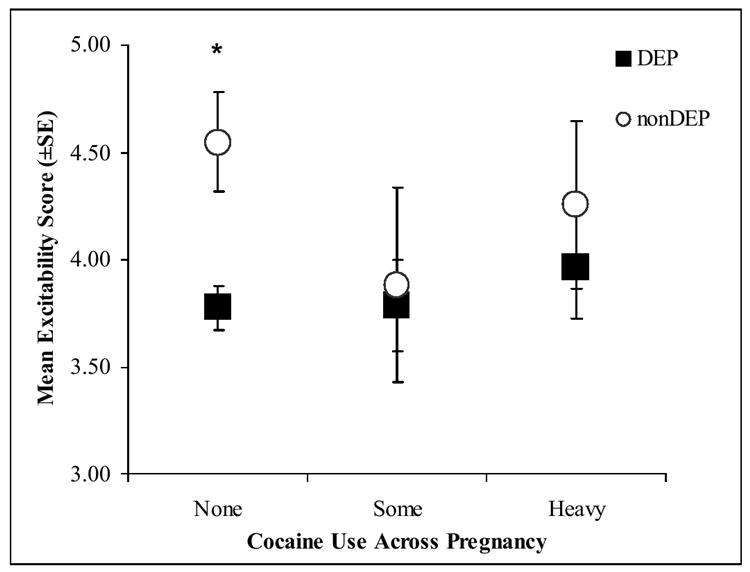
Secondary post-hoc analyses were conducted on the NNNS variables using 3 cocaine groups: none, some (<1.5 days per week) and heavy (≥1.5 days per week) cocaine use. Women who denied cocaine use but whose infants showed positive cocaine toxicology from meconium samples were eliminated from the secondary analyses. Figures 2a-d present the significant adjusted mean scores on the NNNS for the DEP groups in each of the three cocaine exposure groups.
Figure 2. NNNS scores for the DEP and nonDEP group in each COC use group.
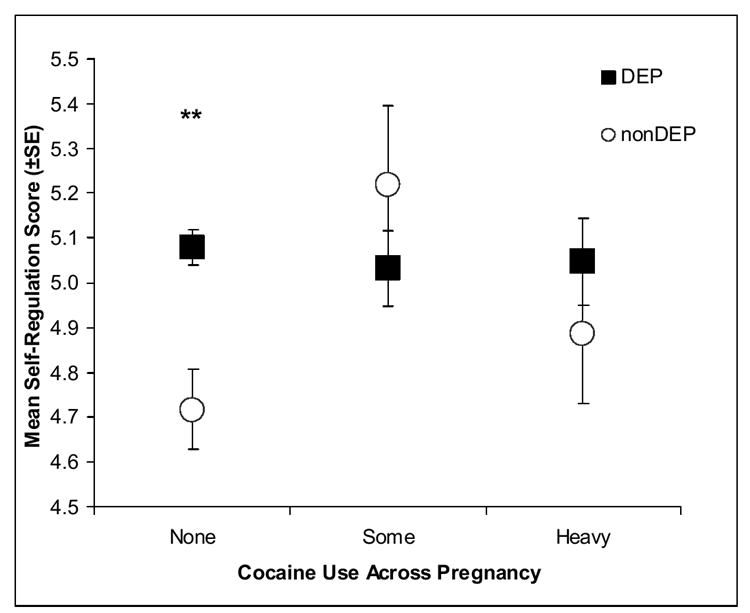
a. Self Regulation
b. Stress
c. Arousal
d. Excitability
*p<.05, **p<.01,; Significant differences on post-hoc Tukey HSD tests
The pattern of results remained the same; only those infants without any prenatal cocaine exposure showed significant DEP differences on the NNNS. The nonCOC/DEP infants compared to nonCOC/nonDEP infants had significantly lower self-regulation (Figure 2a), more stress signs (Figure 2b), more arousal (Figure 2c), and more excitability (Figure 2d).
Due to the large discrepancy in mental health treatment received between COC and nonCOC women, the NNNS analyses were repeated with only those women not receiving mental health treatment. Three interaction effects remained significant: self-regulation, stress, and arousal.
