Abstract
Background
Spinal pain is a common problem, and disability related to spinal pain has great consequence in terms of human suffering, medical costs and costs to society. The traditional approach to the non-surgical management of patients with spinal pain, as well as to research in spinal pain, has been such that the type of treatment any given patient receives is determined more by what type of practitioner he or she sees, rather than by diagnosis. Furthermore, determination of treatment depends more on the type of practitioner than by the needs of the patient. Much needed is an approach to clinical management and research that allows clinicians to base treatment decisions on a reliable and valid diagnostic strategy leading to treatment choices that result in demonstrable outcomes in terms of pain relief and functional improvement. The challenges of diagnosis in patients with spinal pain, however, are that spinal pain is often multifactorial, the factors involved are wide ranging, and for most of these factors there exist no definitive objective tests.
Discussion
The theoretical model of a diagnosis-based clinical decision rule has been developed that may provide clinicians with an approach to non-surgical spine pain patients that allows for specific treatment decisions based on a specific diagnosis. This is not a classification scheme, but a thought process that attempts to identify most important features present in each individual patient. Presented here is a description of the proposed approach, in which reliable and valid assessment procedures are used to arrive at a working diagnosis which considers the disparate factors contributing to spinal pain. Treatment decisions are based on the diagnosis and the outcome of treatment can be measured.
Summary
In this paper, the theoretical model of a proposed diagnosis-based clinical decision rule is presented. In a subsequent manuscript, the current evidence for the approach will be systematically reviewed, and we will present a research strategy required to fill in the gaps in the current evidence, as well as to investigate the decision rule as a whole.
Background
Chronic spinal pain is an increasingly common problem in Western Society [1]. Spinal disorders exact great costs, in terms of both direct medical costs and indirect costs related to disability and lost productivity [1-3]. A number of researchers have attempted to improve our ability to identify the causes of spinal pain as well as to diagnose and treat patients with this problem. In spite of this, accurate diagnosis, leading to specific, targeted treatments, of patients with spinal pain has been elusive.
It has been repeated over the years that only in 15% of patients with spinal pain can a definitive diagnosis be made [4-6]. However, if one surveys the spine literature, one finds a variety of methods for detecting many of the factors that are believed to be of importance, most of which have known reliability and validity, although there are some that do not. Each of these methods may only help the clinician to identify one particular potential contributing factor in the overall clinical picture of the spine pain patient. However, it may be possible that, by utilizing many of the various diagnostic procedures available to the spine clinician, one can develop a specific working diagnosis that encompasses all of the dimensions for which there may be contributing factors and from which a management strategy may be designed that addresses each of the most important factors in each individual patient.
The purpose of this paper is to present the theoretical model of a diagnosis-based clinical decision rule (DBCDR) for the diagnosis and non-surgical management of patients with spinal pain. The model considers a number of known or suspected factors that contribute to the clinical picture and allows for the development of a management strategy that is derived from the multifactorial diagnosis. This paper presents the conceptual model of this clinical decision rule and its application in the clinical setting. In a subsequent manuscript we will systematically review the evidence regarding the components of the approach, and present those areas of research needed to investigate its validity and usefulness to spine clinicians.
Discussion
The three essential questions of diagnosis
The DBCDR is based on what the authors refer to as the 3 essential questions of diagnosis. It is suggested here that the answers to these questions supply the clinician with the most important information required to develop a specific diagnosis from which a management strategy can be derived. The 3 questions are:
The first question of diagnosis: Are the patient's symptoms reflective of a visceral disorder or a serious or potentially life-threatening illness?
There are several diseases for which spinal pain may be among the initial symptoms. It is important for any physician seeing patients with spinal pain to be aware of these disorders and to be skilled enough in differential diagnosis to detect, or at least suspect, their presence. The most important diagnoses are listed in Table 1.
Table 1.
Visceral or potentially serious or life threatening diseases that can present as spinal pain.
| Disorder | Detected by |
| Cancer | Previous history of CA, no position of relief, fever, constitutional symptoms, weight loss |
| Benign tumor | Localized severe pain, no position of relief, dramatic relief with NSAID, pain on percussion |
| Infection | History of fever and/or chills, fever on examination, pinpoint tenderness, redness or heat |
| Fracture | History of trauma, history of osteoporosis, pain on percussion |
| Seronegative spondyloarthropathy | Hx of iritis, AM stiffness, improvement with exercise, family Hx |
| GI disease | GI complaints, relation of pain to certain foods, abdominal examination |
| GU disease | GU complaints, bleeding, spotting, unusual discharge, GU examination |
| Myelopathy | Gait difficulties, bowel/bladder dysfunction, UMN signs, spasticity, sensory level |
| Cauda equina syndrome | Bowel/bladder difficulties, saddle anesthesia, decreased anal sphincter tone |
CA – cancer; NSAID – non-steroidal anti-inflammatory drugs; Hx – history; AM – morning; GI – gastrointestinal; GU – genitourinary; UMN – upper motor neuron
The second question of diagnosis: From where is the patient's pain arising?
In asking this question, the clinician is not necessarily attempting to determine the precise tissue of origin, but rather is trying to identify characteristics about the pain source that allow treatment decisions to be made. There are a number of tissues in the spine that have the potential to generate pain. However, while a few studies have suggested that clinical factors can be used to detect specific pain generators in some instances [7,8], to a large extent, methods that allow clinicians to make an unequivocal tissue-specific diagnosis have been elusive. There is good evidence, however, that historical factors and examination procedures can allow one to identify certain characteristics in each individual patient that may be useful in making treatment decisions. Proposed here are 4 signs of greatest importance in seeking the answer to the second question of diagnosis (Table 2). A variety of clinical tests have been developed that allow the clinician to attempt to identify the origin of the patient's pain. Part 2 of this paper reviews the reliability and validity of these clinical procedures.
Table 2.
Proposed pain provocation signs and their means of detection.
| Pain Provocation Sign | Detection | Suspected Source |
| Segmental Pain Provocation Signs | Palpation, pain provocation tests | Zygapophyseal joint |
| Centralization Signs | End range loading examination | Intervertebral disc |
| Neurodynamic Signs | Neurodynamic Tests | Neural structures |
| Muscle Palpation Signs | Palpation | Myofascial tissues |
Centralization signs
Centralization signs are detected through methods originally developed by McKenzie [9,10]. The examination procedure involves moving the spine to end range in various directions and monitoring the mechanical and symptomatic response to these movements. Traditionally, the findings of this examination have been thought to identify the intervertebral disc as the source of pain, and some experimental evidence supports this [11-13], though further work in this area is needed. Nonetheless, the centralization sign has been demonstrated to be useful in prognosis [14] and in helping the clinician to make decisions regarding the best form of treatment for this particular aspect of the clinical picture [15].
Segmental pain provocation signs
Examination for pain provocation signs involves the clinician attempting to reproduce the patient's pain by apply maneuvers designed to stress segmental tissues. In the cervical, thoracic and lumbar spine, this involves segmental palpation. This palpation is designed to assess for pain response, not necessarily movement abnormality [16-25]. In the sacroiliac area, pain provocation tests are used [8,13,26], and the usefulness of these tests is enhanced when they are performed in conjunction with examination for centralization signs [27] (see below). Some evidence suggests that these signs are reflective of pain arising from joint structures [8,24,28]. However, as will be seen with the other signs discussed here, it is interesting, and sometimes useful, to speculate about the precise pain generating tissue responsible for producing pain with segmental palpation, but it is not necessary to know the precise pain generating tissue in making treatment decisions based on the identification of these signs.
