Abstract
Background
It has been suggested that interleukin-6 (IL-6) may modulate androgen receptor (AR) action to accelerate prostate cancer (PCa) progression. Selenium compounds are highly recommended as a promising chemopreventive agent for PCa. This study was to determine if selenium can repress IL-6 mediated AR action in PCa progression.
Methods
Cell proliferation, prostate-specific antigen, gene transfer, and western blot assays were used to study the effects of sodium selenite and methylseleninic acid on IL-6 mediated AR action on an AR expressing human prostate cancer cell line, LNCaP.
Results
We found that sodium selenite, but not methylseleninic acid, significantly (p < 0.05) inhibited IL-6-induced trans-activating activity of AR and cell proliferation in LNCaP cells. Interestingly, although sodium selenite did not show effect on activation of both STAT3 and ERK1/2 in the presence of IL-6, an increased expression of c-Jun was detected in cells after treatment with sodium selenite. Indeed, we showed overexpression of c-Jun blocked IL-6-induced AR activation.
Conclusions
Taken together, our results suggest that sodium selenite not methylseleninic acid can inhibit IL-6-mediated AR activation by increased c-Jun in LNCaP cells. Sodium selenite may be a proper selenium form for further testing its potency on intervening IL-6-mediated PCa progression.
Keywords: Selenium, IL-6, prostate cancer, c-Jun, androgen independent growth
1. Introduction
Prostate cancer is the most commonly diagnosed solid tumor and the second leading cause of cancer mortality in men in the Western world [1]. Clinical data demonstrate that serum interleukin-6 (IL-6) levels in patients with hormone-refractory prostate cancer are elevated and usually accompanied by high levels of prostate specific antigen (PSA) [2,3]. Recent studies implicate that IL-6 may function as an autocrine or paracrine growth factor since it stimulates proliferation of various cancer cells, including prostate cancer cells [3–8]. IL-6 has been shown to activate androgen receptor (AR) -dependent gene expression in prostate cancer cells in the absence of androgens [4,9,10]. Intracellular signaling pathways, such as those mediated by mitogen-activated protein kinases (MAPK), and signal transducer and activators of transcription-3 (STAT3), are involved in the signaling cross-talking with the AR. Activation of STAT3 has been implicated in the activation of AR induced by IL-6 in an androgen independent manner in LNCaP cells, an androgen responsive human prostate cancer cell line [9,10].
Selenium is a chemopreventive agent, for which there are considerable epidemiological and clinical studies indicating a protective role against several different types of cancer, including that of the prostate. An inverse association between selenium levels in the serum or toenails and the subsequent risk of developing prostate cancer has recently been reported [11,12], which stimulates a great deal of interests in understanding the mechanism of selenium-mediated chemoprevention. Selenium is an essential trace element and exists in both organic and inorganic forms. Sodium selenite and methylseleninic acid (MSeA) are the two most studied selenium compounds for prostate cancer prevention. MSeA is a synthetic organic compound of selenium, relatively stable in solution, and presumably produces methylselenol as an anti-cancer effector [13]. Selenomethionine (Se-Met) can be metabolized and converted into methylselenol in the liver and is currently undergoing a prostate cancer intervention clinical trial SELECT [14]. Although numerous studies have suggested the inhibitory role of selenium in the growth of prostate cancer cells in vitro, a comprehensive understanding of the mechanism underlying selenium anticancer effects on IL-6 mediated prostate cancer cell growth, is currently lacking. Selenium compounds have been shown to inhibit cell proliferation, induce apoptosis and prevent tumor progression [15–17].
2. Materials and methods
2.1. Cell culture and reagents
Human prostate cancer LNCaP cells (American Type Culture Collection, Manassas, VA) was maintained in RPMI 1640 (Mediatech, Hercules, CA) containing 5% fetal bovine serum (FBS, Biofluids, Rockville, MD) at 37°C and 5% CO2. For experiments, cells were cultured for 24 h in serum-free RPMI 1640 in order to avoid potential interference of existing steroids in FBS. Cells were treated in RPMI 1640 with 5% charcoal-stripped FBS supplemented with different chemicals as indicated. Mibolerone (Mib, New England Nuclear, Boston, MA), a nonmetabolizable synthetic androgen, was used at 1 nmol/l concentration. Recombinant human IL-6 (Leinco Technologies, Inc, St. Louis, MO) was used at 25 ng/ml in all experiments.
2.2. Growth responses and PSA Levels
Cells were plated in 24-well plates at 2 × 104 cells/well. Forty-eight to 72 h after plating, cells were treated with MSeA or sodium selenite (Sigma) at different doses in the presence or absence of Mib or IL-6 as indicated. MTS assay, a non-radioactive cell proliferation assay, composed of [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] (Promega, Madison, WI) was used to determine cell proliferation 72 h after treatments. To measure secreted PSA levels, 400 μl of spent medium from cells treated for 72 h were collected. PSA protein levels were determined using specific immunoassays (Mayo Immunochemical Core Facility) as previously reported [18].
