Abstract
Inflammation elicits a splenic lymphopoiesis of unknown physiologic significance but one that juxtaposes developing B cells and exogenous Ag. We show that immature and transitional 1 (immature/T1) B cells constitutively express activation-induced cytidine deaminase and B lymphocyte-induced maturation protein 1 in amounts that support accelerated plasmacytic differentiation and limited class-switch recombination. In vivo, activation of immature/T1 B cells by TLR ligands or bacterial vaccine rapidly induces T1 cells to divide, proliferate, and secrete IgM, IgG, or IgA Ab; in vitro, proliferation and differentiation are substantially enhanced by B cell-activating factor. We propose that inflammation-induced extramedullary lymphopoiesis represents a specialized mechanism for innate Ab responses to microbial pathogens.
Inflammation mobilizes developing lymphocytes from the bone marrow (BM) by suppressing CXCL12 and stem cell factor levels and initiates extramedullary lymphopoiesis in the spleen (1). Concurrently, immature (im) granulocyte numbers increase in the BM and expand into developmental niches vacated by emigrant lymphocytes. We have proposed that this redirection of leukopoiesis represents an innate immune response to microbial pathogens (1).
If expanded BM granulopoiesis is an adaptive response, for what purpose and benefit are lymphocyte progenitors mobilized to the periphery? Unlike granulocytes, mature B cells are capable of mitotic expansion and have half-lives measured in weeks or months, rather than hours or days. Thus, mobilization of B cell progenitors and the establishment of extramedullary lymphopoiesis could be inconsequential. On the other hand, the signals that retain developing myeloid and lymphoid cells in the BM appear well regulated as do the localization and persistence of lymphoid progenitors in the periphery (1). This regulation suggests that inflammation-induced extramedullary B lymphopoiesis may have a significant physiologic role.
We demonstrate that the splenic lymphopoiesis that follows inflammatory stimuli generates large numbers of im and translational 1 (im/T1) B cells that express low, but significant, levels of activation-induced cytidine deaminase (AID). In these cells, AID expression is independent of T cells, CD154, or the IL-1R-associated kinase 4 (IRAK4). This intrinsic AID expression is developmentally regulated; AID message is greatly diminished or undetectable in pro/pre-B, T2, or mature B cells. Splenic im/T1 B cells from CD154-/- mice contain germline γ3 transcripts (γ3 GLT) and the molecular intermediates of IgM→IgG3, IgG2a, IgG2b, and IgA class-switch recombination (CSR). Splenic im/T1 B cells from CD154-/- mice also carry low levels of message for B lymphocyte-induced maturation protein 1 (BLIMP-1) (2) and respond to TLR ligands by rapid entry into cell cycle and production of IgM and IgG Ab; immunization with a bacterial vaccine efficiently differentiates im/T1 B cells into CD138+ plasmacytes. Taken together, these unique properties suggest that the peripheral im/T1 B cell compartment elicited by inflammation is specialized for T cell-independent (Ti) humoral responses to microbial infection in extravascular tissues.
Materials and Methods
Mice
Female C57BL/6 (BL/6), nude, and CD154-deficient congenic (CD154-/-; Ref. 3) mice were purchased from The Jackson Laboratory and maintained in our colony. Mice deficient for linker of activated T cells (LAT-/-) bred onto the BL/6 genetic background (4) were the gift of Dr. W. Zhang (Duke University, Durham, NC). BM samples from BL/6 mice deficient in IRAK4-/- (5) or AID-/- (6) were provided by Drs. J. Aliberti (Duke University) and T. Imanishi-Kari (Tufts University, Boston, MA), respectively. Mice constitutively expressing GFP in all tissues ((BL/6 × BL/6-Tg(ACTB-EGFP)1Osb/J) F1 animals, GFP transgenic (Tg)) (7) were obtained from Dr. M. Kondo (Duke University); mice carrying an IgH transgene (H50GTg) were bred and maintained locally (8). Mice were housed under specific pathogen-free conditions at the Duke University Animal Care Facility with sterile bedding, water, and food. Mice used in these experiments were 6-16 wk old. All experiments involving animals were reviewed and approved by the Duke University Institutional Animal Care and Use Committee.
Ags and immunizations
Mice were immunized by single, i.p. injections of 25 μg of (4-hydroxy-3-nitrophenyl)acetyl (NP) chicken gammaglobulin (CGG) in IFA (Sigma-Aldrich) as described previously (1). NP-CGG contained 10-15 mol NP/mol CGG. A bacterial vaccine was prepared by boiling small volumes of washed Escherichia coli (DH5α) in HBSS; this vaccine (5 × 107 bacteria; ≤200 μl) was given i.v.
Flow cytometry
FITC-, PE-, PE-Cy5-, PE-Cy7-, biotin-, or allophycocyanin-conjugated mAb specific for mouse B220, CD4, CD8, CD21, CD23, CD93, IgM, GL7, TER-119, Gr-1, CD11b, and CD180 were purchased (BD Bioscience or eBioscience). PE-, biotin-, and Texas Red-conjugated Ab for mouse IgD, IgM, and λ L chain were purchased from Southern Biotechnology Associates. Streptavidin-allophycocyanin-Cy7 (eBioscience) identified biotinylated mAb.
Mice were killed at various times after injection/immunization, and cells were harvested from spleen, BM, and/or blood. RBC were lysed in ammonium chloride buffer before immunolabeling. Typically, ≤106 nucleated cells were suspended in 50-100 μl of labeling buffer (HBSS with 2% FCS and labeled mAb) and incubated on ice for 20 min. 7-Aminoactinomycin D (Molecular Probes) or propidium iodide (Sigma-Aldrich) was included to identify dead cells. Labeled cells were analyzed/sorted in a FACSVantage with DIVA option or FACScan (BD Biosciences). Flow cytometric data were analyzed with FlowJo software (Tree Star).
Specific B cell populations from the BM and spleen cells were identified with fluorochrome mAb specific for CD21, CD23, CD93, B220, IgM, or IgD; pro/pre-B, im/T1, T2, mature follicular (MF), marginal zone (MZ), and germinal center (GC) B cells were identified/isolated based on distinctive expression phenotypes (9-12). Dead cells and cells expressing the Gr1, CD11b, CD4, CD8, or Ter119 Ags (Lin+) were excluded in a dump channel. In some experiments, populations of CD93+GL7+B220low and CD93-GL7highB220high splenic B cells (10, 13) from naive or immunized BL/6 mice were analyzed. Cells from AID-deficient controls were sorted for analyses of VDJ mutation frequencies. Typical purities for isolated B cell populations were ≈95% following a single sort and ≥98% after double-sorting.
Adoptive transfer of B cells
im/T1 and MF B cells were enriched from the spleens of naive GFP-Tg mice by MACS (purity, ≈90% of B cells). Sorted B cell populations (5-7 × 106) were transferred into congenic BL/6 recipients (1). Fifteen minutes after transfer, selected recipients were given PBS or bacterial vaccine i.v. Five days after immunization, B220 and CD138 expression by GFP+ splenocytes from immunized and naive recipients were determined by flow cytometry.
Cell culture
Sorted B cells (≈3 × 104) were cultured with 5 μg/ml LPS (Sigma-Aldrich), 5 μg/ml CpG (InvivoGen), or 50 μg/ml anti-IgM Ab F(ab’)2 (Jackson ImmunoResearch Laboratories) in the presence or absence of B cell-activating factor (BAFF; 500 ng/ml; R&D Systems) at 37°C in humidified air supplemented with 5% CO2. In some experiments, cells were labeled with CFSE (1) before culture. After culture for 1-3 days, cells and supernatants were analyzed by RT-PCR, ELISA, and ELISPOT assays.
