Abstract
Objective
To compare the estimated effects of dialysis center profit status on patient survival using alternative estimation strategies with retrospective data.
Data Sources/Study Setting
Patient and provider-level retrospective data from the United States Renal Data System (USRDS), 1996–1999.
Study Design
Observational risk adjustment and instrumental variable methods.
Data Collection/Extraction Methods
Study collected measures from various USRDS files describing clinical characteristics, survival, and the profit status of the initial dialysis center for incident end-stage renal disease (ESRD) patients aged 67+. USRDS facility files were used to assess dialysis center profit status and measure patient distances to dialysis centers.
Principal Findings
Found survival effect related to profit status in the range of previous research using risk-adjusting covariates similar to those used in previous models. Adding further risk-adjusting covariates halved this effect. The relative proximity of for-profit and nonprofit dialysis centers to the patient residence was the strongest determinant of the profit status of the patient's initial dialysis center. The effect of profit status on survival was eliminated using the two-stage least squares variant of instrumental variable estimation with the relative proximity of for-profit and nonprofit dialysis centers to the patient's residence as the instrument.
Conclusions
Using only the variation in initial dialysis center profit status that was related to the relative proximity of for-profit and nonprofit dialysis centers to the patient, we found no relationship between dialysis center profit status and patient survival. These results are in contrast to results obtained using risk-adjustment methods with a limited set of risk-adjusting covariates.
Keywords: Dialysis, instrumental variables, survival, profit status, risk adjustment
The percentage of for-profit dialysis centers increased from approximately 60 percent in 1991 to nearly 80 percent in 2001 (U.S. Renal Data System 2004). The effect of dialysis center “profit” status on provision of care and outcomes for patients with end-stage renal disease (ESRD) has been the topic of several research efforts. It has been reported that for-profit dialysis centers use fewer resources than their nonprofit counterparts (Held 1990, 1991; de Lissovoy et al. 1994; Farley 1996; Hirth et al. 2000; Ozgen and Ozcan 2002) and it has been suggested that patients treated at for-profit dialysis centers are at greater risk as a result (Levinsky 1999; Devereaux et al. 2002; Meyer and Kassirer 2002). Several researchers estimated the difference in survival rates between for-profit and nonprofit dialysis centers (McClellan et al. 1998; Garg et al. 1999; Irvin 2000; Port et al. 2000). Because of the inability to randomize patients between dialysis centers, these researchers used the profit status of the dialysis centers treating ESRD patients found in observational databases as the basis of their comparisons. A meta-analysis by Devereaux et al. 2002 of several of these studies showed a greater 1-year mortality risk for ESRD patients on hemodialysis treated at private for-profit centers relative to private nonprofit centers. The authors attribute this increased risk to the lower resource levels used in for-profit dialysis centers and surmised that 2,500 dialysis deaths could be avoided each year in the United States if only private nonprofit centers provided dialysis services.
It is well known that exploiting “treatment” variation from observational databases for outcome estimation is fraught with inferential difficulties (Byar 1991; Jollis et al. 1993; Doll 1994; Hornberger and Wrone 1997). Inappropriate inferences will occur if patients are selected for treatment in a manner associated with expected outcomes—treatment selection bias, or treatment is simply correlated with unmeasured variables that also affect outcome—confounding bias. If we define the profit status of the dialysis center used by a patient as the “treatment,” treatment selection bias occurs if ESRD patients with less severe conditions are systematically directed to either for-profit or nonprofit centers. For example, if less severe patients are directed toward for-profit centers the estimated survival effect from using a for-profit center for dialysis relative to a nonprofit centers will have a positive bias relative to its true effect reflecting the higher initial health status of patients using for-profit centers. Confounding (or omitted variable) bias occurs if profit status is simply correlated to unmeasured variables that also affect survival. For example, the “ecological” characteristics of populations, such as intrapersonal factors, interpersonal processes and primary groups, institutional factors, community factors, and public policy (McLeroy et al. 1988), may vary across geographic areas with the predomination of either for-profit or nonprofit dialysis centers. For example, Subramanian et al. (2001) demonstrated significant variation in health status across states after controlling for differences in individual characteristics.
Previous studies assessing the effect of dialysis profit status on patient outcomes dealt with selection and confounding problems by specifying a set of “risk” adjusters or measured confounders in outcome equations to control for patient differences directly. The measured confounders used varied with the information available in the study. Using data collected from two prospective studies of ESRD patients performed by the United States Renal Data System (USRDS) in the early 1990s, Garg et al. (1999) specified patient age at ESRD onset, race, gender, education status, employment status, whether the patient was a nursing home resident, census region of residence, residence in a census-defined metropolitan area, primary cause of ESRD, and 14 comorbid conditions. Irvin (2000) used the universe of Medicare patients in the USRDS database in 1996 that were treated at either freestanding for-profit or nonprofit dialysis centers and specified patient age, race, gender, primary cause of ESRD, region of the country (south, far west, east, other), the presence of certificate of need legislation in a state and the zip code-level variables per capita income, years schooling, and urban percentage. Port et al. (2000) used USRDS data from 1995 and 1996 and controlled for age, race, gender, primary cause of ESRD, geographic region, and the presence of 15 comorbid conditions. Pushkal and Powe (2001) argued that the risk adjusters specified by these authors were sufficient to eliminate selection and confounding problems. However, the inferences drawn from these results remain conditional on the validity of this argument.
In this study, we focused on patients with at least 2 years of Medicare coverage before ESRD incidence (aged 67 years and older at incidence) and collected data from the USRDS on survival, clinical characteristics at dialysis initiation, residence zip code, and Medicare claims. We also obtained the universe of dialysis centers in each year from USRDS facility files that include center profit status and zip code. These data enabled us to use additional strategies to avoid selection and confounding problems. First, we measured an expanded set of potential confounders (previous patient health care utilization, distance from the patient to the nearest dialysis center, and state-level indicators of patient residence) unavailable in previous studies. In addition, we used an instrumental variable (IV) estimation approach used earlier by McClellan et al. (1994) in their estimation of the effects of invasive surgery on the survival of patients with acute myocardial infarction. For each patient in their study, McClellan et al. (1994) measured differential distance as the distance from the patient's residence at diagnosis to the nearest provider of a given classification (e.g., a high treatment provider) minus the distance from the patient's residence at diagnosis to the nearest provider not in that classification (e.g., a low treatment provider). For differential distance to be useful in IV estimation it must be related to treatment choice and not be related to patient outcomes directly or to unmeasured confounding variables. In this study, we calculated differential distance for each patient as the distance to the nearest for-profit center minus the distance to the nearest nonprofit center and then estimated the effect of the profit status of the patient's initial dialysis center on patient survival using both regression methods that risk-adjust for measured confounders and IV estimation and contrasted the findings in terms of the assumptions underlying each approach.