2.3. Western blot analysis
Cells were seeded at 1 × 106 cells/plate in 100-mm dishes. Cells grown in log phase were co-treated with IL-6 and various concentrations of MSeA or sodium selenite for different time points. The cells were collected by centrifugation and washed with cold PBS. Cell lysates were prepared in a lysis buffer (PBS containing 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS plus freshly added protease inhibitors, 100 μg/ml phenylmethylsulfonyl fluoride, 30 μl/ml aprotinin, and 1 mmol/l sodium orthovanadate) and used for Western blot analysis. The sample filters were immunoblotted with specific primary antibody to c-Jun, STAT3, phospho-STAT3 (Cell Signaling, Beverly, MA), ERK1/2, phospho-ERK1/2 (Santa Cruz Biotechnology, Santa Cruz, CA) and horseradish peroxidase-conjugated secondary antibodies and visualized by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).
2.4. Transcriptional reporter assays
LNCaP cells were plated into 12-wells plate. Cells at 50–70% confluence were transfected with the appropriate constructs (6-kb PSA promoter-pGL3, hK2-3 x ARE-SV40 minimal promoter pGL3 or empty pGL3 vector) tagged with luciferase gene by using the method described previously [19]. Twenty-four hours after transfection, cells were treated with MSeA or sodium selenite in combination with Mib or IL-6. Whole cell lysate was prepared for luciferase assay according to the manufacturer’s instructions (Promega). CMV-β-galactosidase (β-gal), 0.2 μg/well, expression vector was also co-transfected for normalization of transfection efficiency. The experiments were done in triplicate and repeated three times, and the standard deviations (SDs) were calculated.
2.5. Statistical analysis
All values are given as means plus SDs. Means of groups were compared with the Student’s t test and p < 0.05 was used as the level of significance.
3. Results
3.1. Inhibition of IL-6-mediated cell growth and down-regulation of IL-6-induced PSA protein expression by sodium selenite
When LNCaP cells were exposed to sodium selenite or MSeA in the presence of IL-6 for 72 h, a significant reduction in cell growth was observed only with selenite exposure but not with MSeA (Fig. 1A). As expected, PSA production was stimulated by IL-6. A significant inhibition of PSA upregulation induced by IL-6 was detected in cells treated with sodium selenite (Fig. 1B). A dose-dependent inhibition of PSA protein expression by sodium selenite was also detected in LNCaP cells treated with mibolerone (Fig. 1C). These data suggest that sodium selenite, but not MSeA, can block IL-6 mediated cell growth and PSA upregulation in LNCaP cells.
Fig. 1.
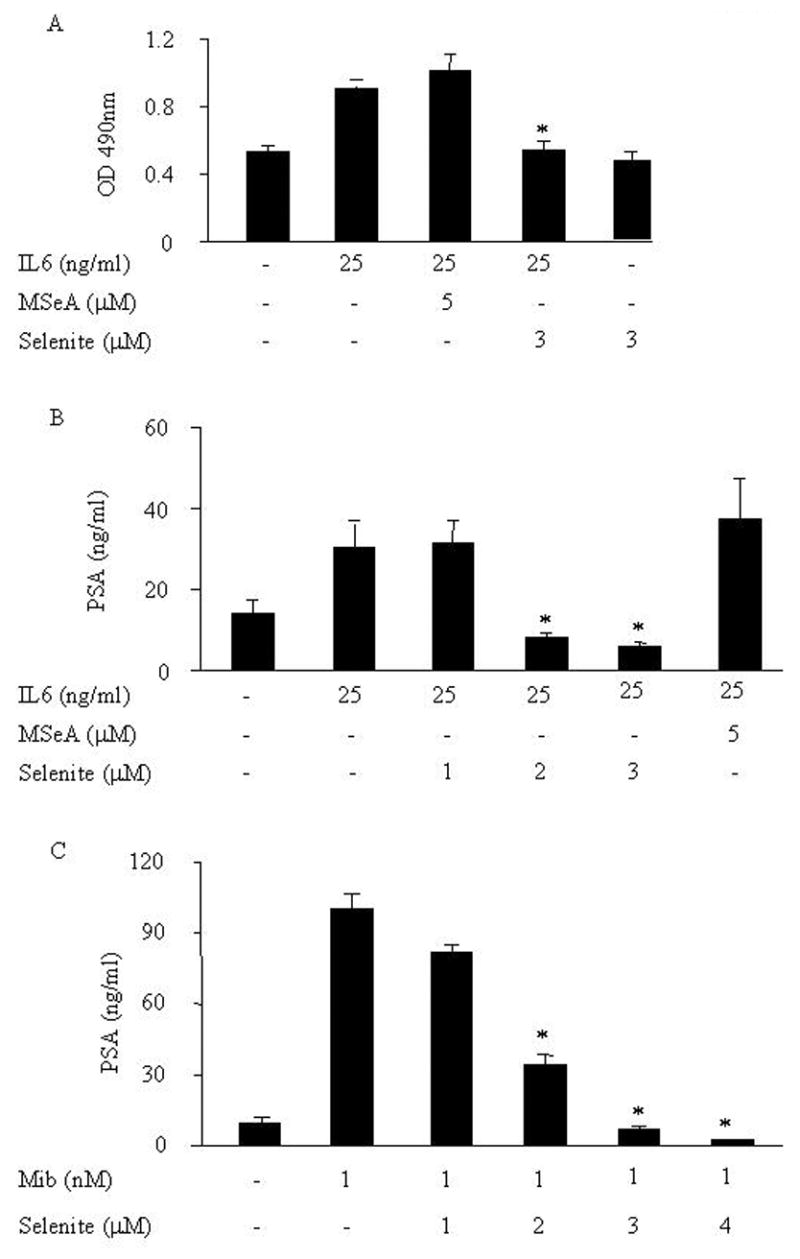
Effects of sodium selenite or MSeA on IL-6-mediated cell growth (A) and IL-6 (B) or Mib (C) induced PSA protein expression in LNCaP cells. (A) After treated with or without IL-6 in the presence or absence of 5 μmol/l MSeA or 3 μmol/l selenite for 72 h, cell growth was measured by the MTS assay. (B) After treated with or without IL-6 in the presence or absence of 1, 2, 3 μmol/l selenite or 5 μmol/l MSeA for 72 h, 400 μl of spent medium was collected and PSA protein expression was determined by immunoassay. (C) After treated with or without Mib in the presence or absence of 1, 2, 3, 4 μmol/l selenite for 72 h, 400 ul of spent medium were collected and PSA protein expression was determined by immunoassay.
