Abstract
Background and aim
To evaluate the diagnostic accuracy of magnetic resonance colonography (MRC) without bowel cleansing in a screening population and compare the results to colonoscopy as a standard of reference.
Methods
315 screening patients, older than 50 years with a normal risk profile for colorectal cancer, were included in this study. For MRC, a tagging agent (5.0% Gastrografin, 1.0% barium sulphate, 0.2% locust bean gum) was ingested with each main meal within 2 days prior to MRC. No bowel cleansing was applied. For the magnetic resonance examination, a rectal water enema was administered. Data collection was based on contrast enhanced T1 weighted images and TrueFISP images. Magnetic resonance data were analysed for image quality and the presence of colorectal lesions. Conventional colonoscopy and histopathological samples served as reference.
Results
In 4% of all colonic segments, magnetic resonance image quality was insufficient because of untagged faecal material. Adenomatous polyps >5 mm were detected by means of MRC, with a sensitivity of 83.0%. Overall specificity was 90.2% (false positive findings in 19 patients). However, only 16 of 153 lesions <5 mm and 9 of 127 hyperplastic polyps could be visualised on magnetic resonance images.
Conclusions
Faecal tagging MRC is applicable for screening purposes. It provides good accuracy for the detection of relevant (ie, adenomatous) colorectal lesions >5 mm in a screening population. However, refinements to optimise image quality of faecal tagging are needed.
Colorectal cancer (CRC) is the second leading cause of cancer death in Western countries.1 The vast majority of CRC develop from benign colonic adenomas/polyps over a time scale of several years.2 Screening programmes targeting precancerous polyps and subsequent endoscopic polypectomy have been shown to considerably reduce CRC mortality. Although colonoscopy has been established as an accurate method for the assessment of the colon, a large discrepancy between the screening potential and clinical reality remains apparent. Even in countries with free access to this diagnostic procedure, participation in cancer screening programmes based on colonoscopy is suboptimal.3
Upcoming non‐invasive alternative methods are mainly based on cross sectional imaging, such as CT and MRI.4,5 By means of post‐processing software, acquired CT or MRI data can be reconstructed into a virtual endoscopic view or fly through. Because of the higher clinical availability of scanners and lower costs, most approaches to date have focused on CT colonoscopy (CTC). Despite promising diagnostic results, the long term impact of CTC as a screening method remains uncertain, with the associated ionising radiation burden raising the possibility of a public health concern.6,7 A compelling rationalisation for pursuing magnetic resonance colonography (MRC) is safety and avoidance of risks associated with exposure to ionising radiation of an otherwise mostly healthy screening population. While the diagnostic effectiveness of CTC has already been assessed in a screening cohort,5 MRC approaches have been evaluated only in high risk populations with a limited number of subjects.8,9,10 In addition, most MRC protocols in the past required bowel purgation similar to the preparation for colonoscopy. However, this part of the procedure is considered very unpleasant by the vast majority of patients11,12 and negatively impacts on acceptance levels. The requirement for bowel cleansing can be reduced by adding contrast agents to regular meals prior to the magnetic resonance examination, thereby modulating the signal characteristics of faecal material (faecal tagging).
The aim of the present study was to assess the diagnostic accuracy of a faecal tagging based MRC protocol in a screening population compared with colonoscopy serving as the gold standard.
Material and method
Subjects
To evaluate a screening population group, a national health insurance company offered their members inclusion in this trial. Randomly selected subjects over 50 years were informed about the study and asked to participate voluntarily. Exclusion criteria were based on a prior history of colorectal cancer or polyps, rectal bleeding, positive faecal occult blood tests or altered bowel habits within the previous 12 months, colonoscopy within the previous 5 years and/or general contraindications to MRI, such as the presence of a pacemaker, metallic implants in the central nervous system or severe claustrophobia. A standardised questionnaire was completed by all potential participants. The study was conducted in accordance with the guidelines set forth by the approving institutional review board. Informed consent was obtained prior to each examination.
Between December 2003 and August 2005, 660 persons were informed of this ongoing study. A total of 414 people responded and eventually participated in the trial. The study group included 223 women and 191 men, aged 50–81 years (median 63). An appointment for MRC was given to the participants within an average time of 15.5 days. Ninety‐four people abandoned the trial after the magnetic resonance examination. Documented reasons included fear and presumable discomfort from the colonoscopy. Thus data for a total number of 320 participants were analysed.
