Abstract
Objectives
To evaluate the effectiveness of four alternative interventions (member mailings, advertising campaigns, free generic drug samples to physicians, and physician financial incentives) used by a major health insurer to encourage its members to switch to generic drugs.
Methods
Using claim-level data from Blue Cross Blue Shield of Michigan, we evaluated the success of four interventions implemented during 2000–2003 designed to increase the use of generic drugs among its members. Around 13 million claims involving seven important classes of drugs were used to assess the effectiveness of the interventions. For each intervention a control group was developed that most closely resembled the corresponding intervention group. Logistic regression models with interaction effects between the treatment group (intervention versus control) and the status of the intervention (active versus not active) were used to evaluate if the interventions had an effect on the generic dispensing rate (GDR). Because the mail order pharmacy was considered more aggressive at converting prescriptions to generics, separate generic purchasing models were fitted to retail and mail order claims. In secondary analyses separate models were also fitted to claims involving a new condition and claims refilled for preexisting conditions.
Results
The interventions did not appear to increase the market penetration of generic drugs for either retail or mail order claims, or for claims involving new or preexisting conditions. In addition, we found that the ratio of copayments for brand name to generic drugs had a large positive effect on the GDR.
Conclusions
The interventions did not appear to directly influence the GDR. Financial incentives expressed to consumers through benefit designs have a large influence on their switching to generic drugs and on the less-costly mail-order mode of purchase.
Keywords: Generic drug, brand-name drug, generic dispensing rate, difference-in-difference analysis, logistic regression, multiple interventions
Because a generic drug “is a chemical copy of a brand-name drug” where the “biggest difference between a generic drug and its brand name counterpart is usually the price” (Center for Drug Evaluation and Research 2004), increasing the use of generic drugs is an opportunity to lower health spending with few clinical consequences. Generic penetration rates were consistently reported at between 40 and 44 percent throughout the 1990s for the population at large (Kaiser Family Foundation 2001). More recent data suggest the rate might be closer to 50 percent (Generic Pharmaceutical Association 2005). Recent estimates indicate that adopting managerial best practices related to generic prescribing could reduce prescription drug spending by about 16 percent (Ritter, Thomas, and Wallack 2003). Hence, health plans and pharmacy benefit managers (PBMs) have launched a variety of initiatives aimed at expanding generic drug dispensing during a period of rapidly growing prescription drug spending (Martinez 2005; Terlep and Naamani-Goldman 2005). These initiatives included generic interchange programs, tiered copayment arrangements, pay-for-performance schemes that target physicians and pharmacists, and promotion of generic products to consumers and physicians. While a broad range of strategies has been adopted by health plans and PBMs, little evidence has been assembled regarding the impacts of these various measures.
In this paper we report on the experience of a natural experiment in expanding generic prescribing by a large health plan, Blue Cross Blue Shield of Michigan (BCBSM). BCBSM adopted an array of policies that were intended to increase the dispensing of generic drugs. These policies included direct communication with plan members, statewide advertising in newspapers that promoted use of generic drugs, a pay-for-performance scheme for physicians, and a program that distributed free samples of generic drugs to physicians. To study the impact of these initiatives we analyzed generic dispensing rates (GDRs); (retail and mail order) in the context of a quasi-experimental study design. Control groups were constructed to obtain as close a match as possible to the characteristics of the plan members subject to the interventions. Our results are among the first to offer empirical evidence on the effects of initiatives to expand utilization of generic drugs in private health plans.
BACKGROUND: BCBSM INTERVENTIONS
In 2001, BCBSM began a campaign to address 4 years of declining use of generic drugs among BCBSM members. The plan undertook these activities because of the large potential savings to BCBSM and its members that would result from an increase in the GDR. The new programs augmented existing efforts, such as maximum allowable cost (MAC) pricing (for most BCBSM benefit designs, when a member selects a brand name drug for which a MAC generic is available, the member must pay the difference in price between the MAC generic and brand, plus a copayment), tiered pharmacy benefit structures, and financial incentives for pharmacies to dispense generic drugs. The targets of the interventions varied across time, geographic location, provider, and employer group. The specific components of the interventions are described below.
Written Communication to Plan Members
Articles on generic drugs were published twice annually between October 2001 and October 2003 in the BCBSM Living Healthy magazine. The articles included facts about generic medications and offered consumers information on how to ask their physician about generic drugs. The articles were sent only to Michigan members.
Statewide Advertising
Advertising ran during May and June 2002 in 89 Michigan newspapers and other local Michigan media outlets such as radio stations. The advertising featured comments by pharmacists and a statement from the U.S. Food and Drug Administration supporting the value of generic medications. Other components of the advertising campaign included sports stadium sponsorships and display materials in pharmacies throughout Michigan.