3.2. Inhibition of IL-6 induced androgen independent AR trans-activation by sodium selenite
PSA is mainly regulated by the AR signals at the transcriptional level. Thus, we used the PSA gene as a model to investigate effects of selenium on AR transactivation activity stimulated by androgens or IL-6. Cells were transfected with the PSA-6kb promoter that contains androgen responsive elements (ARE). AR binds directly to the ARE of target genes for androgen action. Following transfection, cells were treated with either sodium selenite or MSeA at various concentrations in the presence of androgen. Effects of both sodium selenite and MSeA in androgen-mediated AR transactivation activity were then measured by a luciferase reporter assay. We found that both sodium selenite and MSeA significantly inhibited the AR transactivation activity in a dose-dependent manner (Fig. 2A). These results, are consistent with data from previous studies by others [20–25], and suggest that both the compounds were equally effective in inhibiting androgen-mediated AR action. We next tested the effect of these compounds on IL-6-mediated AR transactivation. Interestingly, we found that only sodium selenite, but not MSeA, significantly inhibited IL-6-induced AR transactivation activity in LNCaP cells (Fig. 2B). Taken together, these results suggest that sodium selenite, but not MSeA, is the potent inhibitor of IL-6-mediated AR transactivating function.
Fig. 2.
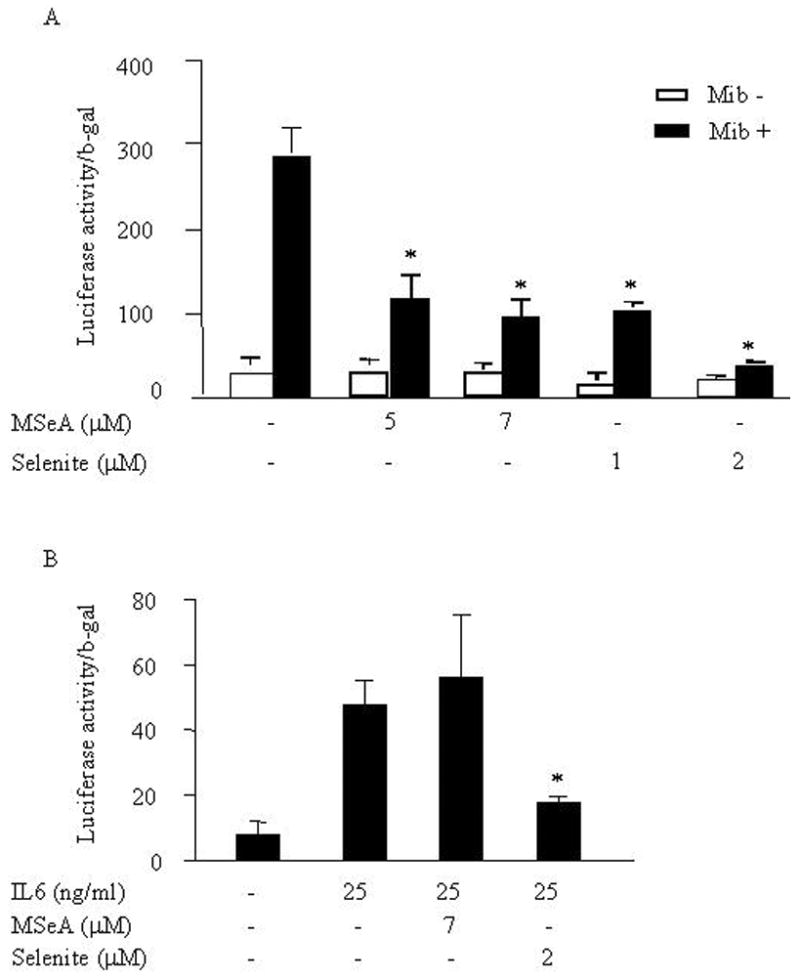
Effects of sodium selenite or MSeA on Mib or IL-6-stimulated AR transactivation activity in LNCaP cells. (A) After co-transfected with PSA-6kb promoter-luciferase reporter (1 μg/well) and CMV-β-gal vectors (0.2 μg/well) for 24 h, LNCaP cells were treated with Mib or without and plus or minus MSeA or selenite as indicated. Twenty-four hours later, cell lysate was collected and transcriptional activity of PSA-6kb and expression of β-gal were determined by luciferase and β-gal assay, respectively. The relative luciferase activities were normalized by β-gals. (B) Effects of sodium selenite and MSeA on IL-6 stimulated AR transactivation activity in LNCaP cells were determined by luciferase and β-gal assays.