Neurodynamic signs
Neurodynamic signs involve the reproduction of pain resulting from tests designed to apply stress to neural structures. In the spine, neurodynamic signs most likely arise from radicular pain, most commonly arising from lateral canal stenosis and disc protrusion [29,30]. Neurodynamic signs are derived from clinical neurodynamic tests [31] and other pain provoking procedures [32]. These can be supported with historical factors as well as neurologic examination [7].
Muscle palpation signs
Muscle palpation signs involve the reproduction of pain from direct palpation of muscles. These signs have typically been thought to implicate the presence of myofascial trigger points (TrPs) [33]. Again, as with other pain generating signs, knowing the precise mechanism is not absolutely necessary in order to make diagnostic and treatment decisions.
Pain referral pattern maps from various muscles have been developed [33] and are in popular use, although only a few of these have been investigated for validity [34,35]. Knowledge of these referred pain patterns, and comparing them with the pain pattern described by the patient, may allow the clinician to make decisions regarding which muscles should be examined with palpation.
The third question of diagnosis: What has gone wrong with this person as a whole that would cause the pain experience to develop and persist?
With this question, the clinician attempts to determine if there are any factors other than the pain generating tissue that serves to maintain or perpetuate the pain experience. It would appear that an essential aspect to effective management of patients with spinal pain would include the identification and management of those factors that place the acute or subacute spinal pain patient at risk of developing ongoing problems or, in the case of the chronic or recurrent spinal pain patient, that contribute to the perpetuation of pain and dysfunction. Table 3 lists the factors believed to be of greatest importance according to current evidence. These factors encompass biomechanical, neurophysiological and psychological processes.
Table 3.
Factors presumed to be of greatest importance in the perpetuation of spinal pain.
| Dynamic Instability (impaired motor control) | Fear |
| Oculomotor dysfunction | Catastrophizing |
| Central pain hypersensitivity | Passive coping |
| Depression |
Dynamic instability (impaired motor control)
Several studies have demonstrated alteration in motor control strategies in patients with spinal pain [36-38]. It is believed that this altered motor control leads to decreased stability of the spine [39-41], thus predisposing the spine to injury and perpetuating chronic spinal pain. Several clinical examination procedures have been suggested to identify impaired motor control in patients with pain in the cervical spine [36,42-46], the lumbar spine [47,48] pelvis [49].
Central pain hypersensitivity (CPH)
CPH is a state in which an alteration has occurred in the manner in which nociceptive information is received, processed and modulated, which serves to heighten the pain experience [50-53]. Increased pain sensitivity and decreased pain thresholds have been found in patients with chronic neck pain [54-56], chronic low back pain (LBP) [57] and chronic headache [58].
The examination for Waddell's nonorganic signs [59] is a popular procedure that was developed for the purpose of identifying a behavioral component of the clinical picture in patients with LBP. A recent systematic review of the literature by Fishbain, et al [60] found good evidence to suggest that these signs are associated with heightened pain perception.
Oculomotor dysfunction
Many studies have found impairment of oculomotor reflexes in patients with chronic neck pain after trauma [61-67] and in patients with chronic tension type headache [68]. Also, treatments aimed at improving oculomotor function have been found to be useful in decreasing neck pain and neck pain-related disability [69,70].
Simple and practical tests for oculomotor reflex function for use in the typical clinical setting are nonexistent. However, Heikklla and Wenngren [63] found significant correlation between the finding of poor performance on oculomotor tests and a test for head repositioning accuracy. Head repositioning accuracy can be measured in the clinic [71].
Fear and catastrophizing
Both fear and catastrophizing have been shown to be important predictors of present pain intensity and disability [72,73] and of future chronicity [74-77]. Several instruments have been validated for measuring these factors [73,78-80].
Passive coping
As with fear and catastrophizing, passive coping has been shown to contribute to present disability [81] and to predict future disability [82] and can be measured in the clinic [83].
Depression
As with the psychological factors discussed previously, depression has been shown to contribute to present disability [75,81] and predict future disability [84-86]. It can be measured via questionnaire [87].
Arriving at a diagnosis and formulating a management strategy
Arriving at a diagnosis
The DBCDR is not a classification method. It is a diagnostic method. However, the "diagnosis", using the DBCDR, is not a traditional diagnosis, in which a diagnostic label is given to a disease entity based on the unique pathophysiology of the particular entity. Rather, it is a collection of signs, sometimes single and sometimes multiple, from which the clinician can make treatment decisions. Also, because of the absence of definitive findings on tests such as imaging in the majority of spinal pain patients, the diagnosis using the DBCDR is a working hypothesis that is tested through treatment. (Figure 1)
Figure 1.
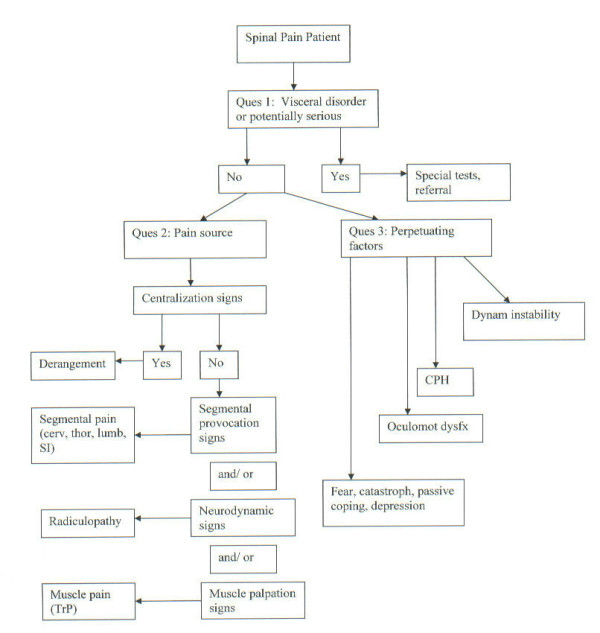
Diagnostic algorithm for the application of the DBCDR.
Some examples of diagnoses using the DBCDR might be:
1. Segmental pain provocation signs (question #2) and significant fear beliefs (question #3); rule out infection (question #1).
2. Centralization signs (question #2) with impaired motor control and CPH (question #3).
3. Segmental pain provocation signs with neurodynamic signs (question #2) and fear and catastrophizing (question #3); rule out myelopathy (question #1).
Formulating a management strategy
Once the clinician has established a working diagnosis (based on the answers to the 3 questions), a management strategy would be developed. An important concept of management in this model is that none of the important factors that may be present in any given spinal pain patient occurs in isolation. Pain generators and perpetuating factors interact in producing the clinical picture that practitioners see. (Figure 2)
Figure 2.
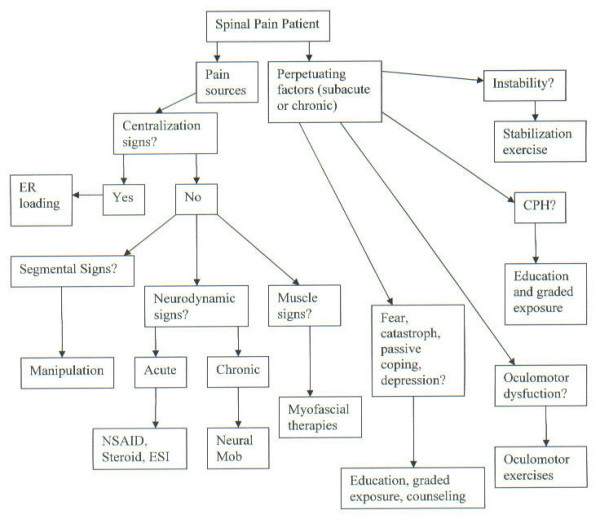
Management algorithm for the application of the DBCDR.