Magnetic resonance colonography
Participants did not undergo bowel cleansing prior to MRC. Instead, a faecal tagging based preparation protocol was applied. A solution containing 5% Gastrografin (Schering, Germany), 1% barium (Polibar, Ezem, USA) and 0.2% locust bean gum (Roeper, Germany) was ingested in portions of 250 ml with each regular meal, starting 48 h prior to the magnetic resonance examination. No dietary restrictions were applied. MRC was performed on a state of the art 1.5 T magnetic resonance system (Magnetom Sonata; Siemens Medical Solutions, Germany). To allow coverage of the entire colon, a set of two large surface array coils were used for signal reception. The examination was performed with the patient in the prone position. For bowel distension, the colon was filled with approximately 2000 ml of warm tap water using hydrostatic pressure (1–1.5 m water column). Prior to rectal filling, 40 mg of scopolamine (Buscopan; Boehringer Ingelheim, Germany) were administered to minimise bowel peristalsis. In the case of known glaucoma or prostate hyperplasia, 1 mg of glucagon hydrochloride (Glucagen; Novo Nordisk, Germany) was given instead. Sedative or analgesic agents were not administered.
T1 weighted three dimensional gradient echo sequences with integrated fat suppression were acquired in the coronal plane. The T1 weighted three dimensional gradient echo sequence was characterised by the following parameters: repetition time (TR) 3.08 ms; echo time (TE) 1.13 ms; flip angle 35°; 50 cm field of view (FOV) in the z‐direction; and matrix size 168×256. Slice thickness was adjusted depending on the patient's size in the range 1.8–2.4 mm to allow an acquisition time of less than 20 s under breath‐hold conditions. After a pre‐contrast data acquisition, a gadolinium compound (Gd‐DOTA; Dotarem, Guerbet, France) was administered intravenously using an automatic injector (Spectris; Medrad, Germany) at a dose of 0.2 mmol/kg body weight and a flow rate of 2 ml/s, followed by rapid injection of 20 ml of normal saline at the same rate. The identical three dimensional sequence was collected after a contrast delay of 75 s. Further data acquisition was based on contrast enhanced fat suppressed axial flash sequences (TR/TE/flip/FOV/matrix 125/1.83/70°/50/168×256) and a TrueFISP sequence in the coronal plane (TR/TE/flip/FOV/matrix 3.79/1.9/70°/40/205×256). After completion of data acquisition, the enema bag was placed on the floor for facilitated emptying of the colon, and the patient was removed from the scanner.
MRC data analysis
Data sets were evaluated on a post processing workstation (Advantage Workstation; GE Healthcare, USA) providing interactive multiplanar viewing and surface rendering three dimensional displays of colorectal structures. Reformations were assessed by scrolling through the three dimensional data sets in all three orthogonal planes as well as in oblique planes and on the basis of virtual endoscopic views. For diagnostic purposes, the colon was divided into five parts: (a) caecum and ascending colon, (b) transverse colon, (c) descending colon, (d) sigmoid colon and (e) rectum. Images were analysed in a consensus mode by two experienced radiologists. Localisation and size of all lesions were recorded. Furthermore, the effectiveness of the faecal tagging in terms of visualisation of stool was assessed by a visual 5 grade ranking for every colonic segment (5 = very good image quality/no stool particles visible, 1 = no diagnostic image quality/large amounts of visible stool). Image quality regarding bowel distension and motion artefacts was evaluated using a visual 3 grade ranking (3 = good, 2 = moderate, 1 = non‐diagnostic image quality).
Colonoscopy
All patients underwent conventional colonoscopy within 4 weeks following the magnetic resonance examination at our Department of Gastroenterology, where more than 1000 colonoscopies are performed every year. A standardised protocol for bowel purgation was applied, starting on the day prior to colonoscopy. An electrolyte solution (4000 ml of Golytely; Braintree Laboratories, USA) was ingested. For the endoscopic procedure, standard equipment was used (CFQ 140; Olympus, Japan). Sedatives (midazolam hydrochloride, Dormicum; Roche, Germany) and analgesics (pethidine, Dolantin; Hoechst, Germany) were administered. The attending gastroenterologist (minimum of 3 years' experience in colonoscopy, more than 200 colonoscopies during the previous 12 months) was unaware of the magnetic resonance findings. Localisation, size of the colorectal lesions and morphology were recorded and polypectomies/biopsies were performed. Immediately after completion of colonoscopy, the results of MRC were revealed. When a colorectal lesion on MRC had been recorded that was not seen on colonoscopy, the analogous colonic segment was reassessed by colonoscopy in the same session. All biopsies were analysed by histopathologically grading the specimen as hyperplastic, adenomatous, inflammatory, lipoid or carcinomatous.