3.3. Both sodium selenite and MSeA had no effects on IL-6-induced activation of STAT3 and ERK1/2
IL-6 can mediate primarily two signal transduction pathways, Janus kinases/STAT3 and ERK 1/2 in prostate cells [26, 27]. To examine if these 2 pathways can be affected by the selenium compounds, LNCaP cells were exposed to either compound in the presence of IL-6 for 30 min. Expression and phosphorylation activation of STAT3 and ERK1/2 were analyzed by Western blot. Consistent with previous studies [27], we detected an increased activation of STAT3 and ERK1/2 via phosphorylation but not the total STAT3 and ERK1/2 protein levels in LNCaP cells after stimulation with IL-6. Neither sodium selenite nor MSeA had any effect on IL-6-induced activation of STAT3 and ERK1/2 (Fig. 3). No alteration of total AR protein was observed following a longer-term exposure (up to 2 h, data not shown).
Fig. 3.
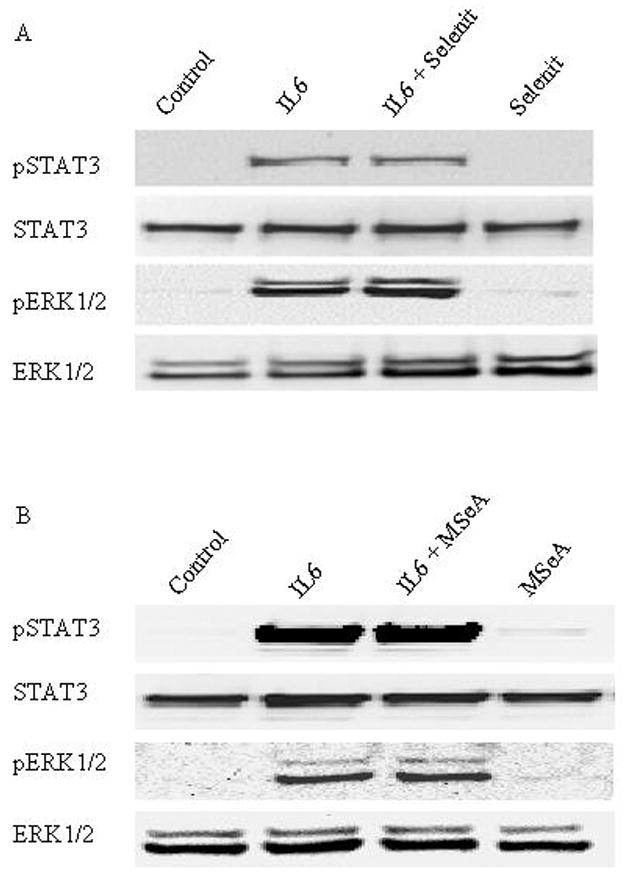
Effects of sodium selenite or MSeA on IL-6-induced expression and activation of STAT3 and ERK1/2 in LNCaP cells. (A) After treated with or without IL-6 in the presence or absence of 3 μmol/l selenite for 30 min, whole cell lysates were prepared and expression of total and phosporylated STAT3 and ERK1/2 were determined by Western blot analysis using specific antibodies. (B) Effects of MSeA on IL-6-induced expression of total and phosphorylated STAT3 and ERK1/2 in LNCaP cells were shown by Western blot analysis.
3.4. Sodium selenite increased c-Jun expression and overexpression of c-Jun inhibited the AR transactivation activity
To dissect the molecular mechanisms underlying selenite-mediated inhibition of AR activation induced by IL-6, we analyzed the effects of both sodium selenite and MSeA on expression of c-Jun in LNCaP cells. As shown in Figure 4A by Western blot analysis, a significant increase of c-Jun protein expression was detected in cells after 24 h treated with sodium selenite, but not in cells treated with MSeA. To further examine the functional contribution of c-Jun to the regulation of AR transactivation, we used a c-Jun expression vector and co-transfected cells with hK2-3ARE, a vector linked with the luciferase gene [28]. As shown in Figure 4B, AR transactivation activity stimulated by IL-6 was significantly inhibited by c-Jun in LNCaP cells in a dose dependent manner. The results strongly suggest that sodium selenite, but not MSeA, has strong inhibitory effect on IL-6-mediated AR transactivation in LNCaP cells and this inhibitory effect is mediated by overexpression of c-Jun.
Fig. 4.