RT-PCR
Total RNA was extracted from 0.5-2 × 104 cells in TRIzol reagent (Invitrogen Life Technologies); mRNA was reverse transcribed (Superscript III; Invitrogen Life Technologies) with oligo(dT) primer for 1 h at 42°C. PCR were performed on serial dilutions (4-fold) of cDNA using TaqDNA polymerase (Denville Scientific). The following PCR primers were used: β-actin, forward, 5′-AGCCATGTACGTAGCCATCC-3′, and reverse, 5′-CTCTCAGCTGTGGTGGTGAA-3′; hypoxanthine phosphoribosyltransferase, forward, 5′-GCTGGTGAAAAGGACCTCT-3′, and reverse, 5′-CACAGGACTAGAACACCTGC-3′; Igβ, forward, 5′-TGTTGGAATCTGCAAATGGA-3′, and reverse, 5′-CACAGGAGGATGGGCTGTAG-3′; AID, external forward, GAGGGAGTCAAGAAAGTCACGCTGG-3′ (AID119), external reverse, GGCTGAGGTTAGGGTTCCATCTCAG-3′ (AID118), internal forward, 5′-ACCTACCTCTGCTACGTGGT-3′ (AIDF2), and internal reverse, 5′-CGGGCACAGTCATAGCAC-3′ (AIDR2); RAG1, forward, 5′-CGGGCACAGTCATAGCAC-3′ (RAG1-F), and reverse, 5′-GTCGATCCGGAAAATCCTGGCAATG-3′ (RAG1-R2); BCL-6, forward, 5′-GAAGAGTTCCTGAACAGCCG-3′, and reverse, 5′-GGAAGTATGGAGCATTCCGA-3′; BLIMP-1, forward, 5′-CTGTCAGAACGGGATGAACA-3′, and reverse, 5′-TGGGGACACTCTTTGGGTAG-3′; TLR2, forward, 5′-TGCTTTCCTGCTGGAGATTT-3′, and reverse, 5′-TGTAACGCAACAGCTTCAGG-3′; TLR4, forward, 5′-ACCTGGCTGGTTTACACGTC-3′, and reverse, 5′-AGAAACATTCGCCAAGCAAT-3′; and TLR9, forward, 5′-ACCCTGGTGTGGAACATCAT-3′, and reverse, 5′-GCTTCAGCTCACAGGGTAGG-3′.
To determine the frequencies of AID expression in im/T1, MF, and GC B cells, single cells were sorted into 96-well plates containing 10 μl of 2× reverse transcriptase buffer with 6 μg of yeast tRNA (Ambion). Sorted cells were frozen at -80°C until use. To detect AID and Igβ expression, cDNA was prepared from single cells by reverse transcription in the presence of 0.5% Nonidet P-40. The resulting cDNA was divided (2 μl of cDNA) for separate reactions with Igβ and AID primers; amplification cycles: AID, primary 40, secondary 40 cycles; Igβ, 50 cycles.
Quantitative PCR was performed as described previously (1). Briefly, mRNA was precipitated in TRIzol and reverse transcribed (Superscript II; Invitrogen Life Technologies). cDNA amplifications were performed in an iCycler thermal cycler (Bio-Rad) with SYBR Green PCR core reagents (Applied Biosystems) and AID, Igβ, or β-actin primers. Amplification conditions were as follows: denaturation at 94°C/10 min; amplification 94°C/15 s, 60°C/45 s. For AID amplification, we used 1 μl of first-round amplification products with AIDF2 and AIDR2 primers. The relative expression levels for growth factor genes were calculated by the comparative threshold cycle (CT) method recommended by the manufacturer (Applied Biosystems) normalized to Igβ message in the same sample. ΔCT values were determined by subtracting C T (target) from CT (Igβ). Expression levels relative to Igβ were defined as 2-ΔCT.
Amplification of germline and class-switch circle transcripts (CT)
To detect γ3 GLT, PCR primers for Iγ3F (Iγ3F, 5′-TGGGCAAGTGGATCTGAACA-3′) and Cγ3 (Cg3R, 5′-GGCTCCATAGTTCCATT-3′) were used (14). Circle-switch transcripts for γ1, γ2a, γ2b, γ3, and α (γ1, γ2a, γ2b, γ3, and α CT) were amplified by a seminested-PCR: first with CμR (5′-AATGGTGCTGGGCAGGAAGT-3′) and Iγ1F (5′-GGCCCTTCCAGATCTTTGAG-3′), Iγ2aF (5′-GGCTGTTAGAAGCACAGTGACAAAG-3′), Iγ2bF (5′-CACTGGGCCTTTCCAGAACTA-3′), and Iγ3F or IαF (5′-CCAGGCATGGTTGAGATAGAGATAG-3′) (13). A second amplification used the CμintR primer (5′-CCATGGCCACCAGATTCTTA-3′) paired with Iγ1F, Iγ2aF, Iγ2bF, Iγ3F, or IαF primers. Amplification conditions were as follows: denaturation 95°C/5 min; amplification 30 cycles 94°C/30 s, 58°C/30 s, and 72°C/1 min. These PCR used AmpliTaq Gold polymerase (Applied Biosystems).
ELISA and ELISPOT assay
Ig concentrations in culture supernatants were determined by a standard ELISA method. Briefly, 96-well plates (BD Biosciences) were coated overnight with goat anti-mouse Ig (H+L) mAb (Southern Biotechnology Associates) or anti-mouse Fcγ (Sigma-Aldrich) (both 10 μg/ml in PBS) at 4°C and blocked for 1 h with 3% BSA. After washing (PBS with 0.1% Tween 20), 25-μl aliquots of serially diluted (4-fold) culture supernatant were loaded, incubated for 3 h at room temperature, and extensively washed. Bound IgM and IgG3 were detected by HRP conjugates of isotype-specific goat Ab (Southern Biotechnology Associates). Bound HRP activity was visualized using tetramethylbenzidine peroxidase (Bio-Rad). Ab-secreting cells in cultures were identified by ELISPOT assay. Cultured B cells were harvested, washed, and resuspended in fresh medium before being plated onto nitrocellulose filters coated with Ab specific for mouse Igκ (Southern Biotechnology Associates). Plated cells were incubated for 3 h at 37°C in humidified air supplemented with 5% CO2. Subsequently, filters were washed and flooded with alkaline phosphatase (AP)-conjugated mAb specific for mouse IgM, IgG3, IgG2a, or HRP-conjugated anti-IgG3 (Southern Biotechnology Associates). Bound AP and HRP activity was visualized using Naphthol AS-MX (Sigma-Aldrich) and 3-amino-9-ethyl carbazole (Sigma-Aldrich), respectively.
Statistics
Statistical significance of data was determined by Student’s t test.
Results
Identification and isolation of specific B cell compartments
Pro/pre-B, im/T1 B, T2, MF, and MZ B cells were isolated from the BM (Fig. 1A) and spleen (Fig. 1B) of naive or immunized mice by established methods. Following precedent (9, 11, 12), we define pro/pre-B cells as Lin-B220lowCD93+CD21-CD23-IgM-IgD-; im/T1 B cells as Lin-B220lowCD93+IgM+IgD-CD21-CD23-; and T2 B cells as Lin-B220lowCD93+CD21lowCD23+IgM+IgDlow. Mature B2 (MF) cells are Lin-B220highCD93-IgM+IgD+CD21+CD23+, whereas MZ B cells are Lin-B220highCD93-D21highCD23lowIgMhighIgDlow (Fig. 1, A and B).
FIGURE 1.