In the model presented here, these are the management decisions that the clinician might make based on the findings of the diagnostic process:
Management strategy in response to Question #1
Any significant findings suggestive of visceral disease or serious or potentially life threatening illness would be investigated with further diagnostic work up and/or referral.
Management strategy in response to Question #2
Centralization signs
The recommended treatment of choice for centralization signs is end range loading maneuvers in the direction of centralization [15], which is part of the McKenzie method [10]. Because centralization signs can be addressed with exercises and self-care strategies that patients can apply themselves, these signs are always addressed first, regardless of the presence of other signs.
The DBCDR does not depend on a known tissue of origin of the patient's pain; however, as centralization signs may be associated with disc pain in the lumbar spine [11], another treatment that may have potential in the presence of these signs is distraction manipulation [88], a type of manipulation that utilizes a special treatment table and which has been shown to reduce intradiscal pressure [89,90]. However, whether and to what extent distraction manipulation may add to self- or practitioner-applied end range loading maneuvers has not been investigated.
Segmental pain provocation signs
In the context of the DBCDR, it is recommended that in patients with segmental pain provocation signs as well as centralization signs, end range loading maneuvers be utilized first, with no action being taken regarding the segmental pain provocation signs until end range loading has been fully explored. In patients with segmental pain provocation signs who do not exhibit centralization signs, manipulation is the recommended treatment. The rationale for this is that it is known that manipulation has segmental effects, [91,92] and segmental hypomobility form a component of a prediction rule for those patients with LBP who are most likely to benefit from manipulation [93]. Because of this, it seems reasonable to consider manipulation as a treatment of choice in the presence of segmental pain signs, and to explore this from a research perspective.
Zygapophyseal [94] or sacroiliac joint [95] injection may also be helpful in patients with segmental pain provocation signs. Radiofrequency denervation may be a last-resort treatment for patients with segmental pain provocation signs[96].
Neurodynamic signs
In the acute stage, especially with disc protrusion, radicular pain is thought to be largely chemical, as a result of inflammation [97]. As such, anti-inflammatory measures would appear to be a useful first line approach. These can come in the form of general approaches such as non-steroidal anti-inflammatory medications (NSAIDs) or oral steroids [98,99]. Epidural steroid injection (ESI) is more specific to radiculopathy [100]; selective nerve root anesthetic block is also an option for radiculopathy patients [101,102]. An anti-inflammatory diet [103] may also be beneficial.
In chronic stages, many patients (one study of lumbar radiculopathy patients [104] suggested approximately 50%) will exhibit centralization signs, in which cases end range loading in the direction of centralization is the recommended first-line approach. In those who do not exhibit centralization signs, or who have residual radicular symptoms, a treatment approach that holds promise is neural mobilization [31] which attempts to mobilize the involved nerve root to improve its mechanics and decrease its sensitivity [105-107]. Distraction manipulation is another option in patients with lumbar radiculopathy [107,108].
Muscle palpation signs
A multitude of treatments has been recommended for pain apparently arising from muscle, ranging from manual techniques such as ischemic compression [109], to muscle lengthening techniques [110], to trigger point injections.
Management strategy in response to Question #3
Dynamic instability (impaired motor control)
Many methods have been recommended to improve motor control in the spine, usually involving "stabilization exercises" [40,111] that may utilize simple floor exercises, or low-technology equipment such as gymnastic balls, or high-technology equipment. General fitness or strength training exercise may also be of benefit [112,113].
Central pain hypersensitivity
As CPH requires ongoing peripheral nociceptive input for its perpetuation [53], effectively treating sources of this nociceptive input (i.e., responding to questions #2), would appear to be useful in addressing CPH.
Also, as CPH is a state in which the nociceptive system has become, in effect, hypersensitive, it would appear that a useful treatment would involve a desensitization process. It may be possible to accomplish this through a graded exposure approach [114].
Oculomotor dysfunction
It appears that oculomotor dysfunction occurs particularly in patients with whiplash injury [61,69] and tension-type headache [68] and is treated with exercises designed to train eye-head-neck movements [115,116].
Fear, catastrophizing, passive coping and depression
Evidence suggests that fear and catastrophizing are directly related to CPH [117,118]. In addition, it appears that passive coping and depression are a part of an overall psychological response on the part of the patient that has a detrimental effect on their ability to recover and resume normal activities [75]. Thus, in the context of the DBCDR, the recommended response to this would be to educate the patient about the presence and nature of CPH. This may reduce fear and catastrophizing, and encourage the patient to take a more active approach to coping, which would, in turn, positively impact depression. This education must then be followed by the graded exposure approach discussed earlier. It should be noted that fear, catastrophizing, passive coping and depression often improve with purely somatic-based treatments, when these treatments are successful in reducing pain [14,119-121].
In some patients in which the fear, catastrophizing, passive coping and depression are not overcome with the interventions discussed above, [75], further intervention by a psychologist or psychiatrist may be necessary.
A comparison of the DBCDR with "classification" schemes for patients with spinal pain
Classically, the diagnosis of spinal pain has revolved around attempting to make a tissue-specific diagnosis based on pathoanatomy. The assumption was that if someone's back or neck hurts, it is the job of the practitioner to identify the tissue that is painful and the pathoanatomic or pathophysiologic process that is producing pain. Once this tissue and this process are identified, the diagnosis can be made. Diagnostic methods such as radiographs, CT, MRI and EMG were developed for this purpose. This is the way diagnoses are made in other areas of medicine, such as with GI or cardiac disorders. However, as research attempted to investigate better ways to make this pathoanatomic diagnosis, it was found that 1) many pathoanatomic entities occur in the absence of spinal pain and 2) many people with spinal pain do not have identifiable pathoanatomic or pathophysiologic processes that would explain their pain.
This led to the categorization of patients into 2 groups – "specific" spinal pain, i.e., those in whom a reliable and valid pathoanatomic diagnosis can be made, and "non-specific", i.e., those in whom the source of pain could not be identified. In recent years, a response to this limited categorization has come in the form of attempting to identify "subgroups" of spinal pain patients. This has resulted in various "classification systems" that have attempted to identify common characteristics in groups of spinal pain patients in the hope that certain treatments can be targeted to certain classifications of patients.
The Quebec Task Force on Spinal Disorders [6] developed a classification system based on signs and symptoms, imaging findings and response to treatment. This system was found to have some utility with regard to surgical decision making [122], it was still limited with regard to helping clinicians make specific treatment decisions in the majority of patients.
One classification system is that of McKenzie [10]. In this classification, three "syndromes" are considered. The clinician attempts to identify in each patient which of these syndromes is present, so that treatment can be applied that is appropriate for that syndrome.
According to McKenzie, the largest of these is the "derangement syndrome". In this group of patients, end range loading maneuvers are used to identify a characteristic pattern of "centralization" of symptoms when loading maneuvers are applied in a certain direction, and "peripheralization" of symptoms when loading maneuvers are applied in another direction (typically the direction opposite of that which produced centralization).
The McKenzie system, at least as it applies to the derangement syndrome, has been found to be efficacious for those patients for whom it applies [15,123,124]. However, only a limited (though sizable) percentage of patients with LBP have derangement. It has been estimated that approximately 70% of patients with acute LBP and approximately 50% with chronic LBP centralize with end range loading maneuvers, thus being classified as "derangement" [125]. It is unknown what percentage of patients with acute and chronic neck pain and thoracic pain can be classified as having derangement. It is also unknown what percentage of spinal pain patients can be classified as having the dysfunction and postural syndromes, and, while the reliability of these classifications have been found to be good [126-128], the validity is unknown.