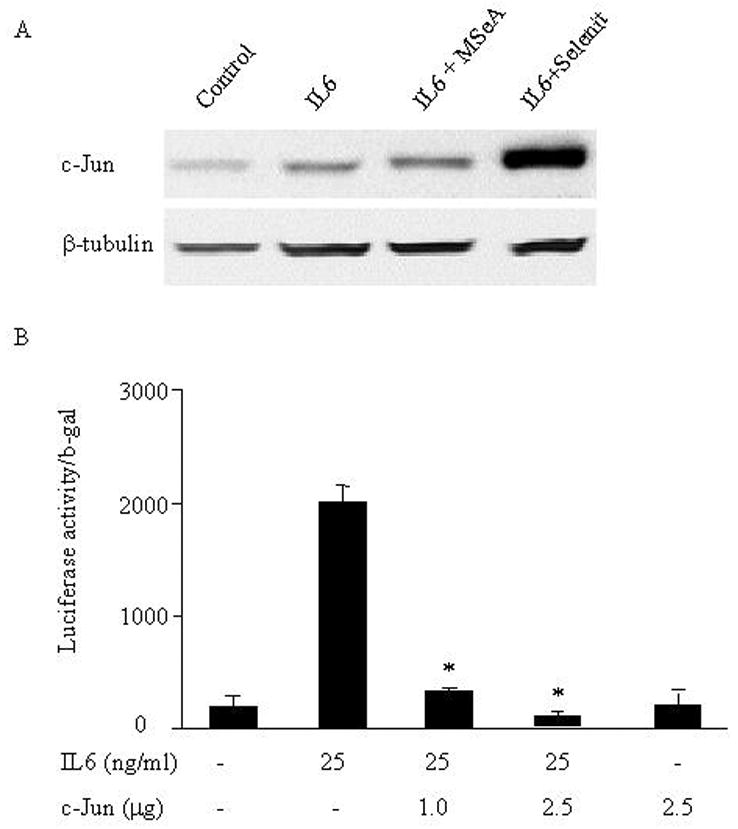
Effects of sodium selenite or MSeA on IL-6-induced c-Jun expression and overexpression of c-Jun on IL-6-mediated AR transactivation in LNCaP cells. (A) Effects of sodium selenite or MSeA on IL-6-induced c-Jun expression. After LNCaP cells treated with or without IL-6 and plus or minus sodium selenite (3 μmol/l) or MSeA (5 μmol/l) for 24 h, the expression of c-Jun in whole cell lysates was analyzed by western blot analysis. β-tubulin was used as an internal control for protein loading and transfer efficiency. (B) Effects of overexpression of c-Jun on IL-6-mediated AR transactivation in LNCaP cells. After cotransfected with hK2-3ARE promoter-luciferase reporter (1 μg/well), CMV-β-gal vectors (0.2 μg/well) and plus or minus c-Jun expression vector (1 or 2.5 μg/well) for 24 h, cells were treated with or without IL-6. Cell lysate was collected 24 h later and transcriptional activity of hK2-3ARE and expression of β-gal were determined by luciferase and β-gal assays, respectively. The relative luciferase activities were normalized by β-gal.
4. Discussion
Our studies suggested that sodium selenium, but not MSeA, inhibits IL-6-mediated androgen receptor activation in prostate cancer cells. Using LNCaP cells, we showed that sodium selenite, but not MseA, down-regulates the expression of PSA and AR transactivation induced by IL-6. In addition, sodium selenite shows an inhibitory effect on IL-6-induced proliferation of LNCaP cells. Moreover, sodium selenite induces c-Jun protein expression which in turn interferes with the AR transactivating activity. Thus, sodium selenite inhibits IL-6-mediated AR activation in LNCaP cells via upregulation of c-Jun, supporting a potential intervention role of sodium selenite in controlling prostate cancer progression.
Immunohistochemical staining of tumor biopsies showed that AR was expressed in most prostate cancer cells. More importantly, expression of AR usually correlates to the clinical development of the patients with prostate cancer. For example, a higher level of AR expression is found in patients with relapsed androgen refractory prostate tumors than those with the primary tumors [29,30] and up to 30% of the patients with recurrent tumors show expression of AR protein [31–33]. The AR in those androgen refractory tumors is still functional and can respond to a number of non-androgen factors including IL-6 [34,35]. The potential role of IL-6 in the development and progression of prostate cancer has been suggested by numerous studies [3–8]. IL-6 has been shown to activate AR -dependent gene expression in prostate cancer cells independent of androgens [34,35]. Barton BE et al. have recently shown that IL-6 signaling causes a change from hyperplasia to neoplasia in rat prostate epithelial cells [36]. Activation of intracellular kinases MERK1/2 and a latent transcription factor, STAT3, has been implicated in the activation of AR-dependent gene expression induced by IL-6 in prostate cancer cells [9,34,35]. In addition, phosphatidylinositol 3-kinase (PI 3-K)/AKT has also been considered as one of early down-stream mediators for activation of the AR by IL-6 [4, 10]. However, conflicting results are shown in the literature regarding the influence of the PI 3-K/AKT pathway on the AR [27]. It has been suggested that activated STAT3 by IL-6 binds AR to enhance AR’s transactivation function [27]. Consistent with previous studies [27], we detected an increased cell proliferation and activation of STAT3 and ERK1/2 via phosphorylation in LNCaP cells after stimulation with a recombinant human IL-6. Here we identified that although both sodium selenite and MSeA equally effective inhibit androgen mediated AR transactivation, only sodium selenite showed inhibitory effects on IL-6-mediated transactivation of AR. Moreover, Wu Y et al., [37] showed that selenium could deactivate AKT in prostate cancer cells. In consistence with this finding we also found selenium compounds used could reduce phosphorylated AKT (data not shown). Yet, we demonstrated MSeA did not inhibit IL-6 mediated AR activation. We concluded that AKT did not play a role in selenite mediated repression of AR activation by IL-6.