Statistical analysis
For statistical analysis, the results of colonoscopy and histopathology served as the standard of reference. Findings were classified into three groups: (a) lesions <5 mm in size, (b) lesions between 5 and 10 mm and (c) lesions >10 mm. Special attention was paid to adenomatous lesions of groups (b) and (c) as these findings are of mayor concern within a screening population. Evaluation was performed on a per lesion and per patient basis. In the case of multiple polyps, patient groups were ranked depending on the largest lesion. Diagnostic accuracy was determined by calculating point estimates for sensitivity and specificity as well as for positive and negative predictive values.
Results
For the 320 participants undergoing both MRC and colonoscopy, colonoscopy was incomplete in 18 cases because of elongated bowel segments, stenoses or insufficient bowel preparation. In one patient, colonoscopy had to be aborted because of a bowel perforation and immediate surgery was performed. Retrospective analysis revealed chronic diverticulitis in this patient, which may have contributed to this complication. MRC was aborted in five cases because of water leakage (n = 4) or severe pain (n = 1). In these five patients, no images were acquired. Colonic segments without colonoscopy or MRC correlation were excluded from data analysis. Hence, a total number of 1532 colonic segments in 315 patients were analysed.
Faecal tagging/MRC image quality
The overall score for all colonic segments, analysed for the effect of faecal tagging, was 3.7 (1.2). Dedicated results are displayed in table 1.
Table 1 Ranking of image quality regarding faecal tagging based on a 5 point scale.
| Ranking | No (%) of colonic segments |
|---|---|
| 1 | 67 (4.4%) |
| 2 | 217 (14.2%) |
| 3 | 280 (18.3%) |
| 4 | 513 (33.5%) |
| 5 | 455 (29.7%) |
5 = very good image quality/no stool particles visible, 1 = no diagnostic image quality because of large amounts of visible stool.
In 4.4% of all colonic segments, there was a large amount of untagged stool, resulting in a ranking score of 1 and impeding reliable assessment of the colorectal wall. Examples of good and poor faecal tagging effectiveness are shown in figs 1 and 2. Analysis of image quality, as well as luminal distension and motion artefacts, resulted in an overall score of 2.8 (0.5). Inadequate distension or severe motion artefacts were found in 5% of colonic segments.
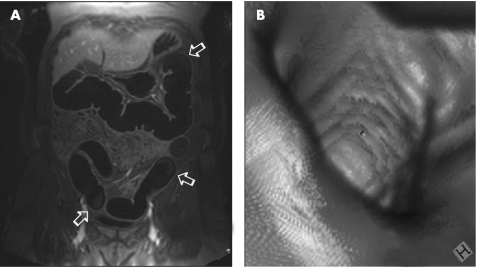
Figure 1 (A) Example of good faecal tagging effectiveness. The remaining stool in the transverse, descending and sigmoid colon (arrows) shows very low signal intensities on T1w images and, therefore, can hardly be discriminated from the dark rectal enema. Thus, an assessment of the bright appearing bowel wall is possible throughout the large bowel. (B) Virtual endoscopic views can be generated by dedicated software systems.
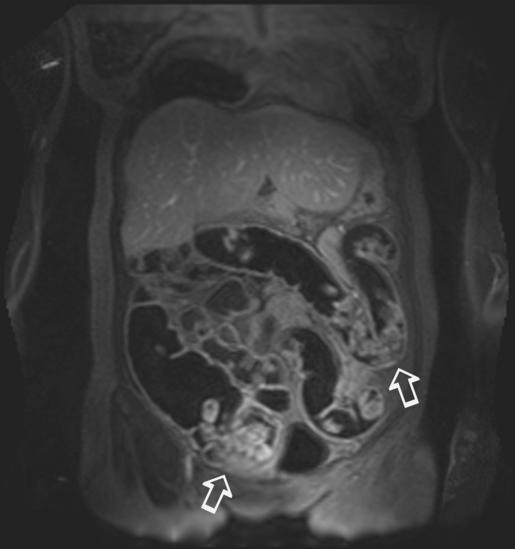
Figure 2 Example of poor faecal tagging effectiveness with large amounts of signal‐intense faecal material (arrows). In this case, a reliable evaluation of the bowel wall throughout the colon is not possible.