METHODS
Data
Most of the data for this study came from four databases maintained by the USRDS in concert with the Centers for Medicare and Medicaid Services (CMS). The CMS Medical Evidence Form Database contains information from the form (Form 2728) that must be filled out for each new ESRD patient to qualify for Medicare benefits. The database contains laboratory values, comorbid conditions at dialysis initiation, date of birth, gender, race, and residence zip code at ESRD diagnosis for each new ESRD patient. The USRDS Modality Sequence File contains information on the initial patient treatment modality and survival. The USRDS 67+ Medicare Inpatient Claims file contains claims for all patients in the USRDS database that were 67 years and older at dialysis initiation including claims for the 2 years prior to each patient's dialysis initiation. The USRDS Facility Database contains the universe of dialysis centers by year and includes their profit status and zip code. To estimate distances from patients to centers we obtained zip code centroid longitudes and latitudes from the ZIPList5 Geocode file from CD Light, LLC. In addition to USRDS data, we used zip code level socioeconomic data from the Bureau of Census's 1990 Census's of Population and Housing Zip Code Summary File.
Sample
For the years 1996–1999, the USRDS databases contained 104,158 incident dialysis patients that were 67–100 years old at initiation of dialysis, had an initial hemodialysis treatment modality, were treated at a nongovernment dialysis center, and had complete and consistent clinical and demographic information in the USRDS databases for all study variables. From this group, 2,489 patients had zip codes that could not be linked to both the 1990 Census zip code file and the ZIPList5 Geocode file. This left 101,669 incident dialysis patients for analysis. A higher percentage of the excluded patients were male, less than 74, white, and had more comorbid conditions, but survival rates were not significantly different between groups.
Measures
One-year survival was used as our dependent variable to be consistent with Devereaux et al. (2002). The USRDS Modality Sequence File provided at least 1 year of survival follow-up after dialysis initiation for each patient in the sample. An indicator variable was set equal to 1 if the patient survived to 1 year after dialysis initiation, 0 otherwise. Independent variables consistent with the Devereaux et al. meta analysis were specified including patient age (1 if age greater than 75, 0 otherwise), gender (1 if male, 0 female), 17 comorbid condition indicator variables (1 if relevant condition is checked on a patient's CMS Medical Evidence Form, 0 otherwise), and eight race indicator variables (1 if patient's race on the CMS Medical Evidence Form matches the definition of the variable, 0 otherwise). Additional risk-adjusting variables were specified. Forty-nine state residence indicator variables were created using data from the CMS Medical Evidence Form (1 if patient resided in respective state at dialysis initiation, 0 otherwise). The USRDS 67+ Medicare inpatient claims files were used to calculate the number of inpatient admissions and the number of inpatient days for each patient in the year before dialysis. The median number of hospital admissions for our sample in the previous year was 1 and the 75th percentile was 2. Thus, we created two indicator variables for inpatient admissions in the year before dialysis initiation (1–2 and 3+ admissions), with patients with 0 admissions as the comparison group. The median number of inpatient days in the previous year for our sample was two and the 75th percentile was 12. We created two indicator variables for inpatient days in the year before dialysis initiation (2–12 and 13+ days) with patients with 1 or fewer inpatient days as the comparison group. We obtained zip code-level measures of the percentage population below the poverty level, per capita income, and the percentage rural population from the 1990 Census of Population and Housing Zip Code Summary File for the residence zip code of each patient in our sample at dialysis initiation. We specified poverty and per capita income variables for each patient using indicator variables based on the respective quartiles of these variables. For the population percentage below poverty, three indicator variables were created (5.8–11.6, 11.6–19.6, 19.6+ percent). Patients living in zip codes with poverty percentages below 5.8 percent were the comparison group. Three indicator variables were created for the per capita income in each patient zip code ($9,322–$11,252, $11,252–$14,250, $14,250+). Patients living in zip codes with per capita income below 9,322 were the comparison group. Because of the large percentage of zip codes that were either fully rural or fully urban we created three indicator variables to represent rural population percentage (>0–50, >50–<100, 100). Patients living in fully urban zip codes (rural percentage=0) were the comparison group.
We used patient residence zip codes at dialysis initiation and dialysis center zip codes and profit status to calculate the distance from each patient to the nearest dialysis center, the distance from each patient to the nearest for-profit center, and the distance from each patient to the nearest nonprofit center. Distance was measured using the straight-line distance from the centroid of the patient's zip code to the centroid of the zip code of dialysis center using the longitudes and latitudes of the zip code centroids. We calculated a measure of general dialysis access as the distance from the patient to the nearest dialysis center regardless of profit status and created four indicator variables based on the quintiles of this measure (>0–1.53, 1.53–3.48, 3.48–8.38, 8.38+ miles). Patients with dialysis centers located in their residence zip code (0 miles) were the comparison group. We then calculated differential distance for each patient as the distance to the nearest for-profit center from the patient's residence minus the distance to the nearest nonprofit center from the patient's residence. In our empirical specification, we created a series of indicator variables that grouped patients by their estimated differential distance.
Analytical Approach
We used the same nonparametric two-stage least squares (2SLS) variant of IV estimation that has been used in previous IV research in health care (McClellan et al. 1994; McClellan and Newhouse 1997; Brooks et al. 2000, 2003; Beck et al. 2003) and in questions of labor supply (Angrist and Evans 1998; Angrist 2001). Despite the specification of a binary dependent variable (1=survive 1 year, 0=otherwise) nonparametric 2SLS yields consistent estimates regardless of the underlying error distributions, whereas alternative two-stage estimators that rely on distributional assumptions (e.g., bivariate probit) are inconsistent if the assumptions are incorrect (Angrist 2001). In the first stage of the 2SLS approach we used ordinary least squares to estimate the following model of for-profit dialysis center choice:
| (1) |
where Pi is the choice for patient “i” (1=for-profit, 0=nonprofit), Xi is a vector of binary variables containing measured confounding variables, ci is the effect of unmeasured confounders that affect both dialysis center choice and survival, and ei is the net impact of the unmeasured factors that affect center choice only. Ai is a vector of indicator variables that group patients based on the differential distance value of patient i. A Chow F-test (Chow 1960) can be used to assess whether Ai describes a significant portion of the variation in Pi (i.e., whether the estimates in the vector a2 are simultaneously equal to 0) and provides a natural test of whether the binary variables representing differential distance affect dialysis center choice.