Dietary supplement of selenium may be an attractive approach to intervene prostate cancer development and progression. The efficacy was suggested Clark et al. [38] who showed 63% reduction of prostate cancer incidence with supplementation of selenite-enriched yeast. Higher serum selenium levels were found to be associated with a reduced (up to 50%) risk of metastasis in prostate cancer [12]. Sodium selenite and MSeA, are comprehensively studied selenium compounds, have been reported to be effective in inhibiting growth of most of the prostate cancer cell lines including LNCaP. Previous studies indicate that MSeA inhibits the expression of PSA protein by androgens in LNCaP cells [20,21,23]. However, in the present study, we provide evidence suggesting that sodium selenite, but not MSeA, down-regulates the expression of PSA and AR transactivation induced by IL-6 in LNCaP cells. Interestingly, both sodium selenite and MSeA showed no inhibitory effects on IL-6-induced activation of STAT3 and ERK1/2, important early events in IL-6-mediated signaling pathways in prostate cancer cells as reported in previous studies [9,34,35]. Instead, it appears that upregulation of c-Jun may play a critical role in sodium selenite induced inhibition of AR activation in response to IL-6.
c-Jun, a member of the basic leucine zipper family, is a main component of AP-1 protein complex which can directly activate or induce transcription of many genes containing AP-1 DNA binding sequences. It was reported that antioxidants such as quercetin can either inhibit or induce the activation and expression of c-Jun depending on cell types [39,40]. For example, angiotensin-II-induced c-Jun N-terminal kinase (JNK) mediated c-Jun activation was inhibited by quercetin in rat aortic smooth muscle cells [41], resulting in a decrease in both phosphorylation and activation of c-Jun. On the other hand, quercetin, resveratrol [42] and perillyl alcohol [43] can enhance the expression of c-Jun which in turn inhibited AR function in human prostate cancer cells. In this study, we showed that sodium selenite, but not MSeA, induces the over-expression of c-Jun in prostate cancer cells. It has been shown that the DNA binding domain of nuclear receptors may interact with the leucine zipper of c-Jun and result in mutual repression [41,44]. Sato et al. [44] demonstrated that there was a direct protein–protein interaction between the DNA- and ligand-binding domains of the AR and the leucine zipper region of c-Jun. Thus, the leucine zipper region of c-Jun by interacting with the DNA binding domain might affect the function of the AR. Meanwhile, this interaction may repel the binding of the coactivator, cyclic AMP response element-binding protein binding protein (CBP), to AR [45]. It is possible that increased c-Jun binding to AR-STAT3 complex might disassociate p300/CBP and interfere with the function of AR-STAT3. We will further address this potential question in the future studies.
In conclusion, we showed that sodium selenite, but not MSeA, significantly inhibited IL-6-induced cell proliferation and trans-activating activity of AR in LNCaP cells, presumably via up-regulation of c-Jun. Thus, sodium selenite may have strong effects in inhibiting progression of prostate cancer. A sodium selenite intervention strategy aimed at dampening the amplitude of AR signaling in androgen dependent and independent pathways could be helpful for controlling prostate cancer in high risk men. Combined with androgen ablation, selenite intervention might also be useful for the prevention of relapse after endocrine therapy. Further studies will focus on the molecular mechanisms of sodium selenite-associated up-regulation of c-Jun and determine if the differential effects of the selenite compound on IL-6-induced AR activation can also be observed in any other dietary selenium compounds.