Diagnostic value of MRC
Following colonoscopy and histopathology, a total of 235 colorectal lesions were found in 121 patients. On magnetic resonance images, 67 of these 235 lesions were diagnosed. Significant differences were determined regarding the ability to detect lesions depending on their size: while only 16 of 153 lesions <5 mm (group a) were depicted, 17 of 23 lesions >10 mm were correctly detected (group c). Concerning lesions of 5–10 mm in size (group b), 34 of 59 lesions were correctly visualised by MRI. All sensitivity values are shown in table 2.
Table 2 Sensitivity of magnetic resonance colonography on a lesion basis compared with the gold standard of colonoscopy.
| <5 mm (group a) | 5–10 mm (group b) | >10 mm (group c) | >5 mm (groups b+c) | All | |
|---|---|---|---|---|---|
| No (C) | 153 | 59 | 23 | 82 | 235 |
| No (MRC) | 16 | 34 | 17 | 51 | 67 |
| Sensitivity | 10.5 | 57.6 | 73.9 | 62.2 | 28.5 |
| False positive findings of MRC | 14 | 8 | 0 | 8 | 22 |
C, Colonoscopy; MRC, magnetic resonance colonography.
For 31 lesions >5 mm missed by MRC (groups b+c), 18 were located in bowel segments with poor faecal tagging effectiveness (n = 13) or insufficient image quality (n = 5).
In the 121 patients with colorectal lesions, 44 were also rated as having colorectal lesions on MRC, resulting in an overall patient based sensitivity of 36.4%. For lesions of 5–10 mm (group b), sensitivity was 60.0% and increased to 70.0% for lesions larger than 10 mm in size (group c) (fig 3). The presence of polyps had been described initially by MRC in 22 patients without confirmation by colonoscopy. These patients were immediately reassessed. However, in only 3 of 22 patients was a colorectal lesion revealed. Thus false positive findings were seen on MRC in 19 patients. The corresponding point estimates for sensitivity, specificity, positive/negative predictive value and accuracy are shown in table 3.
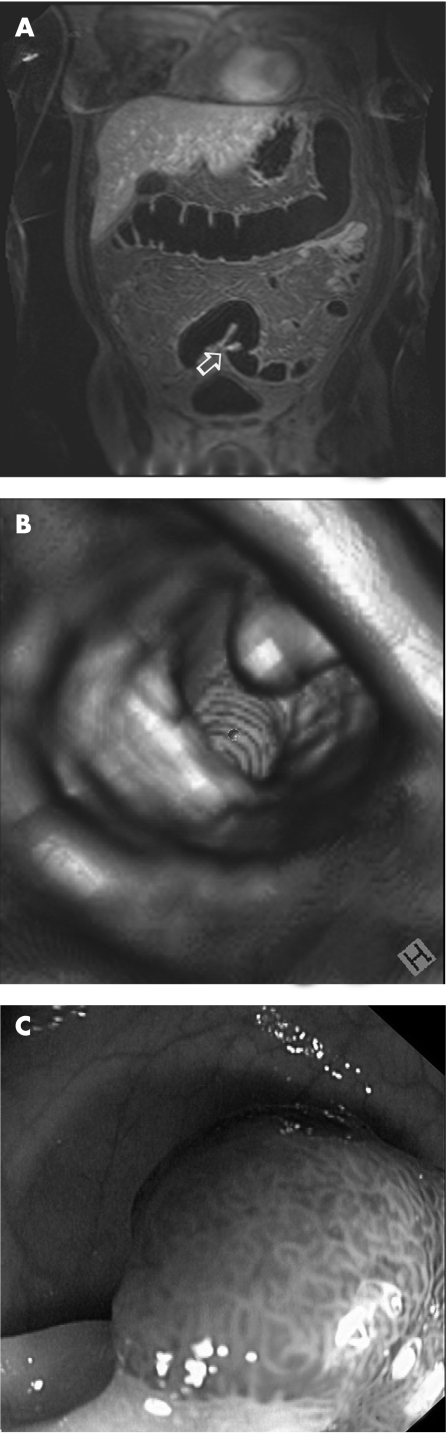
Figure 3 (A) Magnetic resonance colonography source image (arrow); (B) virtual reconstruction; and (C) optical colonoscopy of a 12mm polyp in the sigmoid colon.