4. DISCUSSION
This study examined the effects of early postpartum maternal depression on infant neurobehavior at one month postmenstrual age in infants prenatally exposed to cocaine compared to non-exposed infants using the NICU Network Neurobehavioral Scale (NNNS). Using the ASI as a screening tool for depression, we were able to determine neurobehavioral differences of small to medium effect sizes (0.2 to 0.5), demonstrating adequate power for the intended analyses [16]. The prevalence of a serious depression as measured by the ASI in the immediate postpartum period in this sample was substantial in both the cocaine (COC) and non-cocaine (nonCOC) groups (19.3% and 15.5%, respectively). These rates of depression are within the range typically reported in samples with low socioeconomic status and in substance abusing samples and indicate a critical need to measure maternal mood symptoms in cocaine using samples.[14, 73]
We originally hypothesized that the infants whose mothers were experiencing depression in the early postpartum would perform less optimally on a structured examination of infant neurobehavior, and that infants in the maternal depression condition who were prenatally exposed to cocaine would have the poorest performance. Our hypothesis was only partially correct. We found that infants whose mothers were experiencing a postpartum depression (DEP) were having more difficulty with self-regulation and excitability and they were more hypotonic than infants of non-depressed women (nonDEP). However, we also found significant interaction effects that did not support our hypothesis that prenatal cocaine exposure would result in an exacerbation of the DEP effects. DEP effects were found on 4 of the 12 NNNS variables only for those infants who were not prenatally exposed to cocaine: infants in the DEP group had poorer self-regulation and more stress signs, excitability, and arousal than infants in the nonDEP group. Of note is that the DEP/COC group (double exposure) did not differ on any of the neurobehavioral measures from the nonDEP/nonCOC (control) group. The pattern of results was the same regardless of the amount or trimester of cocaine exposure.
Infants in the nonCOC/DEP group also had the lowest birthweights, shortest birth-lengths, and smallest head circumferences than the other three groups. Women in the nonCOC/DEP group were also the youngest mothers. The significant neurobehavioral results persisted after controlling for the effects of maternal age, birthweight, polydrug use, SES and research site. Regression analyses were conducted to further test the relationship between maternal psychiatric symptom severity measured on the ASI and infant neurobehavior after controlling for the effect of infant birthweight. The results remained the same, namely that maternal symptom level was related to infant NNNS scores (self-regulation, stress, arousal excitation, and hypotonia) only for those in the nonCOC group.
The results indicate that for infants not prenatally exposed to cocaine, maternal depression is associated with greater difficulty with state regulation and reactivity during the examination. These data are in agreement with prior studies that reported that infants of depressed mothers were more irritable, had more sleep problems, were more tense, and reacted more under the stress of testing conditions than infants of non-depressed women [27, 36, 62, 63, 90].
We were unable to determine if the infants were exposed to maternal depression in utero as the psychiatric problems section of the ASI interview did not ask specifically about depression during the pregnancy. A significant depression in the first 30 days after delivery is likely to indicate that the depression started prior to or during the pregnancy as antenatal depression is one of the most predictive factors for postpartum depression [4, 18, 33, 40, 69, 70]. Therefore, we cannot be sure if the depression effects found in this study are due solely to postnatal maternal depression or if prenatal depression exposure contributes to the effects.
Behavioral findings in prenatally cocaine-exposed infants have not always been consistent. Some studies did not find cocaine effects on newborns and young infants, while others reported difficulty in arousal regulation, including greater excitability, poor state regulation, more rapid changes in arousal with stimulation, increased arousal from sleep, and increased physiological lability [7, 9, 20, 32, 41, 57, 75]. Maternal depression was not reported in these papers. Based on our findings, inconsistencies in results may be related to other factors that were not measured or reported, such as maternal mood and depression.
Only two studies looking at infant outcomes have reported maternal mood variables in cocaine using samples. Singer et al (2002) found that maternal psychological distress predicted smaller neonatal head circumference after controlling for the effects of prenatal cocaine use, other drug use and confounding variables [85]. In 1993, Woods and colleagues reported higher maternal depressive scores in cocaine-using women and used the depression scores as a covariate when looking at the effects of prenatal cocaine exposure on infant behavior [92]. This study did not find any differences between cocaine exposed and unexposed infants. The results of this study are consistent with ours in that controlling for maternal depression led to the absence of cocaine main effects.
Our results suggest that early postpartum maternal depressive symptoms have a significant effect on infant neurobehavior and that prenatal cocaine exposure might buffer or alter these effects. One possible explanation for the difference in results in infants exposed to cocaine before birth is the affect of cocaine on developing monoamine systems.