Acknowledgments
We thank Dr. Junxuan Lu of Hormel Institute for providing us with the MSeA compound. This work is supported by an NIH grant CA89000.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbas F, Scardino PT. The natural history of clinical prostate carcinoma. Cancer. 1997;80:827–33. doi: 10.1002/(sici)1097-0142(19970901)80:5<827::aid-cncr1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Drachenberg DE, Elgamal AA, Rowbotham R, Peterson M, Murphy GP. Circulating Levels of Interleukin-6 in Patients With Hormone Refractory Prostate Cancer. Prostate. 1999;41:127–33. doi: 10.1002/(sici)1097-0045(19991001)41:2<127::aid-pros7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Culig Z, Steiner H, Bartsch G, Hobisch A. Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem. 2005;95:497–505. doi: 10.1002/jcb.20477. [DOI] [PubMed] [Google Scholar]
- 4.Hammacher A, Thompson EW, Williams ED. Interleukin-6 is a potent inducer of S100P, which is up-regulated in androgen-refractory and metastatic prostate cancer. Int J Biochem Cell Biol. 2005;37:442–50. doi: 10.1016/j.biocel.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Steiner H, Cavarretta IT, Moser PL, et al. Regulation of growth of prostate cancer cells selected in the presence of interleukin-6 by the anti-interleukin-6 antibody CNTO 328. Prostate. 2006;66:1744–52. doi: 10.1002/pros.20492. [DOI] [PubMed] [Google Scholar]
- 6.Squarize CH, Castilho RM, Sriuranpong V, Pinto DS, Jr, Gutkind JS. Molecular cross-talk between the NFkappaB and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia. 2006;8:733–46. doi: 10.1593/neo.06274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wehbe H, Henson R, Meng F, Mize-Berge J, Patel T. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res. 2006;66:10517–24. doi: 10.1158/0008-5472.CAN-06-2130. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794–800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T, Wang LH, Farrar WL. Interleukin-6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res. 2000;60:2132–35. [PubMed] [Google Scholar]
- 10.Culig Z, Bartsch G. Androgen axis in prostate cancer. J Cell Biochem. 2006;99:373–81. doi: 10.1002/jcb.20898. [DOI] [PubMed] [Google Scholar]
- 11.Brinkman M, Reulen RC, Kellen E, Buntinx F, Zeegers MP. Are men with low selenium levels at increased risk of prostate cancer? Eur J Cancer. 2006;42:2463–71. doi: 10.1016/j.ejca.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Yoshizawa K, Willett WC, Morris SJ, et al. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. Journal of national cancer institute. 1998;90:1219–29. doi: 10.1093/jnci/90.16.1219. [DOI] [PubMed] [Google Scholar]
- 13.Ip C, Dong Y, Ganther HE. New concepts in selenium chemoprevention. Cancer Metastasis Rev. 2002;21(3–4):281–9. doi: 10.1023/a:1021263027659. [DOI] [PubMed] [Google Scholar]
- 14.Klein EA, Thompson IM, Lippman SM, et al. SELECT: the selenium and vitamin E cancer prevention trial. Urol Oncol. 2003;21:59–65. doi: 10.1016/s1078-1439(02)00301-0. [DOI] [PubMed] [Google Scholar]
- 15.Venkateswaran V, Klotz LH, Fleshner NE. Selenium modulation of cell proliferation and cell cycle biomarkers in human prostate carcinoma cell lines. Cancer Res. 2002;62:2540–45. [PubMed] [Google Scholar]
- 16.Hu H, Jiang C, Ip C, Rustum YM, Lu J. Methylseleninic acid potentiates apoptosis induced by chemotherapeutic drugs in androgen-independent prostate cancer cells. Clin Cancer Res. 2005;11:2379–88. doi: 10.1158/1078-0432.CCR-04-2084. [DOI] [PubMed] [Google Scholar]
- 17.Corcoran NM, Najdovska M, Costello AJ. Inorganic selenium retards progression of experimental hormone refractory prostate cancer. J Urol. 2004;171:907–10. doi: 10.1097/01.ju.0000092859.16817.8e. [DOI] [PubMed] [Google Scholar]
- 18.Xing N, Chen Y, Mitchell SH, Young CYF. Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Carcinogenesis. 2001;22:409–14. doi: 10.1093/carcin/22.3.409. [DOI] [PubMed] [Google Scholar]
- 19.Ren FG, Zhang SB, Mitchell SH, Butler R, Young CYF. Tea polyphenols down-regulate the expression of the androgen receptor in LNCaP prostate cancer cells. Oncogene. 2000;19:1924–32. doi: 10.1038/sj.onc.1203511. [DOI] [PubMed] [Google Scholar]
- 20.Cho SD, Jiang C, Malewicz B, et al. Methyl selenium metabolites decrease prostate-specific antigen expression by inducing protein degradation and suppressing androgen-stimulated transcription. Mol Cancer Ther. 2004;5:605–11. [PubMed] [Google Scholar]
- 21.Husbeck B, Bhattacharyya RS, Feldman D, Knox SJ. Inhibition of androgen receptor signaling by selenite and methylseleninic acid in prostate cancer cells: two distinct mechanisms of action. Mol Cancer Ther. 2006;5:2078–85. doi: 10.1158/1535-7163.MCT-06-0056. [DOI] [PubMed] [Google Scholar]
- 22.Chun JY, Nadiminty N, Lee SO, Onate SA, Lou W, Gao AC. Mechanisms of selenium down-regulation of androgen receptor signaling in prostate cancer. Mol Cancer Ther. 2006;5(4):913–18. doi: 10.1158/1535-7163.MCT-05-0389. [DOI] [PubMed] [Google Scholar]