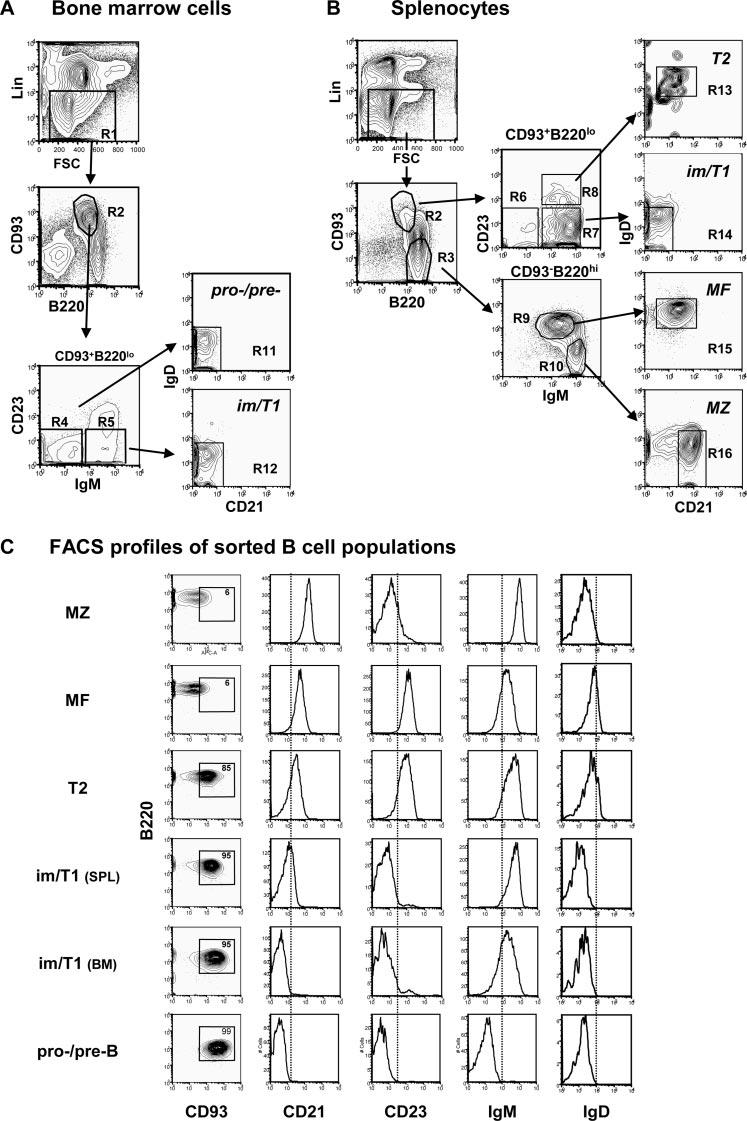
Identification and isolation of B cell populations. Gating strategy for the identification of pro/pre-B, im/T1 B, T2, MF, and MZ B cells from BM (A) and spleen (B). Cells that express markers (CD4/8, CD11b, Ter119, and Gr-1) of other hemopoietic lineages (Lin+) or take up propidium iodide are excluded (R1), and the remaining cells are dived by CD93 and B220 expression into compartments of developing (R2) or mature (R3) B cells. Both compartments are further divided by IgM and CD23 expression (R4-R10); these compartments may be refined by CD21 and IgD expression (R11-R16). By these criteria, we define pro/pre-B cells as Lin-B220lowCD93+CD21-CD23-IgM-IgD- (R11); im/T1 B cells as Lin-B220lowCD93+IgM+IgD-CD21-CD23- (R12(BM) and R14(SPL)); and T2 B cells are Lin-B220lowCD93+CD21lowCD23+IgM+IgDlow (R13). Mature B2 (MF) cells are Lin-B220highCD93-IgM+IgD+CD21+CD23+ (R15), whereas MZ B cells are Lin-B220highCD93-CD21highCD23lowIgMhighIgDlow (R16). C, Typical results for the isolation of specific B cell subsets by flow cytometry. From 20 independent sorts using the labeling strategy outlined in A and B, MZ, MF, T2, im/T1, and pro/pre-B cells could be isolated at purities of ≈95%; secondary sorts gave purities ≥98% (data not shown). B cells within each compartment expressed B220, CD93, CD21, CD23, IgM, and IgD in characteristic fashion. Note that im/T1 cells from spleen (SPL) expressed higher levels of CD21 and IgM than did im/T1 cells recovered from BM. These differences likely represent increased maturity in the splenic im/T1 population.
Representative postsort FACS analyses of these B cell subsets reveal typical purities of ≈95% and diagnostic phenotypes, including modulation of B220, CD21, and CD93 expression during B cell maturation (Fig. 1C). We note that IgM expression by BM im/T1 B cells is lower than the im/T1 splenic compartment, suggesting increased maturity of the peripheral pool. IgD and CD23 first appear on T2 B cells and are increased in the MF compartment and reduced on MZ B cells (Fig. 1C). IgM expression by MZ B cells is characteristically elevated relative to MF B cells (Fig. 1C).
Inflammation expands splenic im/T1 B cell numbers
Inflammation increases the number of developmentally im B cells (Lin-CD93+GL7intB220lowIgM- and Lin-CD93+GL7intB220lowIgM+) in the spleen (1, 13, 15). To determine whether all stages of B cell development expand equivalently, we enumerated splenic pro/pre-B, im/T1, T2, MF, and MZ B cells in naive and adjuvant-immunized mice. Fourteen days after immunization, splenic pro/pre-B and im/T1 B cell numbers were 2- to 8-fold higher than in naive controls, constituting 3-6% (1.5-2.0 × 106) of splenic B cells (Fig. 2A) (1, 13). In contrast, adjuvant did not change splenic T2, MF, and MZ B cell numbers (Fig. 2, A and B). Inflammation-induced increases in splenic pro/pre-B and im/T1 B cells were comparable in both BL/6 and congenic nude mice (16) (Fig. 2C).
FIGURE 2.
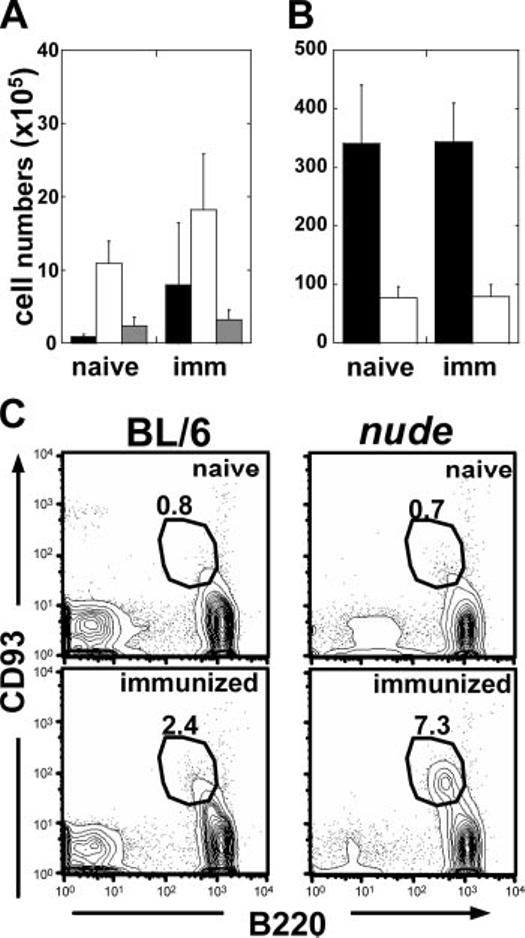
Immunization elicits increase of developmentally immature B cells independent of T cell signaling. Splenocytes from naive (n = 4) and immunized (d14; n = 5) BL/6 mice were harvested and labeled with Abs for B220, CD21, CD23, CD93, IgM, and IgD to enumerate developing pro/pre-B (■), im/T1 (□)(A), and T2 B cells ( ) or mature MF (■) and MZ (□) B cells (mean ± SD) (B). C, FACS profiles of CD93 and B220 from naive (n = 6) and immunized BL/6 (n = 9) and nude (n = 9) mice. Numbers represent the percentage of CD93+B220low spleen cells. Results typical of three independent experiments are shown.
) or mature MF (■) and MZ (□) B cells (mean ± SD) (B). C, FACS profiles of CD93 and B220 from naive (n = 6) and immunized BL/6 (n = 9) and nude (n = 9) mice. Numbers represent the percentage of CD93+B220low spleen cells. Results typical of three independent experiments are shown.