Evidence from rodent, rabbit and primate studies suggest that prenatal exposure to cocaine is associated with decreased dopaminergic activity and a resulting decrease in serotonin receptor density (5-HT1a) in the dorsal raphe nucleus[2, 21]. The action of serotonin in the raphe nucleus exerts an inhibitory effect on the ascending serotonin system into the cortex; therefore a depletion of raphe serotonin results in an increase in serotonin input to the cortex[22, 23, 74]. It is possible that a cocaine induced abundance of serotonin in the cortex would contribute to a “buffer” of serotonin and other monoamines for infants that are exposed to maternal depression.
Other potential mechanisms may be involved in an interaction effect of depression and cocaine, including other neurotransmitter systems, neuroendocrine alterations, and Hypothalamic-Pituitary-Adrenal Axis (HPA) responses. Glucocorticoids, the main end product of the HPA system, mediate monoaminergic activity particularly in the limbic areas of the brain and may be a primary site for further investigation of potential interactions of prenatal cocaine and depression exposure on offspring development.
Other variables we considered include the exclusion of more women in the COC group than the nonCOC group due to inadequate data on the ASI, more days in mental health treatment for women in the COC group in the first 30 days postpartum, and other demographic and neonatal growth variables. More days of treatment for the women in the COC/DEP group than the nonCOC/DEP group could influence newborn behavior if treatment was associated with greater symptom reduction and improvement in mother-infant interactions. However, there was not a significant difference in COC vs nonCOC for meeting depression criteria in the first 30 days postpartum. The interaction results remained significant when reanalyzing the data without participants in outpatient treatment. There was also no significant difference for number of days using cocaine in the postpartum period between those women receiving outpatient mental health treatment and those who were not.
There was limited power in the DEP groups to look at associations with other variables that might also contribute to the results obtained, including maternal anxiety, home environment, time between pregnancies, and general health conditions of the infant at time of testing.
In conclusion, these data provide evidence of an interaction between maternal depression and prenatal cocaine exposure on infant neurobehavioral outcomes. Cocaine exposed infants did not differ from unexposed infants on neurobehavioral scores in depressed groups. Depression effects were only found in infants not exposed to cocaine during gestation. These results suggest a possible buffering effect of cocaine on the effects of maternal depression on infants. Despite the limitations discussed, these findings suggest that past research findings as well as future research on the effects of prenatal substance exposure would benefit from consideration and measurement of maternal mood.
Acknowledgments
Research Project Support: This research was supported by NIH cooperative agreements U10 HD 27904, U10 HD 21397, U10 HD 21385; U10 HD 27856, NICHD contract N01-HD-2-3159 and intra-agency agreements with the National Institute on Drug Abuse (NIDA), Administration on Children, Youth and Families (ACYF), and the Center for Substance Abuse Treatment (CSAT), and NIMH T32MH019927.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- 1.Results from the 2002 National Survey on Drug Use and Health: National Findings in (DHHS Publication No. SMA 03-3836, NHSDA Series H-22) Office of Applied Studies, Substance Abuse and Mental Health Services Administration; Rockville, MD: 2003. [Google Scholar]