Additional initiatives included a generic drug marketing conference titled “How to Promote Generic Drugs” and the development of a generic drug website (TheBrand 2005). BCBSM also advertised the value of generic medications in seven Michigan business journals and three Michigan consumer magazines. Finally, a competition was held among pharmacies to increase GDRs during the fourth quarter of 2001 with the winners featured in a media campaign.
Physician Incentive (PI)/Detailing
In August of 2000, BCBSM began a PI program intended to reward physicians for reducing pharmacy costs for their patients, one component of which was to increase their prescribing of generic drugs. Six physician groups representing over 1,000 primary care physicians within Michigan were chosen for their strength of internal leadership and cohesiveness as a group practice. As part of the program, four full-time pharmacists are assigned to support the physician groups. Key information shared with physicians included detailed clinical and cost information on generic drugs. Reward payments were made to physician practices every 6 months; the amount of the payment was based on the estimated total ingredient cost saved per utilizing member over the 6-month measurement period (some drugs, such as those recommended for treating selected chronic diseases, were excluded from the calculations so there would be no incentive to underuse these drugs). Typical payments made to practices ranged from $250 to $500 per physician employed for a 6-month period.
Generic Sampling (GS)
GS began in October 2000 and focused on up to 500 physicians per year who had low rates of generic prescribing. This intervention included one-on-one meetings with program pharmacists who provided the physicians detailed clinical and cost information on generic drugs. In addition, free samples of generic medications were given to these physicians. The intervention took place in 12 states across the United States (including Michigan) and focused on four of the largest therapeutic categories as measured by drug spending and generic prescribing opportunity—antihypertensives, antidepressants, gastrointestinal, and nonsteroidal antiinflammatory drugs.
Additional Interventions
In addition to the above four interventions, there were several others including: a financial incentive payment to all pharmacies in Michigan to dispense generic equivalents and the development of benefit designs with lower copayments for generics. Pharmacy payments ranged from $0.50 to $5.00 per generic prescription. Because the pharmacy incentive program was in operation since 1994, it predated our data collection period and thus cannot be assessed. The difference in brand to generic copayment amounts has been shown elsewhere (Motheral and Fairman 2001; Huskamp et al. 2003) to be associated with GDRs and was accounted for in all analyses.
METHODS
Overview
We analyzed the introduction of the interventions as a quasi-experiment, i.e., we constructed a closely matched control group of insured individuals who were not subject to interventions to compare with enrollees who were exposed to the interventions (Cook and Campbell 1979). The outcome of central interest was the GDR among mail order and retail prescriptions for drugs containing molecules (active ingredients) available in either generic or brand name forms. The market share of generic drugs can be increased by either increasing the use of generics among drugs that have generics available or by increasing prescribing of drugs that have generics available as an alternative to drugs not available in generic form. Because it captures cross molecule transitions to and from generics, the GDR was preferred to measures such as the generic substitution rate (GSR) that considers only claims for which both generic and brand name forms are available. Furthermore, the GSR is not particularly relevant to health plans in states such as Michigan with generic substitution laws that drive up GSRs. However, to test the sensitivity of our results we repeated the analysis using GSR in place of GDR and found little difference in the results.
We compared changes in the overall GDR and the rate for specific drug molecules. Because of the longitudinal nature of the study, our estimated impacts represent the difference in the GDR over time for the intervention group relative to that of the control group.
The analysis of the four BCBSM interventions involved three different data sets: (1) to study the written communication to plan members (hereafter called mailings) and advertising interventions we compared the claims of in-state BCBSM members to out-of-state members from two national and two regional employer groups (only Michigan members were eligible to receive the mailings or to be exposed to the advertising on a regular basis); (2) to study the GS intervention we compared claims of members associated with physicians in the GS program to claims of members associated with other Michigan physicians; (3) to evaluate the PI program we compared claims of members associated with the group of practices that received the incentive to claims of members associated with the group of closest matching Michigan practices that did not receive the incentive. The first dataset includes in-state and out-of-state members, the second only the Michigan members, and the third is a subset of Michigan members. Because the first analysis makes comparisons between states, we were concerned that differences in generic substitution policies between Michigan and other states could confound the analysis. In Michigan, if a prescription is written for a medication that has a generic equivalent alternative (i.e., an A-rated drug), the pharmacist is allowed to dispense the generic version if the physician has not designated “dispense as written” (DAW) on the prescription. Because most states (40 in 2003) and all neighboring states of Michigan (where most control group members likely reside) had policies similar to Michigan's during the study period, we expect varying generic substitution policies to have minimal influence on the results.
Our original sample for these analyses involved the entire population of BCBSM subscribers for the period January 1, 2000–December 31, 2003. Intervention and control groups were restricted to those members whose benefit designs were known at the time of each prescription purchase. These members were employees of two national employer groups and two smaller, regional employer groups.