So it appears that the McKenzie classification is useful in identifying at least one aspect of a diagnosis in most spinal pain patients. However, half the chronic LBP patients and an unknown percentage of cervical and thoracic pain patients do not have derangements and require some other methods with which to make a diagnosis. Nonetheless, the McKenzie approach plays a key role in the DBCDR presented here.
Another classification system was initially developed by Delitto [129] and used historical factors, symptom behavior and clinical signs to categorize spinal pain patients. This system evolved into one in which patients with LBP are placed into one of four categories [130]:
1. Immobilization
2. Mobilization
3. Specific exercise
4. Traction
A similar classification system was developed for neck pain patients by Childs, et al [131]. In this system, patients are classified into 5 categories [131]:
1. Mobility
2. Centralization
3. Conditioning and increased exercise tolerance
4. Pain control
5. Reduce headache
So, with these classification schemes, patients are categorized into one of four or five groups, rather than into one of two groups (i.e., "specific" and "nonspecific"). Evidence suggests that this has been a significant step forward, as patients with LBP who are treated according to this classification scheme have better outcomes than patients who are treated according to the two-category scheme [130]. It is unknown how this classification scheme applies to the cervical spine.
While this classification is a significant advancement in that it attempts to identify those patients who are most likely to respond to specific treatments, it has its limitations. For example, patients with LBP are placed in the "mobilization" (or manipulation) category based on the following features [93]:
1. Symptom duration < 16 days
2. No symptoms distal to knee
3. < 19 on a Fear-Avoidance Beliefs Q
4. At least 1 hypomobile lumbar segment
5. At least 1 hip with > 35 degrees of internal rotation
In the classification system for the cervical spine, patients are placed in the "mobility" category based on these features:
1. Recent onset of symptoms
2. No radicular or referred symptoms into the upper quarter
3. Restricted range of motion (ROM)
4. No signs of nerve root compression or peripheralization of symptoms with ROM
Evidence suggests that there are many patients who respond positively to manipulation who do not fit into these groups. For example, patients with chronic LBP and neck pain often respond to manipulation [132], as do many with radiculopathy or pain below the knee [106,107,133].
The proposed DBCDR is different from these other systems in that it is not a classification system; there are no classifications in which patients are placed. Rather, those factors that are known or suspected to contribute to the clinical picture of spinal pain and all are considered in each patient, with the recognition that many patients have a variety of factors involved in his or her clinical picture, and thus defy easy classification. It also attempts to find those clinical features that play a role in each individual case, and apply treatments designed to address those features. Thus, the clinician in not limited to 3, 4 or 5 classifications, but is free to manage each patient according to those clinical features that are deemed most relevant in each case. Further research is needed to determine whether the DBCDR is truly a useful "rule" in helping clinicians make diagnostic and treatment decisions.
Summary
The traditional management of patients with spine related disorders has typically been driven more by the training of the individual clinician than the needs of the patient. Research on treatments for spinal pain has placed patients into homogeneous groups, as if all patients had the same diagnosis. This has led to the conclusion among many that no treatment for spinal pain has a great deal to offer [5,134]. A novel approach to the diagnosis and management of patients with spine-related disorders is presented that may help to improve the precision of medical decision making and thus improve treatment outcome. It is based on what the authors refer to as the 3 essential questions of diagnosis.
The answers to these questions may allow the clinician to develop a working diagnosis from which a management strategy can be developed. This strategy is designed to address the most important factors suspected to be contributing in each case.
The theoretical model of this proposed diagnosis-based clinical decision rule is presented here. In a subsequent paper, the evidence as is currently exists related to this model will be systematically reviewed and a research strategy will be presented, the purpose of which is to determine whether the model has sufficient reliability, validity and efficacy to recommend it as an alternative approach to patients with spinal pain.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
DRM conceived of the idea of the diagnosis-based clinical decision rule and was the principal author of the manuscript.
ELH was responsible for help with design and presentation and with conceptualization of the presented research strategy.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The authors would like to thank Tovah Reis of the Brown University library and Mary Ott of the New York Chiropractic College library for help with information gathering.
Contributor Information
Donald R Murphy, Email: rispine@aol.com.
Eric L Hurwitz, Email: ehurwitz@hawaii.edu.
References
- Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Pai S, Sundaram LJ. The economic burden of low back pain: a review of studies published between 1996 and 2001. Orthop Clin North Am. 2004;35:1–5. doi: 10.1016/S0030-5898(03)00101-9. [DOI] [PubMed] [Google Scholar]
- Maetzel A, Li L. The economic burden of low back pain: a review of studies published between 1996 and 2001. Best Pract Res Clin Rheumatol. 2002;16:23–30. doi: 10.1053/berh.2001.0204. [DOI] [PubMed] [Google Scholar]
- Mooney V. Where is the pain coming from? Spine. 1987;12:754–759. doi: 10.1097/00007632-198710000-00008. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Weinstein JN. Low back pain. New Eng J Med. 2001;344:363–370. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- Spitzer WO. Scientific approach to the assessment and management of activity-related spinal disorders: A monograph for clinicians. Report of the Quebec Task Force on Spinal Disorders. Spine. 1987;12:1–59. [PubMed] [Google Scholar]
- Wainner RS, Fritz JM, Irrgang JJ, Boninger ML, Delitto A, Allison S. Reliability and diagnostic accuracy of the clinical and patient self report measures for cervical radiculopathy. Spine. 2003;1:52–62. doi: 10.1097/00007632-200301010-00014. [DOI] [PubMed] [Google Scholar]
- Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10:207–218. doi: 10.1016/j.math.2005.01.003. [DOI] [PubMed] [Google Scholar]
- McKenzie RA. The Cervical and Thoracic Spine: Mechanical Diagnosis and Therapy. Waikanae, New Zealand: Spinal Publications; 1990. [Google Scholar]
- McKenzie RA, May S. The Lumbar Spine: Mechanical Diagnosis and Therapy. 2. Waikenae, NZ: Spinal Publications; 2003. [Google Scholar]
- Donelson R, Aprill C, Medcalf R, Grant W. A prospective study of centralization of lumbar and referred pain a predictor of symptomatic discs and anular competence. Spine. 1997;22:1115–1122. doi: 10.1097/00007632-199705150-00011. [DOI] [PubMed] [Google Scholar]