- 2.Akbari HM, Kramer HK, Whitaker-Azmitia PM, Spear LP, Azmitia EC. Prenatal cocaine exposure disrupts the development of the serotonergic system. Brain Res. 1992;572:57–63. doi: 10.1016/0006-8993(92)90450-n. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong KL, O’Donnell H, McCallum R, Dadds M. Childhood sleep problems: association with prenatal factors and maternal distress/depression. J of Paediatr and Child Health. 1998;34:263–6. doi: 10.1046/j.1440-1754.1998.00214.x. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson AK, Rickel AU. Postpartum depression in primiparous parents. J Abnorm Psychol. 1984;93:115–9. doi: 10.1037//0021-843x.93.1.115. [DOI] [PubMed] [Google Scholar]
- 5.Batson HW, Brown LS, Jr, Zaballero AR, Chu A, Alterman AI. Conflicting measurements of depression in a substance abuse population. J Subst Abuse. 1993;5:93–100. doi: 10.1016/0899-3289(93)90127-w. [DOI] [PubMed] [Google Scholar]
- 6.Bauer CR, Shankaran S, Bada HS, Lester B, Wright LL, Krause-Steinrauf H, Smeriglio VL, Finnegan LP, Maza PL, Verter J. The Maternal Lifestyle Study: drug exposure during pregnancy and short-term maternal outcomes. Am J Obstet Gynecol. 2002;186:487–95. doi: 10.1067/mob.2002.121073. [DOI] [PubMed] [Google Scholar]
- 7.Bendersky M, Lewis M. Arousal modulation in cocaine-exposed infants. Dev Psychobiol. 1998;34:555–64. [PMC free article] [PubMed] [Google Scholar]
- 8.Brazelton TB. Neonatal Behavioral Assessment Scale. Lippincott; Philadelphia, PA: 1984. [Google Scholar]
- 9.Brown J, Bakeman A, Coles CD, Sexson WA, Demi AS. Maternal drug use during pregnancy: Are preterms and full-terms affected differently? Dev Psychol. 1998;34:540–554. doi: 10.1037//0012-1649.34.3.540. [DOI] [PubMed] [Google Scholar]
- 10.Campbell SB, Cohn JF. Prevalence and correlates of postpartum depression in first-time mothers. J Abnorm Psychol. 1991;100:594–9. doi: 10.1037//0021-843x.100.4.594. [DOI] [PubMed] [Google Scholar]
- 11.Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59:11–4. [PubMed] [Google Scholar]
- 12.Chasnoff IJ, Burns WJ, Schnoll SH, Burns KA. Cocaine use in pregnancy. N Engl J Med. 1985;313:666–9. doi: 10.1056/NEJM198509123131105. [DOI] [PubMed] [Google Scholar]
- 13.Chasnoff IJ, Burns KA, Burns WJ. Cocaine use in pregnancy: perinatal morbidity and mortality. Neurotoxicol Teratol. 1987;9:291–3. doi: 10.1016/0892-0362(87)90017-1. [DOI] [PubMed] [Google Scholar]
- 14.Chaudron LH, Kitzman HJ, Peifer KL, Morrow S, Perez LM, Newman MC. Prevalence of maternal depressive symptoms in low-income Hispanic women. J Clin Psychiatry. 2005;66:418–23. doi: 10.4088/jcp.v66n0402. [DOI] [PubMed] [Google Scholar]
- 15.Cleveland A, Westergaard GC, Trenkle MK, Higley JD. Physiological predictors of reproductive outcome and mother-infant behaviors in captive rhesus macaque females (Macaca mulatta) Neuropsychopharmacology. 2004;29:901–10. doi: 10.1038/sj.npp.1300361. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Second. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1988. p. 567. [Google Scholar]
- 17.Cohn JF, Tronick EZ. Three-month-old infants’ reaction to simulated maternal depression. Child Dev. 1983;54:185–93. [PubMed] [Google Scholar]
- 18.Da Costa D, Larouche J, Dritsa M, Brender W. Psychosocial correlates of prepartum and postpartum depressed mood. J Affect Disord. 2000;59:31–40. doi: 10.1016/s0165-0327(99)00128-7. [DOI] [PubMed] [Google Scholar]
- 19.Desai HD, Jann MW. Major depression in women: a review of the literature. J Am Pharm Assoc. 2000;40:525–37. [PubMed] [Google Scholar]
- 20.DiPietro JA, Suess PE, Wheeler JS, Smouse PH, Newlin DB. Reactivity and regulation in cocaine-exposed neonates. Infant Behavior and Development. 1995;18:407–414. [Google Scholar]
- 21.Dow-Edwards DL. Cocaine effects on fetal development: A comparison of clinical and animal research findings. Neurotoxicol and Teratol. 1991;13:347–352. doi: 10.1016/0892-0362(91)90082-8. [DOI] [PubMed] [Google Scholar]
- 22.Dow-Edwards DL. Modification of acoustic startle reactivity by cocaine administration during the postnatal period: comparison with a specific serotonin reuptake inhibitor. Neurotoxicol and Teratol. 1996;18:289–96. doi: 10.1016/s0892-0362(96)90029-x. [DOI] [PubMed] [Google Scholar]