Table 3 Diagnostic value of magnetic resonance colonography on a patient basis.
| <5 mm (group a) | 5–10 mm (group b) | >10 mm (group c) | >5 mm (groups b+c) | All | |
|---|---|---|---|---|---|
| No (C) | 56 | 45 | 20 | 52 | 121 |
| No (MRC) | 3 | 27 | 14 | 33 | 44 |
| Sensitivity (%) | 5.4 | 60.0 | 70.0 | 63.5 | 36.4 |
| Specificity (%) | 95.0 | 97.8 | 100 | 97.9 | 90.2 |
| NPV (%) | 82.3 | 93.6 | 98.0 | 93.1 | 69.4 |
| PPV (%) | 18.8 | 81.8 | 100.0 | 84.6 | 69.8 |
| Accuracy (%) | 79.0 | 92.4 | 98.1 | 92.1 | 69.5 |
| False positive findings of MRC | 13 | 6 | 0 | 6 | 19 |
C, colonoscopy; MRC, magnetic resonance colonography; NPV, negative predictive value; PPV, positive predictive value.
Note: If multiple lesions of different sizes were found in a patient, the patient group was ranked depending on the largest lesion. If MRC showed both false positive and positive findings in a patient, the patient group was ranked according to the positive findings.
Histopathological analysis graded colorectal lesions as hyperplastic (n = 127), adenomatous (n = 74), inflammatory (n = 24), lipoid (n = 9) and carcinomatous (n = 1). Regarding the histopathological differentiation of false negative results on MRC, it was shown that most of the missed polyps were hyperplastic (table 4).
Table 4 Lesions missed by magnetic resonance colonography correlated with histopathology.
| Histopathology | n (%) all lesions | n (%) lesions >5 mm |
|---|---|---|
| Hyperplastic | 118 of 127 (92.9%) | 21 of 28 (75%) |
| Adenomatous | 23 of 74 (31.1%) | 7 of 42 (16.7%) |
| Inflammatory | 22 of 24 (91.2%) | 1 of 3 (33.3%) |
| Lipoid | 9 of 9 (100%) | 2 of 2 (100%) |
However, MRC was able to correctly display 35 of 42 adenomatous lesions >5 mm (groups b+c), resulting in a sensitivity of 83.3%. Reanalysing the cases of the seven missed adenomatous lesions revealed poor faecal tagging in four cases and motion artefacts in two cases. For adenomatous polyps >10 mm (group c), sensitivity was as high as 81.3% (table 5).
Table 5 Analysis of adenomatous lesions.
| <5 mm (group a) | 5–10 mm (group b) | >10 mm (group c) | >5 mm (group b+c) | All | |
|---|---|---|---|---|---|
| Lesion based | |||||
| No (C) | 32 | 26 | 16 | 42 | 74 |
| No (MRC) | 12 | 22 | 13 | 35 | 51 |
| Sensitivity | 37.5% | 84.6% | 81.3% | 83.3% | 68.9% |
| Patient based | |||||
| No (C) | 24 | 21 | 15 | 35 | 55 |
| No (MRC) | 10 | 17 | 13 | 28 | 37 |
| Sensitivity | 41.7% | 81.0% | 86.7% | 80% | 67.3% |
C, colonoscopy; MRC, magnetic resonance colonography.
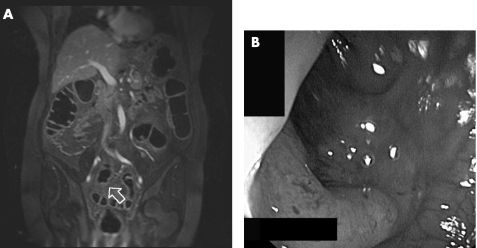
Furthermore, one additional lesion was rated as an adenocarcinoma, which was concordant with the endoscopic finding (fig 4).
Figure 4 (A) Magnetic resonance colonography source image (arrow); and (B) optical colonoscopy of a stenotic carcinoma in the sigmoid colon.