We studied seven classes of drugs: ACE inhibitors, α-blockers, calcium channel blockers, oral hypoglycemics, proton pump inhibitors, selective norepinephrine reuptake inhibitors, and selective serotonin reuptake inhibitors. These classes of drugs were chosen because they were used to treat prevalent chronic diseases, they involved high spending, and each class had both generic and brand name drugs. These drugs represent approximately 27 percent of BCBSM's total spending on prescription drugs.
Data
Of the original 52,517,782 pharmacy claims in the time period under study, about 25 percent (n = 9,790,064) involved the above seven drug classes and were subsequently used in our analyses. Of these 93 percent were from the two national employer groups and the other 7 percent from the two Michigan-based employer groups. A subset of 1,342,922 claims was associated with a PI intervention or control group practice. Retail claims made up the bulk (88 percent) of all claims in the data.
Study Design
Because we were studying multiple interventions, we used a complicated quasi-experimental design requiring multiple control groups with varying numbers of physicians, patients, and claims (Table 1). For each intervention, we chose the control group that most closely resembled the intervention group. Because all the members residing in Michigan were subject to the mailings and the advertising, we used as a control group non-Michigan residents employed by one of the four employers for which we had benefit design information. Because a subset of Michigan physicians received GSs, we assessed the impact of the GS program using claims associated with these physicians as the intervention group and claims for other Michigan physicians as the control, thereby enabling the effectiveness of these interventions to be evaluated within Michigan (if we had used data external to Michigan we would have had to account for differences between states in regulations governing the resale of generic samples). Because strict criteria had been used by BCBSM to select physician groups for the PI program (practices had to demonstrate a strength of leadership and ability to implement change as BCBSM felt that such attributes were necessary to respond rapidly and aggressively to the intervention), we considered only groups in Michigan with the most closely matching characteristics (geography; group size; percent of employees salaried; group cohesiveness and leadership assessment; urban/rural location; and per-member per-month spending) as controls. To describe location, Michigan was partitioned into Southeastern, Southwestern, and Northern regions such that every physician group resided within only one region. The cohesiveness and leadership rating was based on BCBSM's qualitative assessments of the groups, which were based on feedback from the pharmacists working with each of the groups. We formed a combined scale that ranged from 0 to 7 with 7 indicating the highest degree of cohesiveness and leadership.
Table 1.
Sample Sizes for Intervention and Control Groups in the Analyses Associated with the Four Interventions
| Intervention Group | Control Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention/Analysis | Source | Sample Size | Source | Sample Size | |||||
| Member mailings and advertising* | Members living in Michigan | Physicians | 31,576 | Members living outside of Michigan‡ | Physicians | 106,732 | |||
| Patients | 188,216 | Patients | 194,171 | ||||||
| Claims | 4,637,462 | Claims | 5,152,602 | ||||||
| Generic sampling† | Patients of physicians selected for generic sample program | Physicians | 289§ | Patients of physicians not selected for generic sampling program | 31,287 | ||||
| Patients | 22,163 | Patients | 166,053 | ||||||
| Claims | 422,449 | Claims | 4,215,013 | ||||||
| Physician incentive | Patients of group practices selected for physician incentive program | Physicians | 1,127 | Patients of matched groups not selected for physician incentive program | Physicians | 980 | |||
| Patients | 39,474 | Patients | 31,934 | ||||||
| Claims | 773,414 | Claims | 569,508 | ||||||
The member-to-physician ratio is higher for the intervention group because the density of BCBSM members is higher in Michigan than in any other state
The intervention group counts members and claims of members if they were associated with a physician that participated in the generic sampling intervention at any point during the study even if the member was mostly seen by nonparticipating physicians (thus the ratios of members and claims to physicians is higher than for the control group)
These are almost all employees of nationally based employers
Physicians were counted in the intervention group for the generic sampling intervention if they received generic samples in any year
Dependent Variables
The main measure of generic dispensing was the overall GDR, stratified by the mode of purchase (retail and mail-order). We felt that stratified models were warranted because the mechanisms driving consumer behavior might differ across the modes of purchase. We also analyzed the GDR separately for claims associated with a new condition (initial fills) and preexisting conditions (refills). We analyzed the data at the claim level within and across all classes of drugs.
Independent Variables
The key independent variables were the interactions of the study group (intervention versus control) and the variables representing the status of the interventions at a given point in time. The mailings were considered active for 60 days after the date of the mailing while the active period for the advertising campaign was just the 35-day duration of the campaign. Because we were able to identify only the year in which physicians received generic samples or were active in the PI program, claims were counted as active with respect to one of these interventions if they were associated with a physician participating in the program in that calendar year.