In the second stage of 2SLS, we estimated a 1-year survival model specified as follows:
| (2) |
where Si is binary variable equal to 1 if patient i survives 1-year past dialysis initiation, 0 otherwise, Xi, Pi, and ci are defined as in equation (1), and ui is the set of unmeasured factors that affect patient survival and not dialysis center choice. b2 represents the average 1-year survival effect of initiating dialysis at a for-profit center relative to a nonprofit center. Standard methods to estimate equation (2)—logistic regression (LR) or linear probability model (LPM) regression—will yield biased estimates of b2 if ci is not equal to 0. 2SLS avoids this bias when estimating equation (2) by replacing the actual for-profit center choice variable in equation (2), Pi, with the predicted for-profit center choice probability from equation (1) for each patient, P^i. Because Xi is also in equation (2), the only variation in P^i that is used to estimate b2 in equation (2) comes from Ai. As Ai is assumed to be unrelated to ci, the 2SLS estimate of b2 provides a consistent estimate of the change in the 1-year postdialysis initiation survival rate from a 1 unit change in the for-profit center use rate. Little theory exists to select the number of patient groups to specify an instrument empirically through Ai. Dividing patients into more groups based on the instrument exploits more variation in treatment choice (here the choice of a for-profit center), but decreases the number of patients in each group, which increases the risk of unmeasured confounders being associated with group membership. Here we followed the norms of past IV research (McClellan et al. 1994; McClellan and Newhouse 1997; Angrist and Evans 1998; Brooks et al. 2000; Angrist 2001; Beck et al. 2003; Brooks et al. 2003) and varied the number of patient groups defined by our instrument (2, 5, 10, 20, 40 groups) and assessed whether our results were robust to this variation.
In the strictest interpretation, the 2SLS estimate of b2 is the local average treatment effect (LATE) described by Imbens and Angrist (1994) and can only be generalized to the patients whose initial dialysis center profit status choices were affected by differential distance (i.e., if their differential distance values were reversed they would have gone to a dialysis center with a different profit status). However, there is little reason to believe that this characteristic would not be broadly shared across most new ESRD patients. In addition, we contrasted the 2SLS estimates to LPM estimates using ordinary least squares. LPM estimation is used because it affords a more direct comparison with 2SLS estimates (McClellan et al. 1994). We also estimated the models using LR to compare with the results in Devereaux et al. (2002). We specified the LPM and LR models without covariates, with the set of risk-adjusters similar to those used by the studies within Devereaux et al. (2002), and with an expanded list of risk-adjusters using data available from the various USRDS databases described above. The IVREG and REGRESS procedures in STATA/SE 9.0 were used for the 2SLS and LPM models, respectively and were estimated using the ROBUST option to account for potential heterogeneity of the error term. The LOGISTIC procedure in SAS System for Windows 9.0 was used for LR estimation.
For differential distance to be useful as an instrument it must (1) be related to choice of dialysis center profit status and (2) not be related to patient outcomes directly or to unmeasured confounding variables. As discussed above, the first property can be observed and tested with observational data. The second property is an assumption, and like the assumptions innate in risk-adjustment approaches inferences made from IV estimates are conditional on its acceptance. A theoretical justification is required to support the acceptance of the second property. McClellan et al. (1994) suggested that the lack of a theoretical link between differential distance and the usual confounders such as unmeasured patient severity provides a degree of face validity. They argued that for this assumption to be violated patients with different distributions of unmeasured confounders with new diagnoses would have had to have made different residence decisions before their diagnoses. In this study for the second property to hold it also has to be assumed that the newly opened for-profit dialysis centers were not placed into areas with distinct distributions of unmeasured confounders. While these assumptions cannot be fully validated with existing data, they can be scrutinized to some extent. Two-stage least squares models in which differential distance is specified with more than two groups are said to be overidentified and a Hausman test statistic (Hausman 1983) can be estimated to test the null hypothesis that the exclusion of the differential distance indicator variables, Ai, from the survival equation was appropriate (a test of whether all the patient groups specified using differential distance simultaneously had no direct on survival or no indirect effect on survival through an unmeasured confounding variable). A large value of the Hausman statistic rejects the null hypothesis. As is done in randomized controlled trials, we also assessed whether measured confounders were balanced across patients grouped by differential distance. Unbalanced measured confounders makes the inferences based on our 2SLS estimates conditional on the assumption that differences in measured confounders are not symptomatic of differences in unmeasured confounders across differential distance groups and it reinforces the need to control directly for these variables in 2SLS analysis (the Xi variables in the equations 1 and 2). In addition, we compared the measured confounders of patients that initiated dialysis in 1999 that lived in zip codes that switched from being closer to a nonprofit dialysis center in 1996 to being closer to a for-profit dialysis center in 1999 with patients that initiated dialysis in 1999 whose relative access to for-profit dialysis centers remained constant over this period. This comparison provides insight as to whether for-profit dialysis centers were placed in a manner suggestive of potential confounding problems (e.g., areas containing younger or less sick patients).
RESULTS
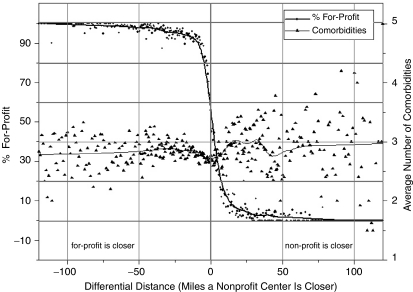
The scatter plot in Figure 1 illustrates the ability of differential distance both to identify variation in the use of for-profit centers and to balance confounders. Each point on the scatter plot contains the percentage initial for-profit and the average number of comorbid conditions listed on the CMS Medical Evidence Form for the set of patients with the same integer-rounded differential distance value. Differential distance is on the x-axis with patients relatively closer to for-profit centers on the left and patients relatively closer to nonprofit centers on the right. The left y-axis is the percentage of patients in each differential distance group that used a for-profit center and this value is depicted by diamonds on the figure. The right y-axis is the average number of comorbid conditions listed on the CMS Medical Evidence Form for the patients at each differential distance and this value is depicted by triangles. The lines through the observations represent nonlinear regression smoothing of the observations. It is clear from Figure 1 that the relative access to for-profit and nonprofit dialysis centers as measured by differential distance had a substantial effect on the choice of a for-profit center. In contrast, no clear relationship existed between differential distance and the average number of measured comorbid conditions. Comorbid condition averages are more dispersed at higher differential distance values, but this reflects the smaller number of patients that lived relatively closer to a nonprofit dialysis center.
Figure 1.
Scatter Plot of for-Profit Percentage and Average Number of Comorbid Conditions for Patients Grouped by Differential Distance, 1996–1999.