Discussion
The present study had three important findings. Firstly, MRC without prior bowel cleansing is feasible and leads to diagnostic image quality in over 90% of all examined colonic segments. Secondly, the overall detection rate of colorectal polyps is only moderate, with sensitivity values of less than 50%. However, sensitivity is more than 80% for adenomatous polyps >5 mm, which are the main targets of colorectal cancer screening. Finally, specificity rates and negative predictive values were found to be more than 90% for lesions >5 mm, which is particularly important for a screening method.
Colonoscopy has been considered the gold standard for colorectal cancer screening. Apart from a high diagnostic accuracy, it provides the possibility of simultaneously performing polypectomy or histological sampling. However, there are some drawbacks inherent in this technique. Depending on the experience of the endoscopist, the procedure may be incomplete in up to 19% of cases, not allowing for a full assessment of the large bowel.13,14 Furthermore, there is a small, but existing, risk of bowel perforation, which was seen in one of our patients. Hence it is controversial whether colonoscopy really represents an ideal gold standard. The underlying rationale is related to the fact that a considerable number of polyps, mostly close to haustral folds, may be missed even in back to back colonoscopies.5,15 Thus there is a general interest in finding alternatives to colonoscopy.
Virtual colonography has been used increasingly over the past decade. There are different indications for this modality. Above all, its implementation in patients with incomplete colonoscopy is widely accepted.16,17 Even in the presence of high grade stenoses or elongated bowel loops, which might not be passed by an endoscope, the pre‐stenotic bowel segments can be assessed by virtual colonography. However, there are only few data on the impact of virtual colonography as a device for colorectal cancer screening. The largest number of screening patients to date has been evaluated in a trial using CT technology.5 Pickhardt et al examined more than 1200 healthy subjects using CT and compared their results with colonoscopy. The sensitivity rates determined for the detection of adenomatous polyps >5 mm and >10 mm were 88.7% and 93.8%, respectively. These findings are almost in agreement with the results of our trial. However, multicentre trials report much lower accuracy of CT based virtual endoscopy, with a sensitivity ranging from 55% to 59% for the detection of colorectal lesions >1 cm.18,19 However, consistency of expertise and technical uniformity may be questioned in a multicentre study.
Also, there still remains the burden of radiation exposure despite the use of low dose protocols for CT colonography.20 This is even more relevant as data acquisition for CT colonography is performed both in supine and prone positions. Recently, lifetime attributable risk estimates for developing a radiation induced malignancy in a regularly screened population using CT have been as high as 1 in 50 patients.21 Furthermore, most current CT protocols obviate the administration of intravenous contrast because of the potential risk of anaphylactic reactions or impairment of renal function.22,23 However, the use of intravenous contrast compounds has been shown to increase specificity rates as colorectal lesions can be more accurately distinguished from residual faecal material.24,25 This is one of the main advantages of MRI, because intravenously applied contrast agents have a far better safety profile and can even be used in patients with renal failure.26,27
MRC was first described by Luboldt et al in 1997.28 Initial approaches were based on so‐called “bright lumen” techniques, which applied gadolinium containing enemas. Ever since, the modality has been modified and improved. One important innovation was the introduction of “dark lumen” MRC.29 An enema consisting of tap water was used and additional intravenous contrast was given. It could be shown that this dark lumen technique was more accurate than the initial bright lumen approaches because a more reliable differentiation between residual faecal material and polyps was possible.29,30 Furthermore, examination times were reduced as data acquisition only needed to be performed either in the prone or supine position. The diagnostic potential of dark lumen MRC has been assessed in two larger trials involving patients with increased risks of colorectal cancer and/or gastrointestinal symptoms.8,9 Ajaj et al examined 122 patients using MRC and compared their findings with subsequent conventional colonoscopy.8 While colorectal lesions <5 mm could not be depicted in this trial, sensitivity and specificity for visualisation of lesions >5 mm were 93% and 100%, respectively. Similar findings were reported by Hartmann et al.9 In their study, 100 patients were included and conventional colonoscopy was again used as the reference. Adenomatous polyps were detected with a sensitivity of 84% for lesions between 5 and 10 mm (100% for lesions >10 mm). Despite the lower prevalence of polyps in our screening population, the results of the present study confirm the findings of Hartmann et al.