TLR ligands activate im/T1 B cells and induce rapid Ab secretion
Expression of the RP105 TLR (Fig. 3A) (17) and TLR9 message (Fig. 3B) is substantial in im/T1 cells, suggesting the possibility of responses to bacterial components. Therefore, we cultured splenic im/T1, MF, and MZ B cells (3 × 104) from BL/6 and CD154-/- mice with LPS or CpG (5 μg/ml) for 24, 48, or 72 h (Fig. 3, C-F). In some experiments, cells were labeled with CFSE (1) before culture. At 72 h, secreted IgM and IgG were quantified by ELISA and Ab-forming cells (AFC) enumerated by ELISPOT. TLR expression and the responses of BL/6- and CD154-deficient B cells to TLR ligands are comparable (data not shown).
FIGURE 3.
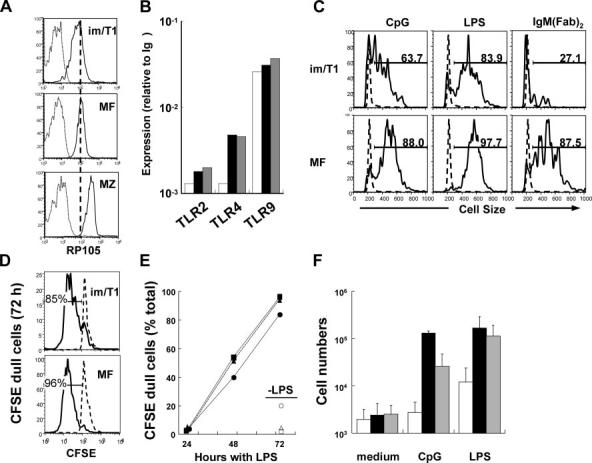
im/T1 B cells express TLR and response to LPS and CpG. Expression of TLR by specific B cell subsets. A, im/T1, MZ, and MF B cells from the spleens of BL/6 mice were labeled with anti-RP105 and analyzed by flow cytometry. The black line represents binding of anti-RP105, and the gray line represents binding of isotype controls in each B cell subset. B, Quantification of TLR2, 4, and 9 mRNA at various stages of B cell development. Bars indicate im/T1 (□), mature MF (■), and MZ ( ) B cells. Results represent the average values from two independent experiments. Splenic im/T1, MF, and MZ B cells were stimulated with CpG, LPS, or anti-IgM F(ab’)2 in vitro for 72 h. C, Blast formation in im/T1 and MF B cells was determined by cell size (forward scatter measured by flow cytometry) after the stimulations. Anti-IgM stimulation did not induce activation in im/T1 B cells as it did in MF B cells, which is consistent with prior work (11). D, CFSE profiles in LPS-stimulated im/T1 and MF B cells. E, Percentages of CFSEdull cells in im/T1 ([hexagonblack]), MF (■), and MZ B (▲) compartments (n = 2-3) during stimulation with LPS in vitro. F, Relative growth of im/T1, MF, and MZ compartments after various stimulations. Bars indicate the average numbers (mean ± SD) of im/T1 (□), mature MF (■), and MZ (
) B cells. Results represent the average values from two independent experiments. Splenic im/T1, MF, and MZ B cells were stimulated with CpG, LPS, or anti-IgM F(ab’)2 in vitro for 72 h. C, Blast formation in im/T1 and MF B cells was determined by cell size (forward scatter measured by flow cytometry) after the stimulations. Anti-IgM stimulation did not induce activation in im/T1 B cells as it did in MF B cells, which is consistent with prior work (11). D, CFSE profiles in LPS-stimulated im/T1 and MF B cells. E, Percentages of CFSEdull cells in im/T1 ([hexagonblack]), MF (■), and MZ B (▲) compartments (n = 2-3) during stimulation with LPS in vitro. F, Relative growth of im/T1, MF, and MZ compartments after various stimulations. Bars indicate the average numbers (mean ± SD) of im/T1 (□), mature MF (■), and MZ ( ) B cells (n = 2∼11) cultured with CpG or LPS for 72 h.
) B cells (n = 2∼11) cultured with CpG or LPS for 72 h.
Activation (Fig. 3C) and CFSE dilution (Fig. 3, D and E) were comparable for im/T1, MF, and MZ B cells cultured with LPS or CpG. In contrast to MF and MZ B cells, however, im/T1 B cell numbers did not increase, indicating concurrent proliferation and death (Fig. 3F).
Despite the increased rates of cell death, LPS induced rapid Ab secretion by im/T1 B cells. After 24 h with LPS, im/T1 (∼20 AFC/103 cells, p = 0.02) and MZ (∼100 AFC/103 cells; p = 0.001) B cells produced higher frequencies of IgM AFC than MF B cells (<1 AFC/103 cells) (Fig. 4A); by 48 h, frequencies of IgM AFC in all cultures were comparable, and by 72 h, LPS-stimulated MF and MZ cultures produced 300-450 AFC/103 cells compared with ∼230 AFC/103 cells in im/T1 cultures (Fig. 4A).
FIGURE 4.
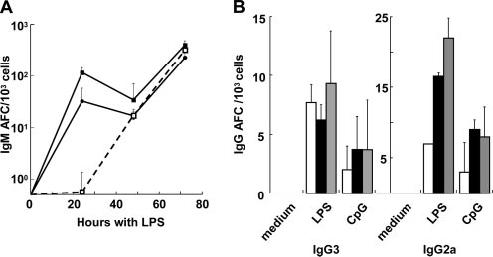
In vitro, splenic im/T1 B cells rapidly produce IgM and IgG Ab in response to LPS or CpG. Splenic im/T1, MF, and MZ B cells (3 × 104) from CD154-/- mice were stimulated with the TLR ligands, LPS, or CpG. A, Kinetics of IgM AFC production during culture with LPS (n = 2-6). Viable cells were harvested at 24, 48, and 72 h. Frequencies of IgM AFC (mean ± SD) per 103 cultured cells were determined by ELISPOT assays. B, Frequencies of IgG AFC in B cell cultures after 72 h in the presence of LPS or CpG. Bars indicate the mean values (±SD) of im/T1 (□), mature MF (■), and MZ ( ) B cells (n = 2-6).
) B cells (n = 2-6).
LPS and CpG also elicited IgG3 and IgG2a AFC in im/T1, MF, and MZ B cells from BL/6 and CD154-/- mice (Fig. 4B). Rapid Ab production by im/T1 and MZ B cells was also evident in the higher amounts of Ab in culture supernatants; at 72 h, im/T1 and MZ B cell supernatants contained twice as much IgM and 4- to 6-fold more IgG3 than MF B cell cultures (data not shown).
BAFF promotes survival and CSR in im/T1 B cells
BAFF expression is increased by inflammation (18). To determine whether BAFF might promote survival and CSR in activated im/T1 B cells (19), we cultured im/T1, MZ, and MF B cells for 72 h with LPS (with or without BAFF) and counted the viable cells and AFC produced (Fig. 5). BAFF increased im/T1 B cell survival ≥4-fold (p = 0.01), a more substantial benefit than for MF and MZ cells (Fig. 5A). With LPS alone, all cultures produced similar frequencies of IgM (230-450 AFC/103 cells) and IgG3 (4-9/103 cells) AFC (Fig. 5B). BAFF had little effect on the frequencies of IgM AFC (150-300/103 cells) and did not alter IgG3 AFC production by MZ or MF B cells (4-12/103 cells) (Fig. 5B). However, BAFF’s effect on the generation of IgG3 AFC by im/T1 B cells was significant with frequencies increasing ≥3-fold (p = 0.01) (Fig. 5B).
FIGURE 5.