- 23.Dow-Edwards DL. Preweaning cocaine administration alters the adult response to quipazine: comparison with fluoxetine. Neurotoxicol and Teratol. 1998;20:133–42. doi: 10.1016/s0892-0362(97)00095-0. [DOI] [PubMed] [Google Scholar]
- 24.ElSohly MA, Stanford DF, Murphy TP, Lester BM, Wright LL, Smeriglio VL, Verter J, Bauer CR, Shankaran S, Bada HS, Walls HC. Immunoassay and GC-MS procedures for the analysis of drugs of abuse in meconium. J Anal Toxicol. 1999;23:436–45. doi: 10.1093/jat/23.6.436. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson A, Coyle MG, LaGasse L, Liu E, Lester B. Neurobehavioral effects of treatment for opiate withdrawal. Ped Research. 2001;49:18A. doi: 10.1136/adc.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Field T. Early interactions between infants and their postpartum depressed mothers. Infant Behavior and Development. 1984;7:517–522. [Google Scholar]
- 27.Field T. Maternal depression effects on infants and early interventions. Prev Med. 1998;27:200–3. doi: 10.1006/pmed.1998.0293. [DOI] [PubMed] [Google Scholar]
- 28.Field T, Diego M, Hernandez-Reif M, Vera Y, Gil K, Schanberg S, Kuhn C, Gonzalez-Garcia A. Prenatal maternal biochemistry predicts neonatal biochemistry. Int J Neurosci. 2004;114:933–45. doi: 10.1080/00207450490461305. [DOI] [PubMed] [Google Scholar]
- 29.Finnegan LP, Kron RE, Connaughton JF, Emich JP. Assessment and treatment of abstinence in the infant of the drug- dependent mother. Int J Clin Pharmacol Biopharm. 1975;12:19–32. [PubMed] [Google Scholar]
- 30.Fulroth R, Phillips B, Durand DJ. Perinatal outcome of infants exposed to cocaine and/or heroin in utero. Am J Dis Child. 1989;143:905–10. doi: 10.1001/archpedi.1989.02150200057018. [DOI] [PubMed] [Google Scholar]
- 31.Garcia Coll CT, Halpern L, Seifer R, Meyer EC, Kilis E, Lester BM, Vohr BR, Oh W. Behavioral intervention and post-natal growth in full-term intrauterine growth retarded (IUGR) infants. Early Human Development. 1996;46:105–16. doi: 10.1016/0378-3782(96)01748-3. [DOI] [PubMed] [Google Scholar]
- 32.Gingras JL, Feibel J, Dalley B, Muelenaer A. Maternal polydrug use including cocaine and postnatal infant sleep architecture: Preliminary observations and implications for respiratory control and behavior. Early Hum Dev. 1995;43:197–204. doi: 10.1016/0378-3782(96)81867-6. [DOI] [PubMed] [Google Scholar]
- 33.Gotlib IH, Whiffen VE, Mount JH, Milne K, Cordy NI. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult and Clin Psychol. 1989;57:269–74. doi: 10.1037//0022-006x.57.2.269. [DOI] [PubMed] [Google Scholar]
- 34.Hasin DS, Hatzenbueler M, Smith S, Grant BF. Co-occurring DSM-IV drug abuse in DSM-IV drug dependence: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:117–23. doi: 10.1016/j.drugalcdep.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Higley JD, Suomi SJ, Linnoila M. CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology (Berl) 1991;103:551–6. doi: 10.1007/BF02244258. [DOI] [PubMed] [Google Scholar]
- 36.Hiscock H, Wake M. Infant sleep problems and postnatal depression: a community-based study. Pediatrics. 2001;107:1317–22. doi: 10.1542/peds.107.6.1317. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson J, Jacobson S. In: Methodological issues in human behavioral teratology, in Advances in Infancy Research. Rovee-Collier C, Lipsitt LP, editors. Plenum Publishing; New York, NY: 1990. pp. 111–148. [Google Scholar]
- 38.Johnson RE, Jones HE, Jasinski DR, Svikis DS, Haug NA, Jansson LM, Kissin WB, Alpan G, Lantz ME, Cone EJ, Wilkins DG, Golden AS, Huggins GR, Lester BM. Buprenorphine treatment of pregnant opioid--dependent women: maternal and neonatal outcomes. Drug and Alcohol Depend. 2001;63:97–103. doi: 10.1016/s0376-8716(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 39.Jones NA, Field T, Fox N, Davalos M, Lundy B, GHart S. Newborns of mothers with depressive symptoms are physiologically less developed. Infant Behavior & Development. 1998;21:537–541. [Google Scholar]