Several variables were used to measure the financial incentive for consumers to purchase generic drugs. In analyses of the GDR we used a “copayment ratio” (brand copayment divided by generic copayment). The ratio of copayments was used as a measure of the financial incentive for members to use generics because benefit designs often used common multiples when setting compayment levels for generic and brand name drugs. We also examined differences (brand–generic) in copayments but found these were not as predictive. For a given claim, the copayment ratio was determined by imputing the price a member would have paid for the corresponding (brand or generic) drug that they did not purchase from the average copayment paid that month by BCBSM members for whom the benefit design was known. For this calculation all copayments are measured per pill dispensed. To account for skewness in the copayments, the logarithm of the copayment ratio was used in all models.
To account for the possibility that providers or patients might become more comfortable with and thus more likely to use a generic drug the longer it has been available, we controlled for the time the corresponding generic drug had been available. If the corresponding generic was not yet available we indicated this using a dummy variable. Time was represented in each model by its natural logarithm. We also controlled for the number of generic drugs available in the same class of drugs as the existence of a large number of tried and proven generics might encourage adoption.
At the claim level we accounted for the nature of the prescription (initial fill or refill), the drug class, and the date of purchase (days since January 1, 2000). A claim was classified as an initial fill for a condition if the member did not purchase any drugs with the same molecule for the past 6 months, indicating that a new set of decisions were made by the physician, member, and pharmacy. The following member characteristics were controlled for: male, age, employer, and salary type (hourly versus salaried). The pharmacy control variable was the previous quarter's reward payment from BCBSM. In the PI analysis we guarded against any imbalances because of imperfect matching by controlling for the characteristics of the physicians' practice used to match the intervention and control groups.
Statistical Analyses
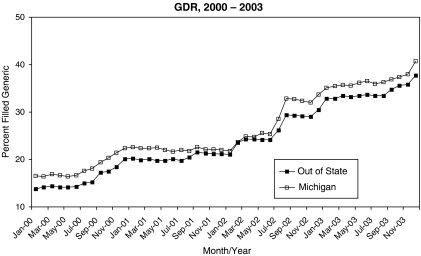
The difference-in-difference (DID) paradigm was the basis of our statistical analyses. The traditional DID approach (Meyer 1995) was used for the mail and advertising campaigns while slight modifications were needed for analyses of the GS and PI initiatives. The modifications accounted for multiple interventions occurring at the same time and physicians being exposed to the GS and PI programs at different times. Following the DID paradigm, we used the longitudinal nature of the study to compare expected outcomes between periods of time when the intervention was active to those when it was not active relative to the corresponding comparison in the control group. The key term in a DID analysis is the parameter estimate for the interaction between the intervention group and the presence or absence of the intervention at a given time. We evaluated the validity of the DID approach by examining trends in the GDR for the mailing and advertising groups. Trends for the intervention and control groups were essentially parallel over the preintervention period, thus indicating the appropriateness of use of the DID approach (Figure 1).
Figure 1.
Plot of the Generic Dispensing Rate for Claims Associated with Blue Cross Blue Shield of Michigan Members That Live in Michigan and Those That Reside Outside Michigan from January 2000 to December 2003. The First 6 Months of This Period Represented the Baseline Period.
For the main analysis of each intervention we fitted two logistic regression models to estimate the effects of the interventions (the DID interaction effect) and other control variables on the probability that a claim: (1) was filled as generic among retail prescriptions (the retail GDR); and (2) was filled as generic among mail order prescriptions (the mail order GDR). In each analysis all identifiable interventions were controlled for (e.g., in the mailing and advertising analysis we control for both the PI and GS programs). However, to minimize the chance that model misspecification will bias the results, we evaluate the effects of the PI and GS interventions using just their matched intervention and control groups. In additional analyses we fitted separate models to claims for initial fills and refills for preexisting conditions and allowed for the interventions to have a persistent effect beyond the period of active intervention (we found no evidence to support the hypothesis that the interventions had a positive effect beyond the period of active intervention). We also tested if the length of time exposed to the PI or GS interventions mattered but found that it did not.
We accounted for the occurrence of multiple claims associated with the same member, physician, and pharmacy by using a mixed effects regression model (estimated using the Glimmix macro in SAS) to ensure that the standard errors reflected the clustered nature of the sample (Bertrand, Duflo, and Mullainathan 2004).