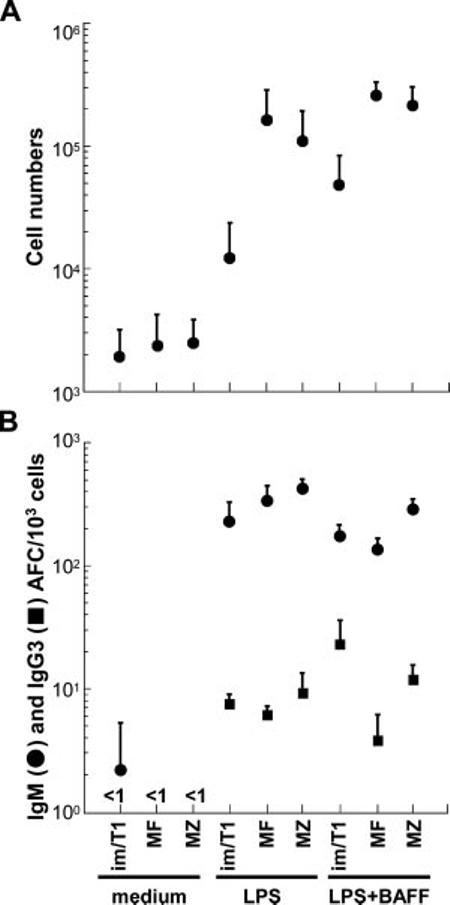
BAFF promotes survival and CSR in im/T1 B cells. A, Viable cell numbers (mean ± SD) present in cultures containing medium alone, LPS, or LPS and BAFF for 72 h (n = 3-8); all cultures initiated with 3 × 104 cells. BAFF is most effective in promoting im/T1 B cell survival. B, Effects of BAFF on frequencies of LPS-induced IgM (●) and IgG3 (■) AFC ((mean ± SD) per 103 cells; 72 h, n = 3-6). Isotype-specific AFC were determined by ELISPOT (n = 3-8). Whereas IgM AFC and IgG3 AFC produced by MF and MZ B cells are little changed, BAFF substantially increased IgG3 AFC production from im/T1 B cells.
Efficient plasmacytic differentiation by im/T1 B cells in vivo
Rapid IgM and IgG AFC responses in vitro to LPS or CpG by BL/6 and CD154-/- im/T1 B cells suggest a capacity for Ti Ab responses. To determine whether im/T1 B cells can respond to microbes in vivo, we enriched (≈90%) im/T1 and MF B cells from GFP-Tg mice (7) and adoptively transferred equal numbers into congenic BL/6 mice. Immediately after transfer, some recipients were immunized with bacterial vaccine; 5 days later, splenic GFP+B220+ and GFP+B220+CD138+ cells in individual recipients were enumerated by flow cytometry (Fig. 6). Whereas GFP+B220+ cell numbers were comparable for all groups, 0.2-0.5% of splenocytes, substantial numbers of GFP+B220+CD138+ cells developed only in immunized recipients (Fig. 6). im/T1 B cells produced CD138+ plasmablasts/-cytes as efficiently as MF B cells (4.7 vs 3.8%, respectively). We conclude that in response to bacterial Ags, im/T1 B cells proliferate and differentiate to AFC in vivo.
FIGURE 6.
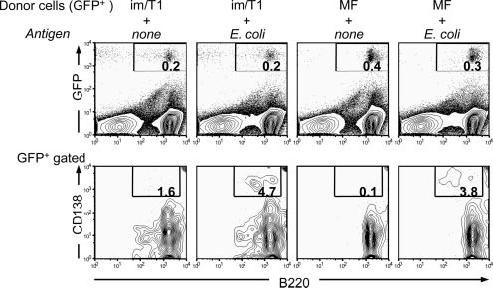
In vivo, im/T1 B cells efficiently differentiate into CD138+ plasmacytes in response to a bacterial vaccine. im/T1 and MF B cells from GFP-Tg mice were enriched by magnetic sorting (≈90% purity) and transferred by i.v. injection to BL/6 recipients; 15 min later, recipients were injected i.v. with 5 × 107 heat-killed E. coli or PBS. After 5 days, splenocytes were harvested and labeled for B220 and CD138 expression. Frequencies of GFP+B220+ spleen cells (upper panels) and GFP+B220+CD138+ plasmacytes/-blasts (lower panels) were determined by flow cytometry. Typical experimental (n = 3) results illustrated.
AID expression in im/T1 B cells
CSR in im/T1 B cells implies AID expression (20). To determine whether peripheral im/T1 B cells express AID, we double-sorted pro/pre-, im/T1, T2, MF, MZ, and GC B cells from the BM (pro/pre-B, im/T1) and spleens (im/T1, T2, MF, MZ, and GC) of naive or immunized mice (9, 10, 21). RAG1 mRNA was abundant in pro- and pre-B cells, rare in im/T1 B cells, and absent from MF B cells (Fig. 7A). In contrast to RAG1, AID transcript levels were higher in im/T1 B cells than in the less mature IgM- compartments. AID mRNA was generally undetectable in T2 and MF B cells but highly expressed in GC B cells. AID message in the im/T1 population did not represent contamination by phenotypically similar GC cells (13), because GC B cells contain BCL-6 mRNA (22) and AID+ im/T1 B cells do not (Fig. 7A). In naive mice, BM and splenic im/T1 B cells express comparable levels of AID mRNA (Fig. 7A).
FIGURE 7.
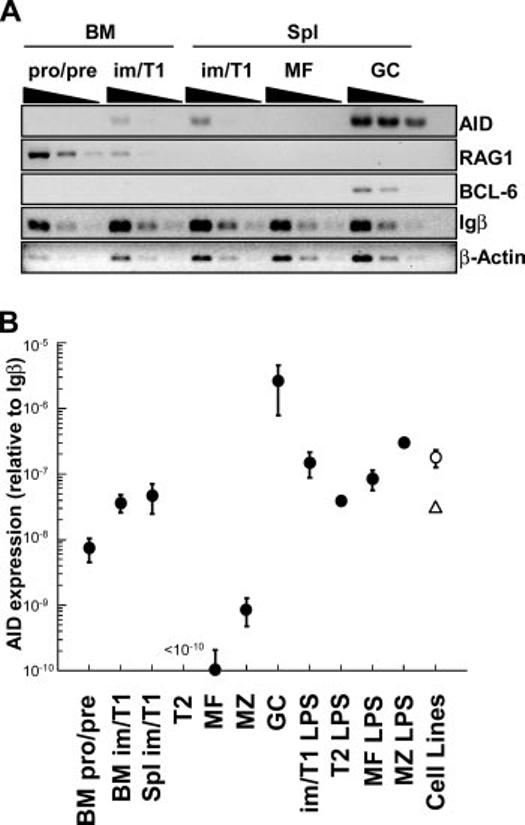
AID expression in im/T1 B cells. A, Semiquantitative RT-PCR (4-fold serial dilutions) for AID, RAG1, BCL-6, Igβ, and β-actin was performed on subsets of developing B cells. AID+ GC B cells (GL7highCD93-B220high) from immunized mice were used as positive controls. cDNA quantities were normalized to Igβ message levels. B, Quantitative RT-PCR of AID mRNA at various stages of B cell development using SYBR Green Real-time PCR. Each point represents the expression of AID relative to Igβ mRNA (mean ± SE) calculated by the CT method (x = 2 - (CTAID - CTIgβ)) (pro/pre-B, im/T1, T2, MF, and MZ, n = 6-10; GC, n = 3; pro/pre-B, im/T1, MF, and MZ plus LPS, n = 2; T2 plus LPS, n = 1 (●); WEHI-231, n = 4 (△); and 103/Bcl-2, n = 8 (○).