Table 1 provides additional information to assess the validity of assumptions underlying the risk adjustment and instrumental variable estimation approaches. The table compares mean values of measured characteristics across patients using two distinct patient grouping methods. The first method groups patients by the profit status of their initial dialysis center (columns 3 amd 4). The second method groups patients by their differential distance to the nearest for-profit dialysis center relative to the nearest nonprofit dialysis center (columns 6 and 7). The p-values for tests of the differences in means across patient groups are also reported (columns 5 and 8, respectively). Because of our large sample size many small differences in the measured characteristics across groups are statistically significant. As a result, we focused on overall differences between patient groups and whether these differences changed with the patient grouping method. When comparing the patients initially dialyzed in for-profit and nonprofit dialysis centers (columns 3 and 4) using the measures available for risk adjustment in earlier studies, we found larger percentages of female and African-American patients and fewer white patients treated at for-profit dialysis centers. Patients initially dialyzed at for-profit centers appeared somewhat healthier with lower comorbidity rates in 13 of the 17 measured conditions listed on the CMS Medical Evidence Form. We also found differences in characteristics across the groups that were unmeasured in previous research. Patients initially dialyzed at for-profit centers had lower previous inpatient utilization and tended to live in states with more extreme average health levels (high health and low health) using the state definitions in Subramanian et al. (2001).
Table 1.
Means of Characteristics of New Dialysis Patients at Nongovernment Dialysis Centers, 1996–1999 for Various Patient Subgroups
| First Center Choice | Differential Distance* | ||||||
|---|---|---|---|---|---|---|---|
| Full Sample | For-Profit | Nonprofit | p-Value† | For-Profit Closer | Nonprofit Closer | p-Value† | |
| Observations | 101,669 | 71,602 | 30,067 | — | 50,852 | 50,817 | — |
| For-profit (%) | 70.43 | 100.00 | 0.00 | <.0001 | 92.78 | 48.05 | <.0001 |
| Male (%) | 50.82 | 50.39 | 51.84 | <.0001 | 51.57 | 50.07 | <.0001 |
| 75 or older (%) | 51.83 | 51.67 | 52.21 | .1164 | 51.56 | 52.09 | .0900 |
| Race (%) | |||||||
| Other | 1.14 | 1.13 | 1.16 | .6645‡ | 1.02 | 1.25 | .0004 |
| Native Americans | 0.74 | 0.70 | 0.84 | .0247‡ | 0.88 | 0.61 | <.0001 |
| Asian | 2.58 | 2.42 | 2.96 | <.0001 | 1.93 | 3.24 | <.0001 |
| African Americans | 22.44 | 23.46 | 20.00 | <.0001 | 19.78 | 25.10 | <.0001 |
| Unknown | 0.48 | 0.43 | 0.62 | .0002‡ | 0.36 | 0.60 | <.0001 |
| Pacific Islander | 0.65 | 0.62 | 0.73 | .0512‡ | 0.57 | 0.73 | .0018 |
| Middle Eastern | 0.26 | 0.29 | 0.18 | .0005‡ | 0.20 | 0.32 | .0002 |
| Indian continent | 0.34 | 0.40 | 0.20 | <.0001 | 0.39 | 0.29 | .0043 |
| White | 71.36 | 70.55 | 73.31 | <.0001 | 74.87 | 67.86 | <.0001 |
| Comorbidity (%) | |||||||
| Cardiac arrest | 1.03 | 1.01 | 1.05 | .5630‡ | 1.10 | 0.95 | .0238 |
| Cardiac failure | 43.08 | 42.63 | 44.15 | <.0001 | 43.07 | 43.09 | .9491 |
| Chronic pulmonary disease | 10.25 | 10.11 | 10.58 | .0268‡ | 10.60 | 9.91 | .0003 |
| Diabetes | 43.95 | 45.05 | 41.34 | <.0001 | 44.78 | 43.12 | <.0001 |
| Dysrhythmia | 9.02 | 8.60 | 10.04 | <.0001 | 9.07 | 8.97 | .5779 |
| Hypertension | 74.46 | 75.39 | 72.23 | <.0001 | 75.31 | 73.60 | <.0001 |
| Alcohol dependence | 0.71 | 0.68 | 0.78 | .0874‡ | 0.68 | 0.74 | .2914 |
| Malignant neoplasm | 7.75 | 7.45 | 8.49 | <.0001 | 7.83 | 7.67 | .3346 |
| Cerebrovascular disease | 12.08 | 11.89 | 12.53 | .0043‡ | 12.03 | 12.13 | .6220 |
| Drug dependence | 0.05 | 0.04 | 0.07 | .1428‡ | 0.03 | 0.07 | .0016 |
| Ischemic heart disease | 33.30 | 32.14 | 36.09 | <.0001 | 33.51 | 33.10 | .1697 |
| Myocardial infarction | 12.06 | 11.57 | 13.21 | <.0001 | 12.15 | 11.96 | .3513 |
| Pericarditis | 0.84 | 0.75 | 1.04 | <.0001 | 0.76 | 0.92 | .0041 |
| Peripheral vascular disease | 18.99 | 18.61 | 19.92 | <.0001 | 19.37 | 18.62 | .0025 |
| Current smoker | 3.35 | 3.30 | 3.46 | .2127‡ | 3.49 | 3.21 | .0127 |
| Inability to ambulate | 5.67 | 5.67 | 5.66 | .9424 | 5.55 | 5.79 | .0980 |
| Inability to transfer | 2.15 | 2.20 | 2.05 | .1451‡ | 2.09 | 2.21 | .1967 |
| Hospital admissions in previous year (%) | |||||||
| 0≤x<2.0 | 69.60 | 70.26 | 68.01 | <.0001 | 69.64 | 69.56 | .7870 |
| 2.0≤x<3.0 | 14.63 | 14.15 | 15.77 | <.0001 | 14.52 | 14.74 | .3241 |
| 3.0≤x | 15.78 | 15.59 | 16.21 | .0143‡ | 15.85 | 15.71 | .5386 |
| Survival (%) | |||||||
| Survive 6 months | 77.29 | 77.22 | 77.46 | .3972 | 76.73 | 77.85 | <.0001 |
| Survive 1 year | 64.88 | 64.79 | 65.10 | .3450 | 64.45 | 65.31 | .0041 |
| Health state (%) | |||||||
| High | 57.13 | 61.24 | 47.35 | <.0001 | 61.39 | 52.87 | <.0001 |
| Mid | 23.47 | 16.40 | 40.31 | <.0001 | 13.67 | 33.27 | <.0001 |
| Low | 19.40 | 22.37 | 12.34 | <.0001 | 24.94 | 13.86 | <.0001 |
Distance to the closest for-profit dialysis center to the patient's residence at diagnosis minus the distance to the closest nonprofit dialysis center to the patient's residence. Patients grouped using the median of the variable (−3.51).