- 40.Josefsson A, Berg G, Nordin C, Sydsjo G. Prevalence of depressive symptoms in late pregnancy and postpartum. Acta Obstet Gynecol Scand. 2001;80:251–5. doi: 10.1034/j.1600-0412.2001.080003251.x. [DOI] [PubMed] [Google Scholar]
- 41.Karmel BZ, Gardner JM. Prenatal cocaine exposure effects on arousal-modulated attention during the neonatal period. Devl Psychobiol. 1996;29:463–80. doi: 10.1002/(SICI)1098-2302(199607)29:5<463::AID-DEV5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 42.Kosten TR, Rounsaville BJ, Kleber HD. Concurrent validity of the addiction severity index. J Nerv Ment Dis. 1983;171:606–10. doi: 10.1097/00005053-198310000-00003. [DOI] [PubMed] [Google Scholar]
- 43.LaGasse LL, Seifer R, Lester BM. Interpreting research on prenatal substance exposure in the context of multiple confounding factors. Clin Perinatol. 1999;26:39–54. vi. [PubMed] [Google Scholar]
- 44.Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111:1318–23. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- 45.Leon DA. Failed or misleading adjustment for confounding. Lancet. 1993;342:479–81. doi: 10.1016/0140-6736(93)91599-h. [DOI] [PubMed] [Google Scholar]
- 46.Lester B, EZ T, L M. Neurodevelopmental consortium, the NICHD Neonatal research network. A neurodevelopmental follow-up battery for substance exposed infants. Ped Research. 1994;35:23A. [Google Scholar]
- 47.Lester B, Tronick E. The effects of prenatal cocaine exposure and child outcome: lessons from the past. Inf Ment Health J. 1994;15:107–120. [Google Scholar]
- 48.Lester BM. The Maternal Lifestyles Study. Ann N Y Acad Sci. 1998;846:296–305. [PubMed] [Google Scholar]
- 49.Lester BM, ElSohly M, Wright LL, Smeriglio VL, Verter J, Bauer CR, Shankaran S, Bada HS, Walls HH, Huestis MA, Finnegan LP, Maza PL. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–17. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- 50.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Lu J, Finnegan LP, Maza PL. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110:1182–92. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 51.Lester BM, Lagasse L, Seifer R, Tronick EZ, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Liu J, Finnegan LP, Maza PL. The Maternal Lifestyle Study (MLS): effects of prenatal cocaine and/or opiate exposure on auditory brain response at one month. J Peds. 2003;142:279–85. doi: 10.1067/mpd.2003.112. [DOI] [PubMed] [Google Scholar]
- 52.Lester BM, Tronick EZ. History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113:634–40. [PubMed] [Google Scholar]
- 53.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Lu J. Summary statistics of neonatal intensive care unit network neurobehavioral scale scores from the maternal lifestyle study: a quasinormative sample. Pediatrics. 2004;113:668–75. [PubMed] [Google Scholar]
- 54.Liu JH, Rebar RW. In: Endocrinology of Pregnancy, in Maternal-Fetal Medicine. Creasy RK, Resnik R, editors. W.B Saunders; Philadelphia: 1998. [Google Scholar]
- 55.Lobel M, Dunkel-Schetter C, Scrimshaw SC. Prenatal maternal stress and prematurity: a prospective study of socioeconomically disadvantaged women. Health Psychol. 1992;11:32–40. doi: 10.1037//0278-6133.11.1.32. [DOI] [PubMed] [Google Scholar]
- 56.Lovejoy MC, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561–92. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- 57.Mayes LC, Bornstein MH, Chawarska K, Haynes CM, Granger AH. Impaired regulation of arousal in three-month-old infants exposed prenatally to cocaine and other drugs. Dev Psychopath. 1996;8:29–42. [Google Scholar]