To quantify AID message, we amplified Igβ, β-actin, and AID cDNA from equal numbers of double-sorted pro/pre-B, im/T1, T2, MF, MZ, and GC B cells using a quantitative PCR (Fig. 7B) (1, 6). As comparators, splenic im/T1, T2, MF, and MZ B cells cultured with LPS (5 μg/ml; 24 h) and the 103/Bcl2 pre-B (23) and WEHI-231 im B cell (24) lines were also evaluated. LPS induces AID expression in mature B cells and WEHI-231 cells express low amounts of AID (25).
Whereas β-actin cDNA levels showed little change relative to Igβ during B cell development (data not shown), AID cDNA levels varied by as much as 105-fold (Fig. 7B). AID expression in pro/pre-B cells was ∼70-fold higher than in MF B cells (p = 0.009) and was increased another 7-fold in im/T1 B cells (BM, p = 0.001; spleen, p = 0.03). Trace amounts of AID cDNA were present in MZ B cells (p = 0.03) but undetectable in T2 (p = 0.22) B cells. As expected, AID expression was greatest in GC (day 16) B cells; in GC B cells, AID message was ≥2 × 105 times more abundant than in MF B cells. Whereas AID message in GC B cells is much elevated (≈50-fold) compared with im/T1 B cells, LPS-activated im/T1, T2, MF, or MZ B cells and the 103/Bcl2 and WEHI-231 cell lines express AID mRNA comparably to im/T1 cells (Fig. 7B).
The lower levels of AID mRNA in im/T1 B cells vs GC B cells could reflect lower frequencies of AID+ cells, diminished AID transcription, or both. We double-sorted im/T1, MF, and GC B cells to high purity (≥99%) from immunized BL/6 mice and determined the frequencies of single AID+Igβ+ cells in each compartment (Table I). The efficiency of single-cell RT-PCR for Igβ was highest in MF B cells (≈80%) with lower frequencies in im/T1 (54%) and GC (42%) B cells. No AID+Igβ+ mature B cells were detected, whereas AID+Igβ+ im/T1 and GC B cells were equally common (12 and 11%, respectively). Therefore, AID expression in im/T1 B cells is low compared with GC B lymphocytes.
Table I.
Frequencies of AID+ B lymphocytes by single-cell RT-PCR
| Positive Cells in Specific B Cell Compartments |
|||
|---|---|---|---|
| RT-PCR | MF | im/T1 | GC |
| Igβ+ | 68/84 (81%) | 50/93 (54%) | 35/84 (42%) |
| AID+ | 2/84 (2%) | 10/93 (11%) | 7/84 (8%) |
| AID+Igβ+ | 0/68 (0%) | 6/50 (12%) | 4/35 (11%) |
AID expression in im/T1 B cells is intrinsic
In MF B cells, AID is elicited by BCR/CD40 and/or TLR/cytokine signals (6). To determine whether these signals are required for AID transcription in im/T1 B lymphocytes, we isolated im/T1 and mature B cells from the BM and spleens of T cell-deficient mice (nude and LAT-/-; Ref. 4), CD154-/- (3), IRAK4-/- (5), H50GTg (8), and their congenic BL/6 controls (Fig. 8). AID expression in im/T1 B cells from nude, LAT-/- (data not shown), CD154-/-, and BL/6 mice was comparable (Fig. 8A). AID mRNA was abundant in CD21- cells from the BM of IRAK4-/- mice (Fig. 8B) and expressed equally in λ- and λ+ im/T1 B cells from H50GTg mice (Fig. 8C). The λ+ Ab of H50GTg mice is specific for the NP, whereas λ- Abs have diverse specificities including self-Ags (8). AID transcription in im/T1 B cells occurs independently of the signals, cognate T cell help, CD40-CD154 signaling, and activation via TLR, known to elicit AID in MF B lymphocytes.
FIGURE 8.
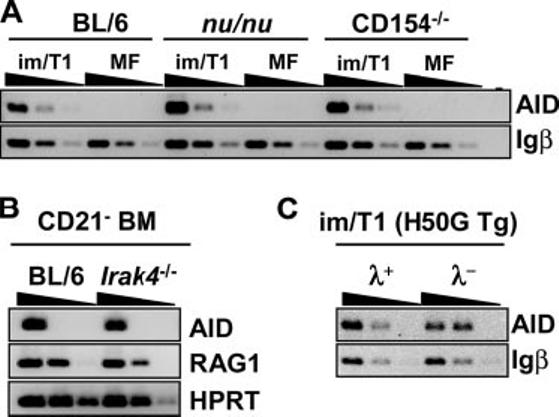
Intrinsic AID expression in im/T1 B cells. A, im/T1 and MF B cells from BL/6, CD154-/-, and nude mice were isolated by flow cytometric sorting. Semiquantitative RT-PCR (4-fold serial dilutions) for AID and Igβ was performed, and AID expression by im/T1 B cells among strains was compared. B, AID expression of CD21- BM cells from IRAK4-/- mice. C, AID expression levels in λ+ and λ- im/T1 B cells. λ+ and λ- im/T1 B cells were sorted from H50g Tg mice. λ+ H50g Tg B cells are NP specific, whereas λ- H50g Tg B cells bind diverse Ags.
CSR in im/T1 B cells
To determine whether early AID expression might mediate CSR in resting im/T1 B cells, we examined splenic im/T1 B cells from CD154-/- mice for γ3 GLT and γ3 CT. γ3 GLT predict IgM→IgG3 CSR (26, 27), and γ3 CT indicate recent and/or active CSR (14). CD154 deficiency eliminates T cell-dependent CSR without affecting B cell development (3, 28).
γ3 GLT were detected in splenic im/T1 and MZ B cells, but not MF B cells, from naive and adjuvant-immunized animals (Fig. 9A). Only trace levels of γ3 GLT could be demonstrated in im/T1 B cells from the BM, a difference that may reflect the preponderance of im (IgMlow) over T1 (IgMhigh) cells in this im/T1 compartment (Fig. 1). Message for the BLIMP-1 differentiation factor was detected in splenic im/T1 B cells as well as in MZ B cells but not in MF B cells (29) (Fig. 9A). The presence of BLIMP-1 in im/T1 cells is consistent with their capacity for rapid plasmacytic differentiation and Ab secretion (Fig. 4, A and B).
FIGURE 9.
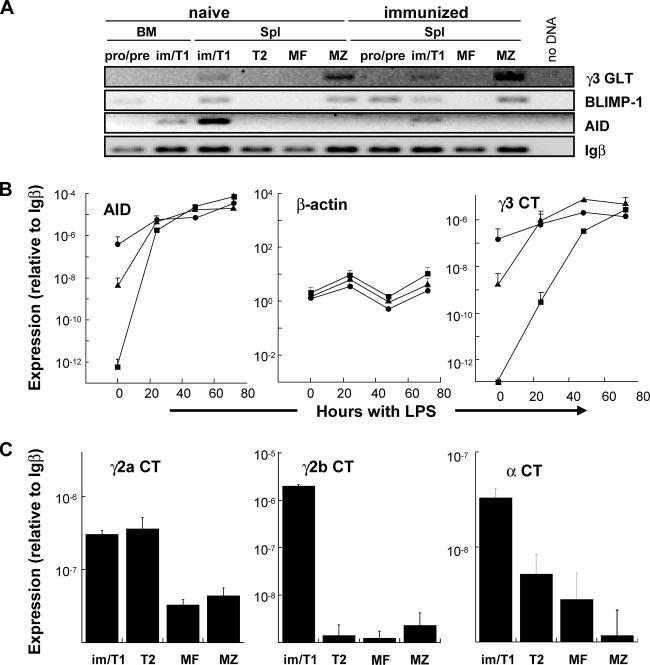
AID+ splenic im/T1 cells express products of IgG and IgA CSR. A, im/T1 B cell from naive and immunized (day 14) CD154-/- mice express AID, BLIMP-1, and γ3 GLT. RT-PCR for AID, BLIMP-1, and γ3 GLT were normalized to Igβ cDNA. Whereas AID expression is confined to the im/T1 compartment, BLIMP-1 is also expressed in MZ and some pro/pre-B cells. γ3 GLT were amplified from MZ and im/T1 B cells. B, Kinetics of LPS-induced AID and γ3 CT expression by im/T1, MF, and MZ B cells. Freshly isolated splenic im/T1 B cells (●) from CD154-/- mice express levels of AID and γ3 CT that are 101- to 106-fold higher than that of MZ (▲) and MF (■) B cells. After 72 h of culture, AID and γ3 CT levels become equivalent in all groups. Relative expression of AID, β-actin, and γ3 CT was determined by quantitative RT-PCR before and after stimulation of cells with 5 μg/ml LPS. Points represent mean (±SD) values for duplicate samples (n = 2). C, Quantification of γ2a, γ2b, and α CT by RT-PCR in im/T1, T2, MF, and MZ B cells from naive C57BL/6 mice. Expression of γ2b and α CT in freshly isolated im/T1 B cells was elevated (>10-fold) in comparison to T2, MF and MZ B cells; quantities of γ2a CT are equally abundant in im/T1 and T2 B cells but ≈10-fold lower in MF and MZ B cells. Points represent mean (±SD) of duplicate samples (n = 2).