Two independent samples t-test.
Satterthwaite's approximation.
Grouping patients based on the median value of the differential distance variable (−3.51) as in columns 6 and 7 produced a large difference in the rate of initial for-profit dialysis center use across groups. Nearly 93 percent of the patients living relatively closer to a for-profit dialysis center were initially dialyzed at a for-profit center, while around 48 percent of the patients living relatively closer to a nonprofit center were initially dialyzed at a for-profit center. In contrast to grouping patients by the profit status of their initial dialysis center, grouping patients by differential distance produced a more balanced distribution of measured comorbidities and previous inpatient utilization. However, grouping patients by differential distance slightly exacerbated differences by race and did little to balance differences across states. This suggests that decisions to locate new for-profit facilities were not independent of the racial composition in local areas which may reflect areas of dialysis need. A larger percentage of African Americans lived relatively closer to nonprofit centers (columns 6 and 7), yet for-profit centers treated a larger percentage of African Americans (columns 3 and 4). To control for these differences in measured characteristics across groups defined by differential distance, we specified the full set of measured covariates within our 2SLS analysis. It should by noted that our 2SLS results are conditional on the assumption that differences in measured covariates across instrument groups are not symptomatic of differences in other unmeasured confounders.
Table 2 compares patients initiating dialysis in 1999 that lived in zip codes that became relatively closer to for-profit dialysis center from 1996 to 1999 with patients initiating dialysis in 1999 who lived in zip codes without changes in the profit status of the closest dialysis center from 1996 to 1999. The only real difference in these groups is the average health levels of the states in which they reside. Patients in zip codes in which for-profit dialysis centers became closest during 1996–1999 lived in states with less extreme average health levels. Given that patients initially dialyzed at for-profit dialysis centers in our sample were mainly from states with extreme health levels, this change probably simply reflects the expansion of for-profits into areas where they were less available and not an effort to select patient populations in a manner that will confound our results.
Table 2.
Patients Initializing Dialysis in 1999 That Lived in Zip Codes That Became Relatively Closer to For-Profit Dialysis Center from 1996–1999 Compared with Patients Initiating Dialysis in 1999 Who Lived in Zip Codes without Changes in Profit Status of the Closest Dialysis Center from 1996 to 1999
| Zip Code Became Closer to For-Profit between 1996– 1999 | ||||
|---|---|---|---|---|
| Patients Initiating Dialysis in 1999 | Yes | No | p-Value* | |
| Observations | 29,704 | 1,621 | 28,083 | — |
| For-profit (%) | 74.28 | 71.31 | 74.46 | .0049 |
| Male (%) | 51.26 | 49.29 | 51.37 | .1035 |
| 75 or older (%) | 54.38 | 54.78 | 54.36 | .7409 |
| Race (%) | ||||
| Asian | 2.60 | 1.60 | 2.67 | .0090 |
| African Americans | 21.20 | 20.79 | 21.23 | .6708 |
| White | 72.93 | 73.97 | 72.87 | .3338 |
| Comorbidity (%) | ||||
| Cardiac arrest | 0.96 | 1.11 | 0.95 | .5214 |
| Cardiac failure | 42.06 | 42.69 | 42.02 | .5964 |
| Chronic pulmonary disease | 10.33 | 9.87 | 10.36 | .5331 |
| Diabetes | 45.24 | 43.62 | 45.33 | .1774 |
| Dysrhythmia | 9.11 | 10.24 | 9.05 | .1048 |
| Hypertension | 76.22 | 75.57 | 76.26 | .5285 |
| Alcohol dependence | 0.59 | 0.49 | 0.59 | .6167 |
| Malignant neoplasm | 7.92 | 7.96 | 7.92 | .9594 |
| Cerebrovascular disease | 12.07 | 11.47 | 12.10 | .4523 |
| Drug dependence | 0.05 | 0.06 | 0.05 | .8366 |
| Ischemic heart disease | 33.86 | 36.15 | 33.73 | .0451 |
| Myocardial infarction | 11.97 | 13.26 | 11.89 | .0985 |
| Pericarditis | 0.73 | 0.49 | 0.74 | .2548 |
| Peripheral vascular disease | 18.90 | 19.12 | 18.89 | .8154 |
| Current smoker | 3.15 | 3.15 | 3.15 | .9844 |
| Inability to ambulate | 5.31 | 4.87 | 5.33 | .4247 |
| Inability to transfer | 1.90 | 1.67 | 1.91 | .4593 |
| Hospital admissions in previous year (%) | ||||
| 0≤x<2.0 | 71.66 | 69.95 | 71.76 | .1183 |
| 2.0≤x<3.0 | 13.81 | 14.25 | 13.78 | .1245 |
| 3.0≤x | 14.54 | 15.79 | 14.46 | .1401 |
| Survival (%) | ||||
| Survive 6 months | 76.34 | 76.50 | 76.33 | .8765 |
| Survive 1 year | 63.82 | 65.45 | 63.72 | .1583 |
| Health state (%) | ||||
| High | 56.97 | 53.92 | 57.14 | .0108 |
| Mid | 22.67 | 29.92 | 22.25 | <.0001 |
| Low | 20.37 | 16.16 | 20.61 | <.0001 |
Two independent samples t-test.
Table 3 contains the partial F-statistics for the first-stage model of the initial use of for-profit dialysis centers (equation 1). The estimates in Table 3 reflect a model in which we specified differential distance using 19 binary variables to represent 20 different patient groups. Patients were placed in the 20 groups based on every fifth percentile of differential distance across the sample. All noninstrument covariate groups described a statistically significant portion of the variation in the initial use of for-profit dialysis centers. Male patients were less likely to go to a for-profit center (p =.0159) and patients over 75 at dialysis initiation were more likely (p =.0069). There were statistically significant differences across races (p<.0001) as Asian Americans had the lowest initial use of for-profit centers and Indian Continent-Americans had the highest use. Patients initially using for-profit centers had higher rates of congestive heart failure (p =.0036), diabetes (p<.0001) and hypertension (p<.0001), but they had lower rates of cardiac dysrhythmia (p =.0225), cancer (p<.0001), ischemic heart disease (p<.0001), myocardial infarction (p =.0058), pericarditis (p =.0009), and tobacco use (p =.0142). Patients living a longer distance from the nearest dialysis center tended to go to for-profit centers at a higher rate (p<.0001). However, after controlling for distance to the nearest dialysis center, patients living in rural areas used for-profit centers at a lower rate (p<.0001).
Table 3.