For easy interpretation the results are presented in terms of the percentage point changes in the GDR attributed to each intervention for different base rates of generic prescribing. These were derived by predicting the GDR at the active level of the intervention or at a higher value of the copayment ratio than the baseline level (see formula and explanation in the Table 3 caption). For example, in the logistic regression model of GDR for retail claims, the log of the copayment ratio has a coefficient of 0.553. Therefore, if the copayment ratio doubles (i.e., the copayment of a brand name drug doubles relative to that for a generic drug) implying a δ of log(2) and the baseline GDR is 0.3 the GDR increases to 0.39 (a 9 percentage-point increase), whereas if the baseline GDR is 0.45 the GDR increases to 0.55 (a 10 percentage-point increase). We used base GDR rates of 30 and 45 percent because they were close to the upper and lower bounds of the GDRs for the intervention and control groups over the last 6 months of 2003. Although the average copayment ratio over our sample was only 1.43, ratios of 2 or greater were commonplace. For example, the percentage of times the copayment ratios exceeded 2 across all applicable retail claims for Lisinopril and Amlodipine Besylate, the two most common molecules in the sample, were 17.1 and 11.8 percent, respectively. The corresponding values for mail order claims were 6.2 and 5.8 percent, respectively.
Table 3.
Estimated Changes in the GDR (Expressed in Percentage Points) as a Function of the Interventions and Copayments for Brand Name Drugs at Two Baseline GDR Rates (30 and 45 percent)
| PptChange at Baseline GDR of | |||||
|---|---|---|---|---|---|
| Mode of Purchase | Intervention | Regression Coefficient | 30% | 45% | p-Value |
| Retail | Mailing | −0.248 | −4.94 | −6.03 | < .0001 |
| Advertising | −0.006 | −0.13 | −0.15 | .875 | |
| Generic sampling | −0.001 | −0.02 | −0.02 | .951 | |
| Physician incentive | −0.016 | −0.33 | −0.40 | .616 | |
| Doubling copayments for brand name drugs | 0.553 | 8.60 | 9.55 | <.0001 | |
| Mail order | Mailing | −0.079 | −1.63 | −1.95 | .232 |
| Advertising | −0.130 | −2.65 | −3.19 | .297 | |
| Generic sampling | −0.083 | −1.71 | −2.04 | .301 | |
| Physician incentive | −0.100 | −2.06 | −2.46 | .406 | |
| Doubling copayments for brand name drugs | 0.19 | 2.84 | 3.27 | .668 | |
Each percentage-point change (PptChange) in the above table was derived using the formula 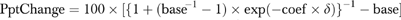 where base is the baseline GDR, coefficient is the estimated regression coefficient of the intervention, δ is the change in the value of the intervention variable (δ=1 for the interventions; δ=log(2) for doubling the copayment ratio), and exp(.) denotes the exponential function. Note that this formula is just a mechanism for translating the parameter estimate to the probability scale; it does not compute the interaction or marginal effect that has been defined as in Ai and Norton (2003) but rather estimates the change in the probability that would have occurred had there not been an intervention.
where base is the baseline GDR, coefficient is the estimated regression coefficient of the intervention, δ is the change in the value of the intervention variable (δ=1 for the interventions; δ=log(2) for doubling the copayment ratio), and exp(.) denotes the exponential function. Note that this formula is just a mechanism for translating the parameter estimate to the probability scale; it does not compute the interaction or marginal effect that has been defined as in Ai and Norton (2003) but rather estimates the change in the probability that would have occurred had there not been an intervention.
GDR, generic dispensing rate
RESULTS
Description of Intervention and Control Groups (Table 2)
Table 2.
Characteristics of Claims Associated with the Intervention and Control Group in Each Analysis
| Model | ||||||
|---|---|---|---|---|---|---|
| Mailings and Advertising | Generic Sampling | Physician Incentive | ||||
| Variable | Intervention | Control | Intervention | Control | Intervention | Control |
| Dependent variables at baseline* | ||||||
| GDR (%) | 16.5 | 14.1 | 15.7 | 16.6 | 17.3 | 17.2 |
| Mail order GDR (%) | 20.7 | 19.2 | 19.0 | 21.0 | 20.3 | 21.4 |
| Retail GDR (%) | 16.2 | 13.5 | 15.5 | 16.3 | 17.1 | 16.8 |
| Claim characteristics† | ||||||
| ACE inhibitor (%) | 20.3 | 18.3 | 20.8 | 20.2 | 20.8 | 20.3 |
| α blocker (%) | 3.9 | 4.1 | 4.1 | 3.9 | 3.8 | 4.3 |
| Calcium channel blocker (%) | 20.5 | 18.9 | 20.4 | 20.5 | 18.9 | 18.4 |
| Oral hypoglycemic (%) | 20.5 | 19.6 | 23.3 | 20.2 | 22.8 | 23.3 |
| Proton pump inhibitor (%) | 18.1 | 19.7 | 18.1 | 18.1 | 18.3 | 17.5 |