We next compared the quantities of message for AID, γ3 CT, and β-actin to Igβ mRNA in im/T1, MZ, and MF B cells from CD154-/- mice by quantitative RT-PCR (Fig. 5B). Message levels were determined for freshly recovered cells (0 h) and cells cultured with LPS for 24, 48, and 72 h (6). Immediately ex vivo, both AID expression and γ3 CT levels were dramatically higher in im/T1 B cells than in MZ or MF B cells. The initial levels of AID message and γ3 CT in im/T1 B cells were reached by MZ and MF B cells only after exposure to LPS for 24 and 48 h, respectively (Fig. 9B). By 72 h, AID mRNA quantities in MF B cells were ≈3 logs higher, and γ3 CT levels were 10-fold higher than in freshly isolated im/T1 cells. Intrinsic γ3 CSR levels in im/T1 B cells are ≈10% of those in MF B cells fully activated by LPS (Fig. 9B).
The relative abundance of γ3 CT in unactivated im/T1 is also reflected in γ2a, γ2b, and α CT; additional RT-PCR indicated that μ→γ2a, γ2b, and α class-switch excision circles were 10- to 1000-fold more abundant in im/T1 than in MF and MZ B cells (Fig. 9C).
Discussion
Inflammation mobilizes developing lymphocytes from BM and expands some, but not all, developing B cell subsets in the spleen (Fig. 2 and Ref. 1). This extramedullary B lymphopoiesis is T cell independent (Fig. 2 and Ref. 16) and places expanded populations of im/T1 B cells constitutively expressing AID, BLIMP-1, and IgG and IgA CT (Figs. 7-9) in anatomic sites that maximize exposure to exogenous Ags (30). Does this peripheral expansion of im/T1 B cells represent a significant and physiological response?
Much to our surprise, we found that im/T1 B cells are capable of mounting humoral responses: they express TLR (Fig. 3) and respond to microbial TLR ligands by proliferation, rapid plasmacytic differentiation, and the accelerated production of IgM and IgG Ab (Figs. 3 and 4). These responses, especially proliferation and IgG production, were substantially enhanced by BAFF (Fig. 5), a B cell survival factor that is elevated by inflammation (31). In vivo, transferred im/T1 B cells efficiently responded to a bacterial vaccine by the generation of CD138+ plasmacytes (Fig. 6). The significant increase of peripheral im/T1 B cells in response to inflammation (Fig. 2) and their capacity to respond to microbial products suggest that im/T1 B cells, like B1 and MZ B cells, may represent a transient B cell compartment specialized for innate humoral immunity. Indeed, the rapidity with which im/T1 B and MZ B cells differentiate into AFC suggests a similar physiologic role (29) for these distinct compartments (Fig. 4). In an environment rich in BAFF, microbial Ags could select locally produced im/T1 B cells for expansion and Ab production. These populations could provide local IgM, IgG, and IgA Ab for opsonization, neutralization, and epithelial transfer.
Mature, Ag-specific B cells respond to Ags with cognate T cell help and initiate AID-dependent SHM and CSR (6). SHM and CSR enhance the protective capacity of Ab by increasing affinity for Ag and entry into extravascular sites. Whereas the predominant site for SHM and CSR is the CD154-dependent GC reaction (32, 33), expression of AID in im/T1 B cells does not require T cell help, CD154, or signaling via IRAK-4 (Fig. 8) but is sufficient to drive low levels of CSR (Fig. 9).
The trace levels of AID message in pro/pre-B cells increase ≈10-fold at the im/T1 stage and then fall to the limit of detection in the T2 and MF compartments (Fig. 7). It is highly unlikely that we have misidentified AID+ im/T1 B cells; these cells do not express BCL-6, a transcription factor necessary for the GC reaction (Ref. 22 and Fig. 7), and appear in CD154-/- and IRAK4-/- mice that cannot form GC (3, 28) or respond to most TLR signals (5) (Fig. 8). im/T1 B cells are CD93+B220low, a phenotype that excludes B1 B cells, a minor population of activated MF B cells, and most plasmacytes (9, 11, 12).
The abundance of AID message in im/T1 B cells (Table I) is comparable to that of LPS-activated (24 h) MF B cells and the 103/Bcl-2 pre-B (23) or WEHI-231 im B cell (24) lines, but only 2-3% of the levels in GC B cells (Fig. 7). Nonetheless, these levels support IgM→IgG and IgA CSR (Fig. 9). We note that Melamed et al. (34) have demonstrated that CSR in B cell precursors rescues B cell development in autoimmune prone μMT mice. This highly selective environment reveals that even the 7-fold lower level of AID expression in pro-B cells is sufficient for rare Ig CSR events.
Does the low level of AID expression in im/T1 B cells also support SHM? WEHI-231 cells are reported to exhibit limited SHM and express low levels of AID (Fig. 7 and Ref. 25) as do AID+ pre-B cells transformed by the Abelson murine leukemia virus (AMuLV) (35). In our hands, AID message levels in WEHI-231 cells and in the AMuLV transformant, 103-Bcl2, are similar to those present in im/T1 B cells (Fig. 7). Mao et al. (20) have reported AID expression and SHM in im B cells from mice with restricted Ab diversity but not C57BL/6 mice. Indeed, we have observed low, but statistically significant, levels of SHM in im/T1 B cells from CD154-/- mice compared with AID-/- im/T1 B cells (16.1 × 10-4 vs 1.6 × 10-4 mutations/bp sequenced; data not shown). Our experiments indicate that AID expression levels and Ig SHM vary significantly between mouse strains, an observation concordant with the “leakiness” of the μMT phenotype on the BALB/c, but not C57BL/6, genetic backgrounds (36). We do not understand the genetic basis for this variability and the role, if any, for SHM in the im/T1 compartment. Experiments to analyze the genetic basis for variable AID expression by im/T1 cells are in progress.
Constitutive AID expression in im/T1 B cells drives CSR, but to what purpose? Recently, Gourzi et al. (35) concluded that AMuLV-induced AID expression in pre-B cells caused sufficient genomic damage to limit infection by the up-regulation of the Rae-1 NKG2D ligand. This is an intriguing idea. However, AID expression in the 103/Bcl-2 AMuLV pre-B cell line is comparable to that of normal im/T1 B cells (Fig. 7), and we note that Gourzi et al. defined virus-induced AID expression by comparison to whole BM cell populations containing relatively few im/T1 B cells (35). While AID expression in im/T1 B cells is likely genotoxic—an effect that may be mitigated by BAFF (Fig. 5)—it seems unlikely to us that developmentally regulated AID expression normally acts to control im/T1 B cell numbers by NK-mediated apoptosis.