- Laslett M, Birgitta O, Aprill CN, McDonald B. Centralization as a predictor of provocation discography results in chronic low back pain, and the influence of disability and distress on diagnostic power. Spine J. 2005;5:370–380. doi: 10.1016/j.spinee.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Young S, Aprill C, Laslett M. Correlation of clinical examination characteristics with three sources of chronic low back pain. Spine J. 2003;3:460–465. doi: 10.1016/S1529-9430(03)00151-7. [DOI] [PubMed] [Google Scholar]
- Werneke M, Hart DL. Centralization phenomenon as a prognostic factor for chronic low back pain and disability. Spine. 2001;26:758–764. doi: 10.1097/00007632-200104010-00012. [DOI] [PubMed] [Google Scholar]
- Long A, Donelson R, Fung T. Does it matter which exercise? A randomized control trial of exercise for low back pain. Spine. 2004;29:2593–2602. doi: 10.1097/01.brs.0000146464.23007.2a. [DOI] [PubMed] [Google Scholar]
- Jull G, Zito G, Trott P, Potter H, Shirley D. Inter-examiner reliability to detect painful upper cervical joint dysfunction. Aust Physiother. 1997;43:125–129. doi: 10.1016/s0004-9514(14)60406-2. [DOI] [PubMed] [Google Scholar]
- Hubka MJ, Phelan SP. Interexaminer reliability of palpation for cervical spine tenderness. J Manipulative Physiol Ther. 1994;17:591–595. [PubMed] [Google Scholar]
- Marcus DA, Scharff L, Mercer S, Turk DC. Musculoskeletal abnormalities in chronic headache a controlled comparison of headache diagnostic groups. Headache. 1999;39:21–27. doi: 10.1046/j.1526-4610.1999.3901021.x. [DOI] [PubMed] [Google Scholar]
- van Suijlekom HA, de Vet HCW, van den Berg SGM, Weber WEJ. Interobserver reliability on physical examination of the cervical spine in patients with headache. Headache. 2000;40:581–586. doi: 10.1046/j.1526-4610.2000.00090.x. [DOI] [PubMed] [Google Scholar]
- Maher C, Adams R. Reliability of pain and stiffness assessments in clinical manual lumbar spine examination. Phys Ther. 1994;74:801–809. doi: 10.1093/ptj/74.9.801. [DOI] [PubMed] [Google Scholar]
- Keating JC, Bergmann TF, Jacobs GE, Finer BA, Larson K. Inter-examiner reliability of eight evaluative dimensions of lumbar segmental abnormality. J Manipulative Physiol Ther. 1990;13:463–470. [PubMed] [Google Scholar]
- Seffinger MA, Najm WI, Mishra SI, Adams A, Dickerson VM, Murphy LS, Reinsch S. Reliability of spinal palpation for diagnosis of back and neck pain. Spine. 2004;29:E413–E424. doi: 10.1097/01.brs.0000141178.98157.8e. [DOI] [PubMed] [Google Scholar]
- Treleaven J, Jull G, Atkinson L. Cervical musculoskeletal dysfunction in post-concussional headache. Cephalalgia. 1994;14:273–279. doi: 10.1046/j.1468-2982.1994.1404273.x. [DOI] [PubMed] [Google Scholar]
- Jull G, Bogduk N, Marsland A. The accuracy of manual diagnosis for cervical zygapophysial joint pain syndromes. Med J of Australia. 1988;148:233–236. doi: 10.5694/j.1326-5377.1988.tb99431.x. [DOI] [PubMed] [Google Scholar]
- Sandmark H, Nisell R. Validity of five manual neck pain provokation tests. Scand J Rehab Med. 1995;27:131–136. [PubMed] [Google Scholar]
- Laslett M, Williams M. The reliability of selected pain provocation tests for sacroiliac joint pathology. Spine. 1994;19:1243–1249. doi: 10.1097/00007632-199406000-00009. [DOI] [PubMed] [Google Scholar]
- Laslett M, Young SB, Aprill CN, McDonald B. Diagnosing painful sacroiliac joints: A validity study of a McKenzie evaluation and sacroiliac provocation tests. Aus J Physiother. 2003;49:89–97. doi: 10.1016/s0004-9514(14)60125-2. [DOI] [PubMed] [Google Scholar]
- Lord SM, Barnsley L, Wallis BJ, Bogduk N. Third occipital nerve headache: a prevalence study. J Neurol Neurosurg Psychiatr. 1994;57:1187–1190. doi: 10.1136/jnnp.57.10.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan K, Litchy WJ, O'Fallon WM, Kurland LT. Epidemiology of cervical radiculopathy A population-based study from Rochester, Minnesota, 1976 through 1990. Brain. 1994;117:325–335. doi: 10.1093/brain/117.2.325. [DOI] [PubMed] [Google Scholar]
- Hurwitz EL, Shekelle P. Epidemiology of low back syndromes. In: Morris CE, editor. Low Back Syndromes: Integrated Clinical Management. New York: McGraw-Hill; 2006. pp. 83–118. [Google Scholar]
- Shacklock M. Clinical Neurodynamics A New System of Musculoskeletal Treatment. Edinburgh: Elsevier; 2005. [Google Scholar]
- Wainner RS, Gill H. Diagnosis and nonoperative management of cervical radiculopathy. J Orthop Sports Phys Ther. 2000;30:728–744. doi: 10.2519/jospt.2000.30.12.728. [DOI] [PubMed] [Google Scholar]
- Travell JG, Simons DG. Myofascial Pain and Dysfunction: The Trigger Point Manual. Baltimore: Williams and Wilkens; 1983. [Google Scholar]
- Cornwall J, Harris AJ, Mercer SR. The lumbar multifidus muscle and patterns of pain. Manual Ther. 2006;11:40–45. doi: 10.1016/j.math.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Hwang M, Kang YK, Kim DH. Referred pain pattern of the pronator quadratus muscle. Pain. 2005;116:238–242. doi: 10.1016/j.pain.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Falla DL, Jull GA, Hodges PW. Patients with neck pain demonstrate reduced electromyographic activity of the deep cervical flexor muscles during performance of the craniocervical flexion test. Spine. 2004;29:2108–2113. doi: 10.1097/01.brs.0000141170.89317.0e. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain a motor control evaluation of transversus abdominis. Spine. 1996;21:2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- Hungerford B, Gilleard W, Hodges P. Evidence of altered lumbopelvic muscle recruitment in the presence of sacroiliac joint pain. Spine. 2003;28:1593–1600. doi: 10.1097/00007632-200307150-00022. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, McGill SM. Mechanical stability of the in vivo lumbar spine implications for injury and chronic low back pain. Clin Biomech. 1996;11:1–15. doi: 10.1016/0268-0033(95)00035-6. [DOI] [PubMed] [Google Scholar]
- McGill S. Low Back Disorders Evidence-Based Prevention and Rehabilitation. Champaign, IL: Human Kinetics; 2002. [Google Scholar]
- O'Sullivan PB. Lumbar segmental 'instability' clinical presentation and specific stabilizing exercise management. Man Ther. 2000;5:2–12. doi: 10.1054/math.1999.0213. [DOI] [PubMed] [Google Scholar]
- Jull GA. Deep cervical flexor muscle dysfunction in whiplash. J Musculoskel Pain. 2000;8:143–154. doi: 10.1300/J094v08n01_12. [DOI] [Google Scholar]
- Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R. Development of motor system dysfunction following whiplash injury. Pain. 2003;103:65–73. doi: 10.1016/S0304-3959(02)00420-7. [DOI] [PubMed] [Google Scholar]
- Jull G, Kristjansson E, Dall' Alba P. Impairment in the cervical flexors a comparison of whiplash and insidious onset neck pain patients. Man Ther. 2004;9:89–94. doi: 10.1016/S1356-689X(03)00086-9. [DOI] [PubMed] [Google Scholar]
- Falla D, Bilenkij G, Jull G. Patients with chronic neck pain demonstrate altered patterns of muscle activation during performance of a functional upper limb task. Spine. 2004;29:1436–1440. doi: 10.1097/01.BRS.0000128759.02487.BF. [DOI] [PubMed] [Google Scholar]
- Falla D, Jull G, Dall'Alba P, Rainoldi A, Merletti R. An electromyographic analysis of the deep cervical flexor muscles in performance of craniocervical flexion. Phys Ther. 2003;83:899–906. [PubMed] [Google Scholar]
- Hicks GE, Fritz JM, Delitto A, Mishock J. Interrater reliability of clinical examination measures for identification of lumbar segmental instability. Arch Phys Med Rehabil. 2003;84:1858–64. doi: 10.1016/S0003-9993(03)00365-4. [DOI] [PubMed] [Google Scholar]