- 23.Lee SO, Yeon Chun J, Nadiminty N, Trump DL, Ip C, Dong Y, Gao AC. Monomethylated selenium inhibits growth of LNCaP human prostate cancer xenograft accompanied by a decrease in the expression of androgen receptor and prostate-specific antigen (PSA) Prostate. 2006;66:1070–75. doi: 10.1002/pros.20329. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Whitfield ML, Xu T, Botstein D, Brooks JD. Diverse effects of methylseleninic acid on the transcriptional program of human prostate cancer cells. Mol Biol Cell. 2004;15:506–19. doi: 10.1091/mbc.E03-07-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong Y, Lee SO, Zhang H, Marshall J, Gao AC, Ip C. Prostate specific antigen expression is down-regulated by selenium through disruption of androgen receptor signaling. Cancer Res. 2004;64:19–22. doi: 10.1158/0008-5472.can-03-2789. [DOI] [PubMed] [Google Scholar]
- 26.Yang L, Wang L, Lin H, et al. Interleukin-6 differentially regulates androgen receptor transactivation via PI3K-Akt, STAT3, and MAPK, three distinct signal pathways in prostate cancer cells. Biochem Biophys Res Commun. 2003;305:462–69. doi: 10.1016/s0006-291x(03)00792-7. [DOI] [PubMed] [Google Scholar]
- 27.Ueda T, Bruchovsky N, Sadar MD. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem. 2002;277:7076–85. doi: 10.1074/jbc.M108255200. [DOI] [PubMed] [Google Scholar]
- 28.Mitchel SH, Murtha PE, Zhang SB, Zhu W, Young CYF. An androgen response element mediates LNCaP cell dependent androgen induction of the hK2 gene. Mol Cell Endocrinology. 2000;168:89–99. doi: 10.1016/s0303-7207(00)00319-1. [DOI] [PubMed] [Google Scholar]
- 29.van der Kwast TH, Schalken J, Ruizeveld de Winter JA, et al. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer. 1991;48:189–93. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]
- 30.Ruizeveld de Winter JA, Janssen PJ, Sleddens HM, et al. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol. 1994;144:735–46. [PMC free article] [PubMed] [Google Scholar]
- 31.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TLJ, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–55. [PubMed] [Google Scholar]
- 32.Brown RSD, Edwards J, Dogan A, et al. Amplification of the androgen receptor gene in bone metastases from hormone-refractory prostate cancer. J Pathol. 2002;198:237–44. doi: 10.1002/path.1206. [DOI] [PubMed] [Google Scholar]
- 33.Koivisto P, Kononen J, Palmberg C, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–19. [PubMed] [Google Scholar]
- 34.Culig Z, Hobisch A, Cronauer MV, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–78. [PubMed] [Google Scholar]
- 35.Hobisch A, Eder IE, Putz T, et al. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58:4640–45. [PubMed] [Google Scholar]
- 36.Barton BE, Murphy TF, Adem P, Watson RA, Irwin RJ, Huang HF. IL-6 signaling by STAT3 participates in the change from hyperplasia to neoplasia in NRP-152 and NRP-154 rat prostatic epithelial cells. BMC Cancer. 2001;1:19. doi: 10.1186/1471-2407-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y, Zu K, Warren MA, Wallace PK, Ip C. Delineating the mechanism by which selenium deactivates Akt in prostate cancer cells. Mol Cancer Ther. 2006;5:246–52. doi: 10.1158/1535-7163.MCT-05-0376. [DOI] [PubMed] [Google Scholar]
- 38.Clark LC, Combs GF, Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–63. [PubMed] [Google Scholar]
- 39.Kobuchi H, Roy S, Sen CK, Nguyen HG, Packer L. Quercetin inhibits inducible ICAM-1 expression in human endothelial cells through the JNK pathway. Am J Physiol. 1999;277:C403–11. doi: 10.1152/ajpcell.1999.277.3.C403. [DOI] [PubMed] [Google Scholar]
- 40.Yoshizumi M, Tsuchiya K, Kirima K, Kyaw M, Suzaki Y, Tamaki T. Quercetin inhibits Shc-and phosphatidylinositol 3-kinase-mediated c-Jun N-terminal kinase activation by angiotensin II in cultured rat aortic smooth muscle cells. Mol Pharmacol. 2001;60:656–65. [PubMed] [Google Scholar]
- 41.Yang-Yen HF, Chambard JC, Sun YL, et al. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62:1205–15. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- 42.Yuan H, Pan Y, Young CYF. Overexpression of c-Jun induced by quercetin and resverol inhibits the expression and function of androgen receptor in human prostate cancer cells. Cancer Lett. 2004;213:155–63. doi: 10.1016/j.canlet.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Chung BH, Lee HY, Lee JS, Young CYF. Perillyl alcohol inhibits the expression and function of the androgen receptor in human prostate cancer cells. Cancer Letters. 2006;236:222–8. doi: 10.1016/j.canlet.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Sato N, Sadar MD, Bruchovsky N, et al. Androgenic induction of prostate-specific antigen gene is repressed by protein-protein interaction between the androgen receptor and AP-1/c-Jun in the human prostate cancer cell line LNCaP. J Biol Chem. 1997;272:17485–94. doi: 10.1074/jbc.272.28.17485. [DOI] [PubMed] [Google Scholar]
- 45.Frønsdal K, Engedal N, Slagsvold T, Saatcioglu F. CREB binding, protein is a coactivator for the androgen receptor and mediates cross-talk with AP-1. J Biol Chem. 1998;273:31853–59. doi: 10.1074/jbc.273.48.31853. [DOI] [PubMed] [Google Scholar]