| SNRI (%) | 5.0 | 6.1 | 3.3 | 5.1 | 3.8 | 4.4 |
| SSRI (%) | 11.7 | 13.4 | 10.0 | 11.9 | 11.6 | 11.8 |
| Copayment ratio‡ | 1.45 ± 1.06 | 1.39 ± 1.12 | 1.46 ± 1.09 | 1.45 ± 1.06 | 1.71 ± 1.21 | 1.65 ± 1.09 |
| Initial fill (%) | 16.5 | 15.5 | 12.6 | 16.9 | 14.0 | 14.8 |
| Spending per claim ($) | 64.9 ± 60.6 | 74.4 ± 70.6 | 65.8 ± 61.2 | 64.8 ± 60.5 | 61.9 ± 57.9 | 63.5 ± 60.0 |
| Member characteristics | ||||||
| Male (%) | 47.1 | 48.4 | 46.8 | 47.1 | 44.8 | 45.2 |
| Age (years) | 66.2 ± 13.9 | 65.5 ± 13.2 | 67.1 ± 13.1 | 66.1 ± 13.9 | 67.4 ± 12.8 | 67.2 ± 12.6 |
| Hourly employee (%) | 70.3 | 83.8 | 72.0 | 70.1 | 47.6 | 43.7 |
| National employer I (%) | 51.6 | 57.5 | 59.1 | 50.8 | 47.3 | 28.3 |
| National employer II (%) | 34.0 | 42.3 | 31.3 | 34.3 | 12.1 | 23.9 |
| Regional employer I (%) | 13.6 | 0.2 | 8.9 | 14.1 | 38.5 | 44.9 |
| Regional employer II (%) | 0.8 | – | 0.7 | 0.8 | 2.1 | 2.9 |
| Physician practice characteristics | ||||||
| Geographic area A (%) | – | – | – | – | 78.3 | 70.3 |
| Geographic area B (%) | – | – | – | – | 4.9 | 6.1 |
| Geographic area C (%) | – | – | – | – | 16.8 | 23.6 |
| Number of physicians | – | – | – | – | 252.7 ± 74.8 | 181.9 ± 27.6 |
| Employees' salaried (%) | – | – | – | – | 85.7 | 22.4 |
| Cohesiveness/leadership rating | – | – | – | – | 3.8 ± 1.2 | 3.8 ± 2.1 |
| Urban/rural location (%) | – | – | – | – | 81.7 | 100 |
| Per-member per-month spending ($) | – | – | – | – | 72.1 ± 26.4 | 46.6 ± 19.3 |
| Pharmacy characteristics | ||||||
| Pharmacy incentive payment per script ($) | 0.15 ± 0.20 | 0.11 ± 0.36 | 0.13 ± 0.15 | 0.16 ± 0.20 | 0.19 ± 0.23 | 0.18 ± 0.23 |
The baseline period is defined as the first 6 months of 2000, predating most of the interventions
The date of the claim was included in the model for the mailings and the advertising campaigns to improve the precision of the analysis, whereas in the models for the generic sampling and physician incentive programs it had to be included to ensure the validity of the inferences
The mean ± standard deviation is presented for the copayment ratio and other continuous predictors
GDR, generic dispensing rate; SNRI, selective norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor
Because of the large number of claims almost all differences between the intervention and control groups were statistically significant. However, in most cases these differences were small and would not be considered meaningful from a business perspective.
In terms of differences between the intervention and control groups for the mailing and advertising campaigns, the most notable differences were in the baseline GDR (16.2 percent versus 13.5 percent, respectively), and in the copayment ratios (1.45 and 1.39, respectively). The percent of claims for hourly employees was slightly higher in the intervention group than in the control group. The spending per claim was substantially higher in the control group ($74.40) than the intervention group ($64.90) suggesting that more expensive medications tended to be used outside of Michigan (this result is consistent with the lower baseline GDR outside of Michigan). Finally, because the control group for this analysis contained only out-of-state residents, it was not surprising that relatively few (around 10,300) control group claims were associated with members that worked for one of the regional (within Michigan) employer groups.
For the GS initiative, the intervention and control groups differed in terms of the proportion of claims that were initial fills (over four percentage points higher in the control group), the pharmacy incentive payment ($0.03 higher per prescription filled in the control group), and the proportion of claims associated with two of the employers (there was an eight percentage point difference for national employer I, and a five percentage point difference for regional employer I). For the PI initiative matching ensured the groups were balanced in terms of geographic region and the cohesiveness/leadership rating. However, despite selecting the closest matching physician groups available, substantial differences existed in the percentage of employees paid via salary at the associated physician group (86 percent for the intervention group, 22 percent for the control group), and the per-member per-month spending of the associated physician group (approximately $26 higher in the intervention group). These groups also differed in terms of the copayment ratio (0.06 higher in the intervention group) and the proportion of claims associated with each employer group (e.g., there was a 19 percentage point difference in the proportion of claims associated with national employer I).