F-Statistics Testing Whether Patient Groups Defined by Model Variables Are Related to the Use of For-Profit Dialysis Centers, 1996–1999
| Variable Group (Degrees of Freedom) | Partial F-statistic* (p-Value) |
|---|---|
| Instrument | |
| Differential distance (17, 101,554) | 2,150.53 (<.0001) |
| Covariates | |
| Gender (1, 101,554) | 5.81 (.0159) |
| Age (1, 101,554) | 7.29 (.0069) |
| Year (3, 101,554) | 59.53 (<.0001) |
| Race (8, 101,554) | 6.26 (<.0001) |
| Comorbidity (17, 101,554) | 8.55 (<.0001) |
| Previous health care use (4, 101,554) | 2.63 (.0326) |
| State (50, 101,554) | 212.00 (<.0001) |
| Actual distance (4, 101,554) | 75.41 (<.0001) |
| Patient zip code rural % (3, 101,554) | 57.46 (<.0001) |
| Patient zip code poverty % (3, 101,554) | 3.52 (.0144) |
| Patient zip code per capita income ($) (3, 101,554) | 5.91 (.0005) |
Against the null hypothesis that the groups defined by the instrumental variables do not describe a significant portion of treatment variation.
Table 4 contains single-equation logistic regression model estimates of the effect of for-profit status of the patient's initial dialysis center on 1-year survival to provide a more direct comparison with the estimates with Devereaux et al. (2002). Three model specifications are provided. Model 1 (first row) is specified without covariates. Model 2 (second row) specifies covariates similar to those specified in the studies summarized within the Devereaux et al. meta analysis (patient age, gender, comorbidity, race). Model 3 (third row) specifies the covariates used in the Devereaux et al. analysis plus state of residence, number of inpatient admissions and inpatient days in the year before dialysis, zip code-level measures of poverty, per capita income, and percent rural. The for-profit status of a patient's initial dialysis center was negatively related to patient survival in all three models but was only statistically significant in Model 2. The effect estimate in Model 2 is similar in magnitude to the estimate found in Devereaux et al. As additional risk adjustment is performed in Model 3, the estimate falls by nearly half and becomes statistically insignificant.
Table 4.
Logistic Regression Model Estimates of the Effect of “For-Profit” Status of Initial Dialysis Center on 1-Year Survival by Covariates Used, 1996–1999
| Covariates | Estimated Effect (Standard Error) | p-Value | Odds Ratio | Odds Ratio 95% Confidence Interval |
|---|---|---|---|---|
| None | −0.0136 (0.0144) | .3454 | 0.986 | 0.959, 1.015 |
| Devereaux* | −0.0559 (0.0149) | .0002 | 0.946 | 0.918, 0.974 |
| Devereaux plus† | −0.0330 (0.0173) | .0560 | 0.967 | 0.935, 1.001 |
Patient age, gender, comorbidity, and race.
Patient age, gender, comorbidity, race, state of residence, number of inpatient admissions and inpatient days in the year before dialysis, zip code-level measures of poverty, per capita income, and percent rural.
Table 5 contains LPM estimates and 2SLS estimates. The LPM estimates are similar to the logistic regression estimates listed in Table 4. The effect of initial dialysis at a for-profit center is negative and statistically significant when covariates similar to those in Devereaux et al. (2002) were specified, but the estimate falls by nearly half and becomes statistically insignificant as more risk-adjusting covariates are added to the model. To assess which of the new risk-adjusters was the main cause for the estimate drop, we ran four additional LPM models that each excluded a single new risk-adjuster. It is clear from comparing the estimates across these specifications that the state-dummy variables had the largest effect on reducing the estimate. Given the differences in the measured covariates across instrument groups (see Table 2), the 2SLS specifications were estimated using the full set of measured confounders. 2SLS models varied with the number of patient groups used to specify differential distance in the model. The 2SLS estimates were obtained using only the variation in the initial use of for-profit dialysis centers related to differential distance and no relationship between the initial use of a for-profit dialysis center and 1-year patient survival is seen across 2SLS model specifications. As is often the case, the 2SLS estimates have larger standard errors than the estimates from the LPM models. However, the 2SLS estimates are close enough to 0 that even using standard errors in the range of the LPM models it would not be possible to reject the null hypothesis. In addition, none of the Hausman test statistics for the overidentified models (models in which differential distance is specified with more than two groups) are statistically significant so we cannot reject the null hypothesis that the exclusion of the differential distance indicator variables from the survival equation was appropriate.
Table 5.
Linear Probability (Ordinary Least Squares) and IV Estimates of the Effect of “For-Profit” Status of Initial Dialysis Center on 1-Year Survival, 1996–1999
| Estimation Method and Specification | Number of Patient Groups Specified Using Differential Distance | Instrument Group Partial F-Statistic (p-Value) | Hausman F-Statistic (p-Value) | Estimated Effect (Std Error, p-Value) |
|---|---|---|---|---|
| LPM; no covariates | NA | NA | NA | −0.0031 (0.00328; .3450) |
| LPM; Devereaux covariates* | NA | NA | NA | −0.01221 (.00319;<.0001) |
| LPM; Devereaux covariates plus† | NA | NA | NA | −0.00715 (0.00366; .0511) |
| LPM; Devereaux covariates plus† without state dummies | NA | NA | NA | −0.01315 (0.00321;<.0001) |
| LPM; Devereaux covariates plus† without previous health care utilization | NA | NA | NA | −0.00759 (0.00368; .0395) |
| LPM; Devereaux covariates plus† without zip code socioeconomic variables | NA | NA | NA | −0.00726 (0.00366;0.0475) |
| LPM; Devereaux covariates plus† without distance to nearest dialysis center | NA | NA | NA | −00702 (0.00366; .0554) |
| 2SLS† | 2 | 18535.00 (<.0001) | NA | 0.00072 (0.00933; .939) |
| 2SLS† | 5 | 7909.59 (<.0001) | 0.64 (0.5877) | 0.00248 (0.00754; .746) |
| 2SLS† | 10 | 3991.69 (<.0001) | 0.99 (0.4379) | −0.00012 (0.00719; .986) |
| 2SLS† | 20 | 2150.53 (<.0001) | 0.82 (0.6625) | −0.00024 (0.007151; .973) |
| 2SLS† | 40 | 1003.79 (<.0001) | 1.23 (0.1583) | 0.00049 (0.007113; .945) |
Also specified patient age, gender, comorbidity, and race.
Also specified patient age, gender, comorbidity, race, state of residence, number of inpatient admissions and inpatient days in the year prior to dialysis, zip code-level measures of poverty, per capita income, and percent rural, distance to nearest dialysis center.
LPM, linear probability model; 2SLS, two-stage least squares.