- Murphy DR, Byfield D, McCarthy P, Humphreys BK, Gregory AA, Rochon R. The hip extension test for suspected impaired motor control of the lumbar spine: a study of interexaminer reliability. J Manipulative Physiol Ther. 2006;29:374–377. doi: 10.1016/j.jmpt.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Mens JMA, Vleeming A, Snijders CJ, Stam HJ. Active straight leg raising test: a clinical approach to the load transfer function of the pelvic girdle. In: Vleeming A, Mooney V, Snijders CJ, Dorman TA, Stoeckart R, editor. Movement, Stability and Low Back Pain The Essential Role of the Pelvis. New York: Churchill Livingstone; 1997. pp. 425–431. [Google Scholar]
- Sterling M, Treleaven J, Edwards S, Jull G. Pressure pain thresholds in chronic whiplash associated disorder further evidence of altered central pain processing. J Musculoskel Pain. 2002;10:69–81. doi: 10.1300/J094v10n03_05. [DOI] [Google Scholar]
- Sterling M, Jull G, Vicenszino B, Kenardy J. Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain. 2003;104:509–517. doi: 10.1016/S0304-3959(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Curatolo M, Arendt-Nielsen L, Petersen-Felix S. Evidence, mechanisms, and clinical implications of central hypersensitivity in chronic pain after whiplash injury. Clin J Pain. 2004;20:469–476. doi: 10.1097/00002508-200411000-00013. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Banic B, Peterson-Felix S, Andersen OK, Radanov BP, Villiger PM, Arendt-Nielsen L, Curatolo M. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 2004;107:7–15. doi: 10.1016/j.pain.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Herren-Gerber R, Weiss S, Arendt-Nielsen L, Petersen-Felix S, Di Stefano, Radanov Bp, Curatolo M. Modulation of central hypersensitivity by nociceptive input in chronic pain after whiplash injury. Pain Med. 2004;5:366–376. doi: 10.1111/j.1526-4637.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- Scott D, Jull G, Sterling M. Widespread sensory hypersensitivity is a feature of chronic whiplash-associated disorder but not chronic idiopathic neck pain. Clin J Pain. 2005;21:175–181. doi: 10.1097/00002508-200503000-00009. [DOI] [PubMed] [Google Scholar]
- Giesbrecht RJSB, Michele C. A comparison of pressure pain detection thresholds in people with chronic low back pain and volunteers without pain. Phys Ther. 2005;85:1085–1092. [PubMed] [Google Scholar]
- Bendsten L. Central sensitization in tension-type headache-possible pathophysiological mechanisms. Cephalagia. 2000;20 doi: 10.1046/j.1468-2982.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- Waddell G, McCulloch JA, Kummel E, Venner RM. Non-organic physical signs in low back pain. Spine. 1980;5:117–125. doi: 10.1097/00007632-198003000-00005. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cole B, Cutler RB, Lewis J, Rosomoff HL, Rosomoff RS. A structured evidence-based review on the meaning of nonorganic physical signs Waddell Signs. Pain Med. 2003;4:141–181. doi: 10.1046/j.1526-4637.2003.03015.x. [DOI] [PubMed] [Google Scholar]
- Gimse R, Tjell C, Bjorgen I, Saunte C. Disturbed eye movements after whiplash due to injuries to posture control system. J Clin Exp Neuropsychol. 1996;18:178–186. doi: 10.1080/01688639608408273. [DOI] [PubMed] [Google Scholar]
- Gimse R, Bjorgen I, Tjell C, Tyssedal J, Bo K. Reduced cognitive functions in a group of whiplash patients with demonstrated disturbances in the posture control system. J Clin Exp Neuropsychol. 1997;19:838–849. doi: 10.1080/01688639708403764. [DOI] [PubMed] [Google Scholar]
- Heikkila HV, Wenngren BI. Cervicocephalic kinesthetic sensibility, active range of cervical motion, oculomotor function in patients with whiplash injury. Arch Phys Med Rehabil. 1998;79:1089–1094. doi: 10.1016/S0003-9993(98)90176-9. [DOI] [PubMed] [Google Scholar]
- Treleaven J, Jull G, LowChoy N. Smooth pusuit neck torsion test in whiplash-associated disorders: relationship to self-reports of neck pain and disability, dizziness and anxiety. J Rehabil Med. 2005;37:219–223. doi: 10.1080/16501970510027989. [DOI] [PubMed] [Google Scholar]
- Wenngren BI, Pettersson K, Lownhielm G, Hildingsson Eye mobility and auditory brainstem response dysfunction after whiplash injury. Acta Otolaryngol. 2002;122:276–283. doi: 10.1080/000164802753648150. [DOI] [PubMed] [Google Scholar]
- Hildingsson C, Wenngren B, Bring G, Toolanen G. Oculomotor problems after cervical spine injury. Acta Orthop Scand. 1989;60:513–516. doi: 10.3109/17453678909150113. [DOI] [PubMed] [Google Scholar]
- Hildingsson C, Wenngren B, Bring G, Toolanen G. Eye motility dysfunction after soft tissue injury of the cervical spine a controlled prospective study of 38 patients. Acta Orthop Scand. 1993;64:129–132. doi: 10.3109/17453679308994552. [DOI] [PubMed] [Google Scholar]
- Rosenhall U, Tjell C, Carlsson J. The effect of neck torsion on smooth pursuit eye movements in tension-type headache patients. J Audiol Med. 1996;5:130–140. [Google Scholar]
- Humphreys BK, Irgens PM. The effect of a rehabilitation exercise program on head repositioning accuracy and reported levels of pain in chronic neck pain subjects. Whiplash Rel Disord. 2002;1:99–112. doi: 10.1300/J180v01n01_09. [DOI] [Google Scholar]
- Fitz-Ritson D. Phasic exercises for cervical rehabilitation after "whiplash" trauma. J Manipulative Physiol Ther. 1995;18:21–24. [PubMed] [Google Scholar]
- Revel M, Andre-Deshays C, Minguet M. Cervicocephalic kinesthetic sensibility in patients with cervical pain. Arch Phys Med Rehabil. 1991;72:288–291. [PubMed] [Google Scholar]
- Severeijns R, Vlaeyen JW, van den Hout MA, Weber WE. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin J Pain. 2001;17:165–172. doi: 10.1097/00002508-200106000-00009. [DOI] [PubMed] [Google Scholar]
- Swinkels-Meewisse IEJ, Roelofs J, Oostendorp RAB, Verbeek ALM, Vlaeyen JWS. Acute low back pain: pain-related fear and pain catastrophizing influence physical performance and perceived disability. Pain. 2006;120:36–43. doi: 10.1016/j.pain.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Nederhand MJ, IJzerman MJ, HErmens HJ, Turk DC, Zilvold G. Predictive Value of fear avoidance in developing chronic neck pain disability consequences for clinical decision making. Arch Phys Med Rehabil. 2004;85:496–501. doi: 10.1016/j.apmr.2003.06.019. [DOI] [PubMed] [Google Scholar]
- Boersma K, Linton S. Psychological processes underlying the development of a chronic pain problem. A prospective study of the relationship between profiles of psychological variables in the fear-avoidance model and disability. Clin J Pain. 2006;22:160–166. doi: 10.1097/01.ajp.0000159582.37750.39. [DOI] [PubMed] [Google Scholar]
- Truchon M, Cote D. Predictive validity of the chronic pain coping inventory in subacute low back pain. Pain. 2005;116:205–212. doi: 10.1016/j.pain.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Gheldof ELM, Vinck J, Blaeyen JWS, Hidding A, Crombes G. The Differential role of pain, work characteristics and pain-related fear in explaining back pain and sick leave in occupational settings. Pain. 2005;113:71–81. doi: 10.1016/j.pain.2004.09.040. [DOI] [PubMed] [Google Scholar]
- Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A fear-avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52:157–168. doi: 10.1016/0304-3959(93)90127-B. [DOI] [PubMed] [Google Scholar]
- Crowley D, Kendall NAS. Development and initial validation of a questionnaire for measuring fear-avoidance associated with pain the fear-avoidance of pain scale. J Musculoskel Pain. 1999;7:3–20. doi: 10.1300/J094v07n03_02. [DOI] [Google Scholar]