Impact of BCBSM Interventions on GDR (Tables 3 and 4)
The four interventions had no positive effects on the GDR for retail purchases (Table 3). For example, when we assumed all claims had a base GDR of 30 percent the mail campaign was associated with a 4.9 percentage point decrease in the GDR for retail purchases. In contrast, a doubling of the ratio of copayments for brand to generic drugs results in an increase of 8.6 percentage points in the GDR. If a base GDR of 45 percent was chosen (about the base rate for the PI groups), then doubling the copayment ratio led to an increase of 9.6 percentage points in the GDR. With the exception of GS that had a negative impact, there was little effect of the interventions or copayments on the GDR for mail order claims. In results not presented we found that the longer a molecule had been available in generic form, and the greater the number of generics available in a class, the greater the likelihood that a generic is dispensed.
When we stratified the analyses by claims associated with initial fills and refills (Table 4), the interventions again had no positive effects on the GDR. The copayment ratio had a significant positive effect for both initial and refilled prescriptions (doubling the ratio from a baseline GDR of 30 percent resulted in 14.3 and 8.3 percentage point increases, respectively).
Table 4.
Estimated Changes in the GDR (Expressed in Percentage Points) for Initial Fills and Refills as a Function of the Interventions and Copayments for Brand Name Drugs at Two Baseline GDR Rates (30 and 45 percent)
| Regression Coefficient | |||||
|---|---|---|---|---|---|
| Type of Fill | Intervention | Regression Coefficient | 30% | 45% | p-Value |
| Initial | Mailing | −0.167 | −3.39 | −4.09 | .008 |
| Advertising | −0.053 | −1.10 | −1.31 | .691 | |
| Generic sampling | 0.005 | 0.11 | 0.12 | .964 | |
| Physician incentive | 0.038 | 0.80 | 0.94 | .756 | |
| Doubling copayments for brand name drugs | 0.892 | 14.30 | 15.29 | <.0001 | |
| Refill | Mailing | −0.235 | −4.69 | −5.72 | <.0001 |
| Advertising | 0.001 | 0.02 | 0.02 | .973 | |
| Generic sampling | 0.009 | 0.19 | 0.22 | .728 | |
| Physician incentive | −0.077 | −1.59 | −1.90 | .034 | |
| Doubling copayments for brand name drugs | 0.537 | 8.34 | 9.28 | <.0001 | |
See the caption for Table 3 for a formula for computing the percentage point change (PptChange) of the interventions
GDR, generic dispensing rate
Finally, when the analyses were stratified by drug class (not presented), we found a smattering of small positive effects. However, because these were neither concentrated in a specific drug class nor for a particular intervention (e.g., the GS initiative was the only intervention to have a positive impact for calcium channel blockers but had negative effects for the other drug classes), we doubt that these are anything more than statistical artifacts.
DISCUSSION
The economic gains from increasing the use of generic drugs have been well documented (Congressional Budget Office 2001; Martinez 2005; Terlep and Naamani-Goldman 2005). As a result, insurers and PBMs have been experimenting with a range of strategies aimed at expanding generic dispensing. The methods utilized include promotion of generic products to consumers and physicians, the use of performance-based payments for pharmacists and physicians, and differential pricing to consumers. In the cost conscious environment of the years 2000–2003, only the consumer pricing strategies had a consistent significant positive effect on GDRs.
Two other important facts emerged from our study. First, we found that the copayment ratios did not have any direct impact on the mail order GDR. This likely reflects the powerful influence of administrative actions, e.g., because mail order pharmacies dispense brand name drugs only if the prescription comes with a “DAW” order this leaves little additional contribution for consumer demand response. (Retail pharmacists also can do this, but are much less likely to do so.) Second, the copayment ratio had more impact on the GDR for initial fills compared with refills. This is intuitively reasonable; members filling a prescription for the first time are not attached to a given therapy and so it is understandable that they would more readily accept a generic medication than a member that has already been treated with a branded medication for some time.
Despite the overall strong results for the effect of changes in the copayment structure, continued increasing of copayments for brand name drugs is not a good strategy. Other data have shown, for example, that implementing such a policy might result in some members opting not to seek treatment when a generic alternative is unavailable, lowering the standard of their health care (Huskamp et al. 2003).
It is striking that the physician pay for performance approach had so little impact on the GDR. This in part may be due to the intervention strategy pursued. The PI program was implemented to target physicians in the best-managed practices, and these tended to have high GDRs even before the intervention. While taking such an approach increases the likelihood that practices will have an internal structure suited to implementing such a program, it also rewards an already high performing physician group. Payments to the PI intervention groups were disproportionately based on past performance, rather than improved performance (a very costly performance improvement strategy). Hence, there was little room for improvement among this group of physicians relative to other physicians despite the fact that we did our best to select a set of matched controls. We suspect that the results for this intervention may have been more positive if lower performing physician groups had been targeted. Indeed, the GS program targeted low performing physicians and had more encouraging results (inconclusive rather than negative).