DISCUSSION
Studies have shown that nonprofit dialysis centers use more resources in the delivery of dialysis than for-profit centers. The effects of these differences are unclear. Some suggest that the lower resource use by for-profit dialysis centers compromises the health outcomes of dialysis patients (Levinsky 1999; Devereaux et al. 2002; Meyer and Kassirer 2002). Others interpreted the higher levels of resource use at nonprofit dialysis centers as inefficiency (Ozgen and Ozcan 2002) with the implication that nonprofit centers employ a level of resources beyond what is necessary for proper patient care. The correct implication of this resource difference hinges on whether patients in dialyzed nonprofit dialysis centers have superior health outcomes to patients treated in for-profit dialysis centers. Unfortunately, it is not feasible to randomize the profit status of the dialysis center that ESRD patients use when they initiate dialysis to assess survival differences across dialysis center profit status. Consequently, many researchers estimated the difference in survival probabilities between for-profit and nonprofit dialysis centers using observational databases (McClellan et al. 1998; Garg et al. 1999; Irvin 2000; Port et al. 2000) and risk-adjusted for measured confounders. A meta-analysis of these risk-adjustment models (Devereaux et al. 2002) showed greater mortality risk for ESRD patients on hemodialysis treated at for-profit centers relative to nonprofit centers. As seen in Tables 4 and 5, we were able to obtain similar estimates using a set of risk-adjusters similar to those used in the models included in Devereaux et al. (2002). However, Table 2 shows that risk factors unmeasured in the previous research—previous inpatient utilization and the health characteristics of the patient's residence state—differed significantly between patients initially dialyzed at for-profit and nonprofit centers, and that controlling for these risk factors lowered the estimated survival differences between for-profit and nonprofit centers by nearly half regardless of estimation model used (Tables 4 and 5).
To further investigate this relationship, we employed the 2SLS variant of instrumental variable analysis. We used the differential distance from a patient's residence to the nearest for-profit and nonprofit dialysis centers to provide an ex-post facto randomization of dialysis center choice. Differential distance described a substantial portion of the variation in the profit status of the dialysis center initially chosen, and grouping patients by differential distance instead of the profit status of their initial dialysis center led to a more balanced distribution of comorbidities and previous health care utilization. We also found no evidence suggesting that new for-profit dialysis centers were sited during our analysis period in a manner that would have led to relationships between differential distance and unmeasured confounders. Differences in race, state of residence, and several comorbidities remained across patients grouped with differential distance. We controlled for these measured confounders directly in our 2SLS models so that our estimates used only the variation in differential distance that was independent of these factors. Inferences from our 2SLS estimates, although, are conditional on the assumption that the differences in measured confounders across instrument groups were not symptomatic of differences in unmeasured confounders across the same groups.
In contrast to the estimates in Devereaux et al. (2002) and our from risk-adjustment model estimates, our 2SLS estimates showed no relationship between initial dialysis at for-profit dialysis centers and 1-year patient survival. These estimates were robust to the grouping method used to specify differential distance. Hausman test statistics indicated little risk that differential distance is directly related to patient survival or unmeasured confounders. Our 2SLS estimates can only be generalized strictly to the set of patients whose initial dialysis center profit status would have been affected by changes in differential distance (Angrist et al. 1996), but the substantial independent effect that differential distance had on initial dialysis center profit status suggests that this characteristic would probably be shared by most elderly ESRD patients. Our results suggest that lower resource use at for-profit dialysis centers did not jeopardize the survival of new elderly ESRD patients and policies designed to reduce the number for-profit dialysis centers may increase costs without increasing patient survival. As the number of dialysis centers increased from 2,154 in 1991 to 4,586 in 2003 (U.S. Renal Data System 2005) it appears that the competitive market for dialysis services forced all dialysis providers to maintain sufficient quality levels regardless of the overriding goals of each organization.
Acknowledgments
This research was performed as part of the Economics Special Study Center within the United States Renal Data System (USRDS). Preliminary results from this research were presented at 36th Annual Meeting and Scientific Exposition of the American Society of Nephrology, San Diego, CA, November 13, 2003. The authors are especially grateful for the financial support (contract DK02401) and encouragement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) especially from Paul Eggers and data and technical support from the folks at USRDS Coordinating Center in Minneapolis. This research could not have progressed without the farsighted database development operations within the USRDS Coordinating Center led by Allan Collins and Shu Chen. We would also like to thank University of Iowa graduate students Wei Zhang, Mingeen Lu, and Yongming Zhao for their excellent research assistance and two journal referees and the HSR editors for helping us craft the text more concisely.
Disclosures: None
Disclaimers: Any remaining errors are attributable to the authors. This paper does not represent policy of either NIDDK or the USRDS Coordinating Center. The views expressed herein are those of the authors and no official endorsement by NIDDK and the USRDS Coordinating Center is intended or should be inferred.
REFERENCES
- Angrist JD. Estimation of Limited Dependent Variable Models with Dummy Endogenous Regressors: Simple Strategies for Empirical Practice. Journal of Business and Economic Statistics. 2001;19(1):2–16. [Google Scholar]
- Angrist JD, Evans WN. Children and Their Parent's Labor Supply: Evidence from Exogenous Variation in Family Size. American Economic Review. 1998;88(3):450–77. [Google Scholar]
- Angrist JD, Imbens GW, Rubin DB. Identification of Causal Effects Using Instrumental Variables. Journal of the American Statistical Association. 1996;91(434):444–72. [Google Scholar]