Alternatively, Ti responses by AID+ im/T1 B cells might produce Ab specificities, including autoreactive paratopes, unavailable in the MF compartment (37). The ability to produce self-reactive Ab could be beneficial since one mechanism used by microbes to evade adaptive immunity is the mimicking of host Ags (38). Autoantibody production by im/T1 B cells might not endanger the host as responses by im/T1 B cells are substantially dependent on BAFF (Fig. 5); reduced BAFF levels, e.g., by the resolution of infection, would likely end autoantibody production (18). Alternatively, autoantibody produced by peripheral im/T1 B cells might be important for the efficient clearance of cellular debris from infection sites.
Finally, if AID drives CSR in im/T1 B cells, how is it that newly formed MF B cells express IgM? CSR in im/T1 cells is undoubtedly rare, and it is likely that elevated BAFF levels are required for im/T1 B cells to survive AID-dependent genomic change (Fig. 5). The low level of AID expression in im/T1 B cells ensures that many, perhaps most, T1 B cells are undamaged and mature to cells expressing germline IgM. Under conditions that elevate BAFF and/or other survival factors, e.g., infection or inflammation (31), we predict that increasing numbers of class-switched T1 B cells will survive and mature. Indeed, CSR optimally rescues B cell development in Fas-deficient, autoimmune-prone mice (34).
Inflammation expands AID+ im/T1 B cells numbers at sites exposed to exogenous Ags. In an environment with high levels of BAFF, activated AID+ im/T1 B cells survive, respond to TLR ligands and Ag, and efficiently produce IgM and IgG Ab. We propose that these phenomena represent an inflammation-induced humoral response to infections that lie beyond the reach of IgM Ab that is unable to diffuse out of the vasculature.
Acknowledgments
We thank Dr. M. Kondo (Duke University) for help with quantitative RT-PCR and for the gift of GFP-Tg mice. We thank Drs. W. Zhang and J. Aliberti (Duke University) for their gifts of LAT- and IRAK4 deficient mice. Dr. T. Imanishi-Kari provided tissues from AID-deficient mice.
Abbreviations used in this paper:
- BM
bone marrow
- AID
activation-induced cytidine deaminase
- AFC
Ab-forming cell
- AMuLV
Abelson murine leukemia virus
- AP
alkaline phosphatase
- BAFF
B cell-activating factor
- BLIMP-1
B lymphocyte-induced maturation protein 1
- CGG
chicken gammaglobulin
- CSR
class-switch recombination
- CT
circle transcript
- CT
threshold cycle
- GC
germinal center
- GLT
germline transcript
- im
immature
- IRAK4
IL-1R-associated kinase 4
- LAT
linker of activated T cell
- MF
mature follicular
- MZ
marginal zone
- NP
(4-hydroxy-3-nitrophenyl)acetyl
- SA
streptavidin
- SHM
somatic hypermutation
- T1
translational 1
- Tg
transgenic
- Ti
T cell independent
Footnotes
This work was supported by National Institutes of Health Grants AI-49326 and AI-24335 and the Duke University Autoimmunity Center of Excellence Grant AI-56363.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J. Exp. Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, Staudt LM. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 3.Renshaw BR, Fanslow WC, III, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand-deficient mice. J. Exp. Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, et al. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki N, Suzuki S, Duncan GS, Millar DG, Wada T, Mirtsos C, Takada H, Wakeham A, Itie A, Li S, et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–756. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- 6.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 7.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. “Green mice” as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 8.Dal Porto JM, Haberman AM, Shlomchik MJ, Kelsoe G. Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J. Immunol. 1998;161:5373–5381. [PubMed] [Google Scholar]
- 9.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 10.Han S, Dillon SR, Zheng B, Shimoda M, Schlissel MS, Kelsoe G. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278:301–305. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- 11.Li YS, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5:527–535. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
- 12.Rolink AG, Andersson J, Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur. J. Immunol. 1998;28:3738–3748. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.Monroe RJ, Seidl KJ, Gaertner F, Han S, Chen F, Sekiguchi J, Wang J, Ferrini R, Davidson L, Kelsoe G, Alt FW. RAG2:GFP knockin mice reveal novel aspects of RAG2 expression in primary and peripheral lymphoid tissues. Immunity. 1999;11:201–212. doi: 10.1016/s1074-7613(00)80095-3. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita K, Harigai M, Fagarasan S, Muramatsu M, Honjo T. A hallmark of active class switch recombination: transcripts directed by I promoters on looped-out circular DNAs. Proc. Natl. Acad. Sci. USA. 2001;98:12620–12623. doi: 10.1073/pnas.221454398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J. Exp. Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gartner F, Alt FW, Monroe RJ, Seidl KJ. Antigen-independent appearance of recombination activating gene (RAG)-positive bone marrow B cells in the spleens of immunized mice. J. Exp. Med. 2000;192:1745–1754. doi: 10.1084/jem.192.12.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogata H, Su I, Miyake K, Nagai Y, Akashi S, Mecklenbrauker I, Rajewsky K, Kimoto M, Tarakhovsky A. The Toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J. Exp. Med. 2000;192:23–29. doi: 10.1084/jem.192.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao C, Jiang L, Melo-Jorge M, Puthenveetil M, Zhang X, Carroll MC, Imanishi-Kari T. T cell-independent somatic hypermutation in murine B cells with an immature phenotype. Immunity. 2004;20:133–144. doi: 10.1016/s1074-7613(04)00019-6. [DOI] [PubMed] [Google Scholar]
- 21.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 23.Chen YY, Wang LC, Huang MS, Rosenberg N. An active v-abl protein tyrosine kinase blocks immunoglobulin light-chain gene rearrangement. Genes Dev. 1994;8:688–697. doi: 10.1101/gad.8.6.688. [DOI] [PubMed] [Google Scholar]
- 24.Gutman GA, Warner NL, Harris AW. Immunoglobulin production by murine B lymphoma cells. Clin. Immunol. Immunopathol. 1981;18:230–244. doi: 10.1016/0090-1229(81)90029-5. [DOI] [PubMed] [Google Scholar]
- 25.Spillmann FJ, Wabl M. Endogenous expression of activation-induced cytidine deaminase in cell line WEHI-231. J. Immunol. 2004;173:1858–1867. doi: 10.4049/jimmunol.173.3.1858. [DOI] [PubMed] [Google Scholar]
- 26.Stavnezer-Nordgren J, Sirlin S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 1986;5:95–102. doi: 10.1002/j.1460-2075.1986.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yancopoulos GD, DePinho RA, Zimmerman KA, Lutzker SG, Rosenberg N, Alt FW. Secondary genomic rearrangement events in pre-B cells: VHDJH replacement by a LINE-1 sequence and directed class switching. EMBO J. 1986;5:3259–3266. doi: 10.1002/j.1460-2075.1986.tb04637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 29.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 30.Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- 31.MacLennan I, Vinuesa C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity. 2002;17:235–238. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 32.Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. Cellular interaction in germinal centers: roles of CD40 ligand and B7-2 in established germinal centers. J. Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- 33.Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melamed D, Miri E, Leider N, Nemazee D. Unexpected autoantibody production in membrane Ig-μ-deficient/lpr mice. J. Immunol. 2000;165:4353–4358. doi: 10.4049/jimmunol.165.8.4353. [DOI] [PubMed] [Google Scholar]
- 35.Gourzi P, Leonova T, Papavasiliou FN. A role for activation-induced cytidine deaminase in the host response against a transforming retrovirus. Immunity. 2006;24:779–786. doi: 10.1016/j.immuni.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 36.Orinska Z, Osiak A, Lohler J, Bulanova E, Budagian V, Horak I, Bulfone-Paus S. Novel B cell population producing functional IgG in the absence of membrane IgM expression. Eur. J. Immunol. 2002;32:3472–3480. doi: 10.1002/1521-4141(200212)32:12<3472::AID-IMMU3472>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 37.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 38.Haynes BF, Fleming J, St. Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]