- 58.Mayes LC. Developing brain and in utero cocaine exposure: effects on neural ontogeny. Dev Psychopath. 1999;11:685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- 59.Mayes LC. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotox and Teratol. 2002;24:385–95. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- 60.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 61.Morrow CE, Bandstra ES, Anthony JC, Ofir AY, Xue L, Reyes ML. Influence of prenatal cocaine exposure on full-term infant neurobehavioral functioning. Neurotox and Teratol. 2001;23:533–44. doi: 10.1016/s0892-0362(01)00173-8. [DOI] [PubMed] [Google Scholar]
- 62.Murray L. The impact of postnatal depression on infant development. J Child Psychol Psychiatry. 1992;33:543–61. doi: 10.1111/j.1469-7610.1992.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 63.Murray L, Fiori-Cowley A, Hooper R, Cooper P. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev. 1996;67:2512–26. [PubMed] [Google Scholar]
- 64.Napiorkowski B, Lester BM, Freier MC, Brunner S, Dietz L, Nadra A, Oh W. Effects of in utero substance exposure on infant neurobehavior. Pediatrics. 1996;98:71–5. [PubMed] [Google Scholar]
- 65.Neuspiel DR, Hamel SC, Hochberg E, Greene J, Campbell D. Maternal cocaine use and infant behavior. Neurotox and Teratol. 1991;13:229–33. doi: 10.1016/0892-0362(91)90015-o. [DOI] [PubMed] [Google Scholar]
- 66.Nguyen TT, Tseng YT, McGonnigal B, Stabila JP, Worrell LA, Saha S, Padbury JF. Placental biogenic amine transporters: in vivo function, regulation and pathobiological significance. Placenta. 1999;20:3–11. doi: 10.1053/plac.1998.0348. [DOI] [PubMed] [Google Scholar]
- 67.O’Connor T, O’Halloran DJ, Shanahan F. The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. Q J Med. 2000;93:323–33. doi: 10.1093/qjmed/93.6.323. [DOI] [PubMed] [Google Scholar]
- 68.O’Hara M, Neunaber DJ, Zekoski EM. Prospective study of postpartum depression: prevalence, course, and predictive factors. J Abnorm Psychol. 1984;93:158–171. doi: 10.1037//0021-843x.93.2.158. [DOI] [PubMed] [Google Scholar]
- 69.O’Hara MW, Zekoski EM, Philipps LH, Wright EJ. Controlled prospective study of postpartum mood disorders: comparison of childbearing and nonchildbearing women. J Abnorm Psychol. 1990;99:3–15. doi: 10.1037//0021-843x.99.1.3. [DOI] [PubMed] [Google Scholar]
- 70.O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues. Biologic and psychosocial factors. Archives of General Psychiatry. 1991;48:801–6. doi: 10.1001/archpsyc.1991.01810330025004. [DOI] [PubMed] [Google Scholar]
- 71.Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. Journal of Pediatrics. 1987;111:571–8. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- 72.Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. American Journal of Epidemiology. 2002;156:797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- 73.Pajulo M, Savonlahti E, Sourander A, Helenius H, Piha J. Antenatal depression, substance dependency and social support. Journal of Affective Disorders. 2001;65:9–17. doi: 10.1016/s0165-0327(00)00265-2. [DOI] [PubMed] [Google Scholar]
- 74.Penington NJ, Kelly JS, Fox AP. A study of the mechanism of Ca2+ current inhibition produced by serotonin in rat dorsal raphe neurons. Journal of Neuroscience. 1991;11:3594–609. doi: 10.1523/JNEUROSCI.11-11-03594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Regalado MG, Schechtman VL, Del Angel AP, Bean XD. Sleep disorganization in cocaine-exposed neonates. Infant Behavior and Development. 1995;18:319–327. [Google Scholar]
- 76.Richardson G, Day N. Studies of prenatal cocaine exposure: assessing the influence of extraneous variables. Journal of Drug Issues. 1999;29:225–236. [Google Scholar]
- 77.Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychology. 1999;18:333–45. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- 78.Rounsaville BJ, Kosten TR, Weissman MM, Kleber HD. Prognostic significance of psychopathology in treated opiate addicts. A 2.5-year follow-up study. Archives of General Psychiatry. 1986;43:739–45. doi: 10.1001/archpsyc.1986.01800080025004. [DOI] [PubMed] [Google Scholar]