The negative results associated with the mailings and advertising interventions suggest that in the presence of financial incentives from benefit designs, state laws governing generic substitution, and efforts to increase use of mail order pharmacies, additional efforts appear to yield little behavior change. It should be noted that the advertising campaign focused entirely on print media. It is unknown whether television advertising would produce different results although the cost of such an initiative would clearly be much higher than existing efforts. We also note that this analysis was also susceptible to differences between states in their generic substitution policies over time. However, most states had the same generic substitution policy and MAC program as Michigan, and the states where differences were observed were not the most likely places for control group members to reside. Therefore, we think it unlikely that our results would have been affected.
Although we had access to claims from all BCBSM members, we had benefit information from only four employers. However, although our results may not be representative of the population of BCBSM members, it is important to realize that these employer groups comprise a large proportion of BCBSM's total business (almost 25 percent) and a diverse range of BCBSM's benefit structures. Because doctor participation in the PI and GS programs is known only by year, our results (especially the investigation of an exposure time–response relationship) may not be as precise as they would be if exact participation periods were known. However, the consistency across interventions and analyses of the null or negative effects of the BCBSM interventions on the GDR makes it unlikely we would have found positive results even with more precise data. Another limitation is that we focused on a narrow range of conversions by only looking at selected drug classes. However, because these were associated with the most common chronic conditions we think it unlikely that the results would be very different even if all drug classes had been considered.
Multiple efforts to increase the utilization of generic prescriptions within four employers in Michigan between 2000 and 2003 had modest effects. However, a major switch to generic prescriptions occurred from the introduction of lower relative copayments for generic drugs.
Acknowledgments
We thank members of the Blue Cross Blue Shield of Michigan plan for their role in developing this project, for access to data, and for reviews of the manuscript. Special thanks are extended to Marc Ciriello and Jeffrey Souza at the Department of Health Care Policy at Harvard Medical School for their help in managing the project and programming respectively. This work was supported, in part, by the Blue Cross Blue Shield Association, an association of independent Blue Cross and Blue Shield Plans.
REFERENCES
- Ai C, Norton E C. Interaction Terms in Logit and Probit Models. Economics Letters. 2003;80:123–29. [Google Scholar]
- Bertrand M, Duflo E, Mullainathan S. How Much Should We Trust Difference-in-Difference Estimates? Quarterly Journal of Economics. 2004;119:249–75. [Google Scholar]
- Center for Drug Evaluation and Research. Report to the Nation 2003. Rockville, MD: FDA; 2004. [Google Scholar]
- Congressional Budget Office. How Increased Competition from Generic Drugs Has Affected Prices and Returns in the Pharmaceutical Industry. Washington, DC: CBO; 2001. [Google Scholar]
- Cook T D, Campbell D T. Quasi-Experimentation: Design and Analyses for Field Settings. Chicago: Rand McNally College Publishing; 1979. [Google Scholar]
- Generic Pharmaceutical Association. [February 25, 2005];2005 Available at http://www.gphaonline.org/aboutgenerics/factsab.
- Huskamp H A, Deverka P, Epstein A M, Epstein R, McGuigan K, Frank R G. The Effect of Incentive-Based Formularies on Prescription Drug Utilization and Spending. New England Journal of Medicine. 2003;349(23):2224–32. doi: 10.1056/NEJMsa030954. [DOI] [PubMed] [Google Scholar]
- Kaiser Family Foundation. Prescription Drug Trends: A Chartbook Update. Menlo Park, CA: Kaiser Family Foundation; 2001. [Google Scholar]
- Martinez B. Wall Street Journal. New York: 2005. Drug Copays Hit $100. [Google Scholar]
- Meyer B. Natural and Quasi-Natural Experiments in Economics. Journal of Business and Economic Statistics. 1995;XII:151–62. [Google Scholar]
- Motheral B, Fairman K A. Effect of a Three-Tier Prescription Copay on Pharmaceutical and Other Medical Utilization. Medical Care. 2001;39:1293–304. doi: 10.1097/00005650-200112000-00005. [DOI] [PubMed] [Google Scholar]
- Ritter G, Thomas C, Wallack S S. Schneider Institute for Health Policy, Brandeis University; 2003. Greater Use of Generics: A Prescription for Drug Cost Savings. [Google Scholar]
- Terlep S, Naamani-Goldman D. Detroit News. Detroit: 2005. Insurers Demand Generics. [Google Scholar]
- TheBrand. [January 1, 2005];2005 Available at http://www.theunadvertisedbrand.com.