- Beck CA, Penrod J, Gyorkos TW, Shapiro S, Pilote L. Does Aggressive Care Following Acute Myocardial Infarction Reduce Mortality? Analysis with Instrumental Variables to Compare Effectiveness in Canadian and United States Patient Populations. Health Services Research. 2003;38(6):1423–40. doi: 10.1111/j.1475-6773.2003.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JM, Chrischilles E, Scott SD, Chen-Hardee SS. Was Breast Conserving Surgery underutilized for Early Stage Breast Cancer? Instrumental Variables Evidence for Stage II Patients from Iowa. Health Services Research. 2003;38(6, part I):1385–402. doi: 10.1111/j.1475-6773.2003.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JM, McClellan M, Wong HS. The Marginal Benefits of Invasive Treatment for Acute Myocardial Infarction: Does Insurance Coverage Matter. Inquiry. 2000;37(1):75–90. [PubMed] [Google Scholar]
- Byar DP. Problems with Using Observational Databases to Compare Treatments. Statistics in Medicine. 1991;10:663–6. doi: 10.1002/sim.4780100417. [DOI] [PubMed] [Google Scholar]
- Chow G. Tests of Equality between Sets of Coefficients in Two Linear Models. Econometrica. 1960;28(3):591–605. [Google Scholar]
- de Lissovoy G, Powe NR, Griffiths RI, Watson AJ, Anderson GF, Greer JW, Herbert RJ, Eggers PW, Milam RA, Whelton PK. The Relationship of Provider Organizational Status and Erythropoietin Dosing in End Stage Renal Disease Patients. Medical Care. 1994;32(2):130–40. doi: 10.1097/00005650-199402000-00004. [DOI] [PubMed] [Google Scholar]
- Devereaux P, Schunemann H, Ravindran N, Bhandari M, Garg AX, Choi PT, Grant BJ, Haines T, Lacchetti C, Weaver B, Lavis JN, Cook DJ, Haslam DR, Sullivan T, Guyatt GH. Comparison of Mortality between Private for-Profit and Private Not-for-Profit Hemodialysis Centers. A Systematic Review and Meta-analysis. Journal of the American Medical Association. 2002;288(19):2449–57. doi: 10.1001/jama.288.19.2449. [DOI] [PubMed] [Google Scholar]
- Doll R. Summation of a Conference, Doing More Good Than Harm: The Evaluation of Health Care Interventions. Annuals of New York Academy of Science. 1994;705:310–3. [Google Scholar]
- Farley DO. Competition under Fixed Prices: Effects on Patient Selection and Service Strategies by Hemodialysis Providers. Medical Care Research and Review. 1996;53(3):330–49. doi: 10.1177/107755879605300307. [DOI] [PubMed] [Google Scholar]
- Garg P, Frick K, Diener-West M, Powe NR. Effect of the Ownership of Dialysis Facilities on Patients' Survival and Referral for Transplantation. New England Journal of Medicine. 1999;341(22):1653–60. doi: 10.1056/NEJM199911253412205. [DOI] [PubMed] [Google Scholar]
- Hausman JA. Specification and Estimation of Simultaneous Equation Models. In: Griliches Z, Intriligator MD, editors. Handbook of Econometrics. Vol. 1. New York: North-Holland Publishing Company; 1983. pp. 392–448. [Google Scholar]
- Held PJ, Garcia J, Pauly MV, Cahn MA. Price of Dialysis, Unit Staffing, and Length of Dialysis Treatments. American Journal of Kidney Disease. 1990;15:441–50. doi: 10.1016/s0272-6386(12)70362-1. [DOI] [PubMed] [Google Scholar]
- Held PJ, Levin NW, Bovbjerg RR, Pauly MV, Diamond LH. Mortality and Duration of Hemodialysis Treatment. Journal of the American Medical Association. 1991;265(17):871–5. [PubMed] [Google Scholar]
- Hirth RA, Chernew ME, Orzol SM. Ownership, Competition, and the Adoption of New Technologies and Cost-Saving Practices in a Fixed-Price Environment. Inquiry. 2000;37:282–94. [PubMed] [Google Scholar]
- Hornberger J, Wrone E. When to Base Clinical Policies on Observational versus Randomized Trial Data. Annals of Internal Medicine. 1997;127(8):697–703. doi: 10.7326/0003-4819-127-8_part_2-199710151-00053. [DOI] [PubMed] [Google Scholar]
- Imbens GW, Angrist JD. Identification and Estimation of Local Average Treatment Effects. Econometrica. 1994;62(2):467–75. [Google Scholar]
- Irvin R. Quality of Care Differences by Ownership in United States Renal Dialysis Facilities. ASAIO Journal. 2000;46:775–8. doi: 10.1097/00002480-200011000-00023. [DOI] [PubMed] [Google Scholar]
- Jollis J, Ancukiewicz M, DeLong ER, Pryor DB, Muhlbbaier L, Mark DB. Discordance of Databases Designed for Claims Payment versus Clinical Information Systems. Annals of Internal Medicine. 1993;119(8):844–50. doi: 10.7326/0003-4819-119-8-199310150-00011. [DOI] [PubMed] [Google Scholar]
- Levinsky NG. Quality and Equity in Dialysis and Renal Transplantation. New England Journal of Medicine. 1999;341(22):1691–3. doi: 10.1056/NEJM199911253412212. [DOI] [PubMed] [Google Scholar]
- McClellan M, McNeil B, Newhouse JP. Does More Intensive Treatment of Acute Myocardial Infarction in the Elderly Reduce Mortality. Journal of the American Medical Association. 1994;272:859–66. [PubMed] [Google Scholar]
- McClellan M, Newhouse JP. The Marginal Cost-Effectiveness of Medical Technology: A Panel Instrumental-Variables Approach. Journal of Econometrics. 1997;77:39–64. [Google Scholar]
- McClellan W, Soucie JM, Flanders WD. Mortality in End-Stage Renal Disease Is Associated with Facility-to-Facility Differences in Adequacy of Hemodialysis. Journal of the American Society of Nephrology. 1998;9:1940–7. doi: 10.1681/ASN.V9101940. [DOI] [PubMed] [Google Scholar]
- McLeroy KR, Bibeau D, Steckler A, Glanz K. An Ecological Perspective on Health Promotion Programs. Health Education Quarterly. 1988;15(4):351–77. doi: 10.1177/109019818801500401. [DOI] [PubMed] [Google Scholar]
- Meyer KB, Kassirer JP. Squeezing More Cost and Care Out of Dialysis: Our Patients Would Pay the Price. American Journal of Medicine. 2002;112:232–4. doi: 10.1016/s0002-9343(01)01100-7. [DOI] [PubMed] [Google Scholar]
- Ozgen H, Ozcan YA. A National Study of Efficiency for Dialysis Canters: An Examination of Market Competition and Facility Characteristics for Production of Mulitple Dialysis Outputs. Health Services Research. 2002;37(3):711–32. doi: 10.1111/1475-6773.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F, Wolfe RA, Held PJ, Bander S, Lazarus M, Lindenfeld S, Nissenson AR, Owen WF, Qunibi W, Riley D, Abboud H, Ruma J, Wick G, Garg P, Frick K, Powe NR, Millikan A. Ownership of Dialysis Facilities and Patients' Survival: Editorials. New England Journal of Medicine. 2000;342(14):1053–6. doi: 10.1056/NEJM200004063421415. [DOI] [PubMed] [Google Scholar]
- Pushkal GP, Powe NR. Profit-Making in the Treatment of Chronic Kidney Disease: Truth and Consequences. Seminars in Dialysis. 2001;14(3):153–6. doi: 10.1046/j.1525-139x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Kawacki I, Kennedy BP. Does the State You Live in Make a Difference? A Multilevel Analysis of Self-Rated Health in the US. Social Science and Medicine. 2001;53(1):9–19. doi: 10.1016/s0277-9536(00)00309-9. [DOI] [PubMed] [Google Scholar]
- U.S. Renal Data System. USRDS 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2004. [Google Scholar]
- U.S. Renal Data System. USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease; 2005. [Google Scholar]