- 79.Sandman CA, Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Belman J, Porto M, Murata Y, Garite TJ, Crinella FM. Psychobiological influences of stress and HPA regulation on the human fetus and infant birth outcomes. Ann N Y Acad Sci. 1994;739:198–210. doi: 10.1111/j.1749-6632.1994.tb19822.x. [DOI] [PubMed] [Google Scholar]
- 80.Sandman CA, Wadhwa PD, Chicz-DeMet A, Dunkel-Schetter C, Porto M. Maternal stress, HPA activity, and fetal/infant outcome. Ann N Y Acad Sci. 1997;814:266–75. doi: 10.1111/j.1749-6632.1997.tb46162.x. [DOI] [PubMed] [Google Scholar]
- 81.Scafidi FA, Field TM, Wheeden A, Schanberg S, Kuhn C, Symanski R, Zimmerman E, Bandstra ES. Cocaine-exposed preterm neonates show behavioral and hormonal differences. Pediatrics. 1996;97:851–5. [PubMed] [Google Scholar]
- 82.Shaffery J, Hoffmann R, Armitage R. The neurobiology of depression: perspectives from animal and human sleep studies. Neuroscientist. 2003;9:82–98. doi: 10.1177/1073858402239594. [DOI] [PubMed] [Google Scholar]
- 83.Shannon C, Schwandt ML, Champoux M, Shoaf SE, Suomi SJ, Linnoila M, Higley JD. Maternal absence and stability of individual differences in CSF 5-HIAA concentrations in rhesus monkey infants. American Journal of Psychiatry. 2005;162:1658–64. doi: 10.1176/appi.ajp.162.9.1658. [DOI] [PubMed] [Google Scholar]
- 84.Singer L, Arendt R, Minnes S. Neurodevelopmental effects of cocaine. Clinics in Perinatology. 1993;20:245–62. [PMC free article] [PubMed] [Google Scholar]
- 85.Singer LT, Salvator A, Arendt R, Minnes S, Farkas K, Kliegman R. Effects of cocaine/polydrug exposure and maternal psychological distress on infant birth outcomes. Neurotoxicology and Teratology. 2002;24:127–35. doi: 10.1016/s0892-0362(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 86.Stratakis CA, Gold PW, Chrousos GP. Neuroendocrinology of stress: implications for growth and development. Hormone Research. 1995;43:162–7. doi: 10.1159/000184269. [DOI] [PubMed] [Google Scholar]
- 87.Wadhwa PD, Porto M, Garite TJ, Chicz-DeMet A, Sandman CA. Maternal corticotropin-releasing hormone levels in the early third trimester predict length of gestation in human pregnancy. Am J Obstet Gynecol. 1998;179:1079–85. doi: 10.1016/s0002-9378(98)70219-4. [DOI] [PubMed] [Google Scholar]
- 88.Weinberg MK, Tronick EZ. Emotional characteristics of infants associated with maternal depression and anxiety. Pediatrics. 1998;102:1298–304. [PubMed] [Google Scholar]
- 89.Westergaard GC, Cleveland A, Trenkle MK, Lussier ID, Higley JD. CSF 5-HIAA concentration as an early screening tool for predicting significant life history outcomes in female specific-pathogen-free (SPF) rhesus macaques (Macaca mulatta) maintained in captive breeding groups. Journal of Medical Primatology. 2003;32:95–104. doi: 10.1034/j.1600-0684.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 90.Whiffen VE, Gotlib IH. Infants of postpartum depressed mothers: temperament and cognitive status. Journal of Abnormal Psychology. 1989;98:274–9. doi: 10.1037//0021-843x.98.3.274. [DOI] [PubMed] [Google Scholar]
- 91.Wisner KL, Peindl KS, Hanusa BH. Psychiatric episodes in women with young children. Journal of Affective Disorders. 1995;34:1–11. doi: 10.1016/0165-0327(94)00097-s. [DOI] [PubMed] [Google Scholar]
- 92.Woods NS, Eyler FD, Behnke M, Conlon M. Cocaine use during pregnancy: Maternal depressive symptoms and infant neurobehavior over the first month. Infant Behavior and Development. 1993;16:83–98. [Google Scholar]
- 93.Zuckerman B, Frank DA, Hingson R, Amaro H, Levenson SM, Kayne H, Parker S, Vinci R, Aboagye K, Fried LE, et al. Effects of maternal marijuana and cocaine use on fetal growth. New England Journal of Medicine. 1989;320:762–8. doi: 10.1056/NEJM198903233201203. [DOI] [PubMed] [Google Scholar]
- 94.Zuckerman B, Bauchner H, Parker S, Cabral H. Maternal depressive symptoms during pregnancy, and newborn irritability. Journal of Developmental and Behavioral Pediatrics. 1990;11:190–4. [PubMed] [Google Scholar]