- Stewart MW, Harvey ST, Evan IM. Coping and catastrophizing in chronic pain: A psychometric analysis and comparison of two measures. J Clin Psychol. 2001;37:131–138. doi: 10.1002/1097-4679(200101)57:1<131::AID-JCLP13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Samwel HJA, Evers AWM, Crul BJP, Kraaimaat FW. The role of helplessness, fear of pain, and passive pain-coping in chronic pain patients. Clin J Pain. 2006;22:245–251. doi: 10.1097/01.ajp.0000173019.72365.f5. [DOI] [PubMed] [Google Scholar]
- Mercado AC, Carroll LJ, Cassidy D, Cote P. Passive coping is a risk factor for disabling neck or low back pain. Pain. 2005;117:51–57. doi: 10.1016/j.pain.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Mercado AC, Carroll LJ, Cassidy JD, Cote P. Coping with neck and low back pain in the general population. Health Psychol. 2000;19:333–338. doi: 10.1037/0278-6133.19.4.333. [DOI] [PubMed] [Google Scholar]
- Hurwitz EL, Morgenstern H, Yu F. Cross-sectional and longitudinal associations of low-back pain and related disability with psychological distress among patients enrolled in the UCLA Low-back pain study. J Clin Epidemiol. 2003;56:463–471. doi: 10.1016/S0895-4356(03)00010-6. [DOI] [PubMed] [Google Scholar]
- Koleck M, Mazaux JM, Rascle N, Brichon-Schweitzer M. Psycho-social factors and coping strategies as predictors of chronic evolution and quality of life in patients with low back pain: A prospective study. Eur J Pain. 2006;10:1–11. doi: 10.1016/j.ejpain.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Cote P. The role of pain coping strategies in prognosis after whiplash injury: passive coping predicts slowed recovery. Pain. 2006;124:18–26. doi: 10.1016/j.pain.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Stansbury JP, Ried LD, Velozo CA. Unidimensionality and bandwidth in the Center for Epidemiologic Studies Depression (CES-D) Scale. J Pers Assess. 2006;86:10–22. doi: 10.1207/s15327752jpa8601_03. [DOI] [PubMed] [Google Scholar]
- Cox JM. Biomechanics, adjustment procedures, ancillary therapies, and clinical outcomes of Cox distraction technique. In: Cox JM, editor. Low Back Pain: Mechanisms, Diagnosis and Treatment. 6. Baltimore: Williams and Wilkens; 1999. pp. 273–376. [Google Scholar]
- Gudavalli MR, Cox JM, Cramer GD, Baker JA, Patwardhan AG. Intervertebral disc pressure changes during a chiropractic procedure. BED- Advances in Bioengineering. 1997;36:215–216. [Google Scholar]
- Gudavalli MR, Cox JM, Cramer GD, Baker JA, Patwardhan AG. Intervertebral disc pressure changes during low back treatment procedures. BED- Advances in Bioengineering. 1998;39 [Google Scholar]
- Cramer GD, Gregerson DM, Knudsen JT, Hubbard BB, Ustas LM, Cantu JA. The effects of side posture positioning and spinal adjusting on the lumbar Z joints a randomized controlled trial with sixty-four subjects. Spine. 2002;27:2459–2466. doi: 10.1097/00007632-200211150-00008. [DOI] [PubMed] [Google Scholar]
- Ianuzzi A, Khalsa PS. Comparison of human lumbar facet joint capsule strains during simulated high-velocity, low-amplitude spinal manipulation versus physiological motions. Spine J. 2005;5:277–290. doi: 10.1016/j.spinee.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs JD, Fritz JM, Flynn TW, Irrgang JJ, Johnson KK, Majkowski GR, Delitto A. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: A validation study. Ann Int Med. 2004;141:920–928. doi: 10.7326/0003-4819-141-12-200412210-00008. [DOI] [PubMed] [Google Scholar]
- Jackson RP, Jacobs RR, Montesano PX. Facet joint injection in low-back pain: A prospective statistical study. Spine. 1988;13:966–971. doi: 10.1097/00007632-198809000-00002. [DOI] [PubMed] [Google Scholar]
- Slipman CW, Lipetz JS, Plastaras CT, Jackson HB, Vresilovic EJ, Lenrow DA, Braverman DL. Fluoroscopically guided therapeutic sacroiliac joint injections for sacroiliac joint syndrome. Am J Phys Med Rehabil. 2001;80:425–432. doi: 10.1097/00002060-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Niemisto L, Kalso E, Malmivaara A, Seitsalo S, Hurri H. Radiofrequency denervation for neck and back pain A systematic review within the framework of the cochrane collaboration back review group. Spine. 2003;28 doi: 10.1097/01.BRS.0000084682.02898.72. [DOI] [PubMed] [Google Scholar]
- Chen C, Cavanaugh JM, Song Z, Takebayashi T, Kallakuri S, Wooley PH. Effects of nucleus pulposus on nerve root neural activity, mechanosensitivity, axonal morphology, and sodium channel expression. Spine. 2004;29:17–25. doi: 10.1097/01.BRS.0000096675.01484.87. [DOI] [PubMed] [Google Scholar]
- van Tulder MW, Scholten RJPM, Koes BW, Deyo RA. Nonsteroidal anti-inflammatory drugs for low back pain a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine. 2000;25:2501–2513. doi: 10.1097/00007632-200010010-00013. [DOI] [PubMed] [Google Scholar]
- Peloso P, Gross A, Haines T, Trinh K, Goldsmith CH, Aker P, Cervical Overview Group Cervical Overview Group. Medicinal and injection therapies for mechanical neck disorders. The Cochrane Database of Systematic Reviews. 2005:CD000319. doi: 10.1002/14651858.CD000319.pub3. [DOI] [PubMed] [Google Scholar]
- McLain RF, Kapural L, Mekhail NA. Epidural steroid therapy for back and leg pain: mechanisms of action and efficacy. Spine J. 2005;5:191–201. doi: 10.1016/j.spinee.2004.10.046. [DOI] [PubMed] [Google Scholar]
- Slipman CW, Lipetz JS, DePalma MJ, Jackson HB. Therapeutic selective nerve root block in the nonsurgical treatment of traumatically induced cervical spondylotic radicular pain. Am J Phys Med Rehabil. 2004;83:446–454. doi: 10.1097/00002060-200406000-00007. [DOI] [PubMed] [Google Scholar]
- DePalma MJ, Bhargava A, Slipman CW. A critical appraisal of the evidence for selective nerve root injection in the treatment of lumbosacral radiculopathy. Arch Phys Med Rehabil. 2005;86:1477–1483. doi: 10.1016/j.apmr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Seaman DR. The diet-induced proinflammatory state a cause of chronic pain and other degenerative diseases. J Manipulative Physiol Ther. 2002;25:168–179. doi: 10.1067/mmt.2002.122324. [DOI] [PubMed] [Google Scholar]
- Kopp JR, Alexander AH, Turocy RH, Levrini MG, Lichtman DM. The use of lumbar extension in the evaluation and treatment of patients with acute herniated nucleus pulposus. A preliminary report. Clin Orthop Relat Res. 1986:211–8. [PubMed] [Google Scholar]
- Coppieters MW, Stappaerts KH, Wouters LL, Janssens K. The immediate effects of a cervical lateral glide treatment technique in patients with neurogenic cervicobrachial pain. J Orthop Sports Phys Ther. 2003;33:369–378. doi: 10.2519/jospt.2003.33.7.369. [DOI] [PubMed] [Google Scholar]
- Murphy DR, Hurwitz EL, Gregory AA, Clary R. A nonsurgical approach to the management of patients with cervical radiculopathy: A prospective observational cohort study. J Manipulative Physiol Ther. 2006;29:279–287. doi: 10.1016/j.jmpt.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Murphy DR, Hurwitz EL, Gregory AA, Clary R. A non-surgical approach to the management of lumbar spinal stenosis: A prospective observational cohort study. BMC Musculoskel Disord. 2006;7:16. doi: 10.1186/1471-2474-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudavalli MR, Cambron JA, McGregor M, Jedlicka J, Keenum M, Ghanayem JA, Patwardhan AG. A randomized clinical trial and subgroup analysis to compare flexion-distraction with active exercise for chronic low back pain. Eur Spine J. 2005 doi: 10.1007/s00586-005-0021-8. DOI 10.1007/s00586-005-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JH, Schneider M. Receptor-tonus technique: an overview. Chiro Technique. 1990;2:13–16. [Google Scholar]
- Lewit K. Man Med. 1986. pp. 101–104.
- Richardson C, Jull G, Hodges P, Hides J. Therapeutic Exercise For Spinal Segmental Stabilization In Low Back Pain Scientific Basis and Clinical Approach. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- Koumantakis GA, Watson PJ, Oldham JA. Trunk muscle stabilization training plus general exercise versus general exercise only: randomized controlled trial of patients with recurrent low back pain. Phys Ther. 2005;85:209–225. [PubMed] [Google Scholar]
- Kay T, Gross A, Sataguida PL, Hoving J, Goldsmith C, Bronfort G, Cervical Overview Group Exercise for mechanical neck disorders. Cochrane Database of Systematic Reviews. 2005. pp. 20051–26. [DOI] [PubMed]
- Staal JB, Hlobil H, Twisk JWR, Smid T, Koke AJA, van Mechelen W. Graded activity for low back pain in occupational health care. Ann Int Med. 2004;140:77–84. doi: 10.7326/0003-4819-140-2-200401200-00007. [DOI] [PubMed] [Google Scholar]
- Fitz-Ritson D. Cervicogenic vertigo and disequilibrium. In: Murphy DR, editor. Conservative Management of Cervical Spine Syndromes. New York: McGraw-Hill; 2000. pp. 221–236. [Google Scholar]
- Murphy DR. Sensorimotor training and cervical stabilization. In: Murphy DR, editor. Conservative Management of Cervical Spine Syndromes. New York: McGraw-Hill; 2000. pp. 607–640. [Google Scholar]
- George SZ, Wittmer VT, Fillingim RB, Robinson ME. Sex and pain-related psychological variables are associated with thermal pain sensitivity for patients with chronic low back pain. J Pain. 2007;8:2–10. doi: 10.1016/j.jpain.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120:297–306. doi: 10.1016/j.pain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Long A, Donelson R, Fung T. Does it matter which exercise? a multicentered RCT of low back pain subgroups. Spine J. 2004;4:14S. doi: 10.1016/j.spinee.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Smeets R, Vlaeyen J, Kester A, Knottnerus J. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7:261–271. doi: 10.1016/j.jpain.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Grotle M, Vollestad NK, Brox IJ. Clinical course and impact of fear-avoidance beliefs in low back pain. Spine. 2006;31:1038–1046. doi: 10.1097/01.brs.0000214878.01709.0e. [DOI] [PubMed] [Google Scholar]
- Atlas SJ, Deyo RA, Patrick DL, Convery K, Keller RB, Singer DE. The Quebec Task Force classification for spinal disorders and the severity, treatment and outcomes of sciatica and lumbar spinal stenosis. Spine. 1996;21:2885–2892. doi: 10.1097/00007632-199612150-00020. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M, Seferiadis A, Carlsson J, Gunnarsson R. Active intervention in patients with whiplash-associated disorders improves long-term prognosis a randomized controlled clinical trial. Spine. 2003;28:2491–2498. doi: 10.1097/01.BRS.0000090822.96814.13. [DOI] [PubMed] [Google Scholar]
- Petersen T, Kryger P, Ekdahl C, Olsen S, Jacobsen S. The effect of McKenzie therapy as compared with that of intensive strengthening training for the treatment of patients with subacute or chronic low back pain a randomized controlled trial. Spine. 2002;27:1702–1708. doi: 10.1097/00007632-200208150-00004. [DOI] [PubMed] [Google Scholar]
- Donelson R. Rapidly Reversible Back Pain: An Evidence-Based Pathway to Widespread Recoveries and Savings. Hanover, NH: Self Care First; 2007. [Google Scholar]
- Razmjou H, Kramer J, Yamada R. Intertester reliability of the McKenzie evaluation in assessing patients with mechanical low-back pain. J Orthop Sports Phys Ther. 2000;30:368–389. doi: 10.2519/jospt.2000.30.7.368. [DOI] [PubMed] [Google Scholar]
- Kilpikoski S, Airaksinen O, Kankaanpaa M, Leminen P, Videman T, Alen M. Interexaminer reliability of low back pain assessment using the McKenzie Method. Spine. 2002;27:E207–E214. doi: 10.1097/00007632-200204150-00016. [DOI] [PubMed] [Google Scholar]
- Clare HA, Adams R, Maher CG. Reliability of McKenzie classification of patients with cervical or lumbar pain. J Manipulative Physiol Ther. 2005;28:122–127. doi: 10.1016/j.jmpt.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Delitto A, Ehrhard RE, Bowling RW. A treatment-based classification approach to low back syndrome Identifying and staging patients for conservative treatment. Phys Ther. 1995;75:20–34. doi: 10.1093/ptj/75.6.470. [DOI] [PubMed] [Google Scholar]
- Fritz JM, Delitto A, Erhard RE. Comparison of classification-based physical therapy with therapy based on clinical practice guidelines for patients with acute low back pain: A randomized clinical trial. Spine. 2003;28:1363–1372. doi: 10.1097/00007632-200307010-00003. [DOI] [PubMed] [Google Scholar]
- Childs JD, Fritz JM, Piva SR, Whitman JM. Proposal of a classification system for patients with neck pain. J Orthop Sports Phys Ther. 2004;34:686–700. doi: 10.2519/jospt.2004.0102. [DOI] [PubMed] [Google Scholar]
- Bronfort G, Haas M, Evans RL. Efficacy of spinal manipulation and mobilization for low back pain and neck pain: a systematic review and best evidence synthesis. Spine J. 2004;4:335–356. doi: 10.1016/j.spinee.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Burton AK, Tillotson KM, Clearly J. Single-blind randomised controlled trials of chemonucleolysis and manipulation in the treatment of symptomatic lumbar disc herniation. Eur Spine J. 2000;9:202–207. doi: 10.1007/s005869900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogduk N. Spinal manipulation for neck pain does not work. J Pain. 2003;4:427–428. doi: 10.1067/S1526-5900(03)00733-8. [DOI] [PubMed] [Google Scholar]