In addition, most hyperplastic lesions were not detected by MRC, even in retrospective re‐analysis, which is concordant with previous studies.9,31 However, we have to consider adenomatous lesions as the main screening target as hyperplastic lesions show no or only little potential for malignant transformation.32,33 Similarly, the relatively poor overall sensitivity of MRC in our trial needs to be discussed in this context; an overall sensitivity of less than 50% for a screening method is not acceptable. However, we found that over two‐thirds of the polyps detected by the reference standard were smaller than 5 mm (independent of their histology). Most authors claim little importance of these small lesions because of their reduced risk of malignant transformation.21,34 In other clinical trials the benchmark for relevant lesions was even considered to be 10 mm.5,35 Taking these guidelines into account, the sensitivity for the detection of adenomatous polyp >5 mm was more than 80% in our study. Thus the outlined MRC concept may be regarded as a suitable screening tool. However, one limitation will be flat adenomas, which are likely to remain elusive.
An important prerequisite for screening modalities is high patient acceptance. Although patient preferences were not particularly evaluated in this trial, virtual colonography may be considered an alternative to colonoscopy. Apparently, patients are less likely to undergo a screening test when negative expectations, including pain and discomfort, are involved.36 Only moderate participation was determined in some screening programmes for colonoscopy in the past.37,38 This can be indirectly confirmed by our data as almost 25% of the initial 414 patients eventually refused to undergo colonoscopy. There have been several studies evaluating patient preferences between conventional colonoscopy and virtual colonography. The outcome has been very inconsistent, with some trials claiming virtual colonography to be more accepted and other studies preferring colonoscopy.39,40 However, there seems to be a definite potential for virtual non‐invasive modalities as there will always be a certain proportion of patients who will refuse to be screened by conventional colonoscopy. This plays an even greater role as bowel cleansing has been determined to be the most unpleasant part of colonic examinations.41 Thus the faecal tagging protocol used for MRC in the present study, which obviates bowel purgation, may be one key criterion for subjects to accept virtual MRC as a screening modality. The present technique is not without its limitations. Whenever a colorectal polyp is found by faecal tagging based MRC, patients have to undergo bowel cleansing for subsequent colonoscopy and polypectomy. However, we found that only approximately 10% of subjects showed an adenomatous polyp >5 mm who then would have had to undergo additional colonoscopy outside the clinical trial.
Clearly, there are certain limitations regarding the outlined MRC concept with faecal tagging. First and foremost, we have to be aware that MRC was not diagnostic in approximately 5% of all colonic segments because of failure of faecal tagging. It is still speculative as to whether these patients were non‐compliant with ingestion of the tagging agent or if other factors, such as bowel transit times, may be the reason for this limitation. Nevertheless, there is still a need for improving the faecal tagging outcome. Similarly, some colonic segments could not be interpreted because of motion artefacts. This was assumedly due to the length of the acquisition times and the need to perform data collection under breath‐hold conditions. With the implementation of new sequence types and image reconstruction algorithms, including parallel imaging, these artefacts will probably impede image interpretation less often in the future. Finally, a relatively large number of patients refused to undergo colonoscopy after MRC. Again, one can wonder if there is a certain bias for this patient group and whether results would have been different if these subjects had been included. However, this fact underlines the fear that some patients have of undergoing an invasive screening test.
Despite the drawbacks of this study, we demonstrated a high detection rate of faecal tagging based MRC for lesions that are important within a screening cohort. Because of the high diagnostic accuracy and the possibility of simultaneous polypectomy, conventional colonoscopy will keep on playing a leading role as a screening tool. However, for patients unwilling to undergo colonoscopy, MRC should be regarded as an attractive alternative.
Abbreviations
CRC - colorectal cancer
CTC - computed tomography colonoscopy
FOV - field of view
MRC - magnetic resonance colonography
TE - echo time
TR - repetition time
Footnotes
This study was supported by grant 70‐3006 from the German Cancer Aid (Deutsche Krebshilfe).
Competing interests: None.
References
- 1.Jemal A, Tiwari R C, Murray T.et al Cancer statistics, 2004. CA Cancer J Clin 2004548–29. [DOI] [PubMed] [Google Scholar]
- 2.Grady W M. Epigenetic events in the colorectum and in colon cancer. Biochem Soc Trans 200533684–688. [DOI] [PubMed] [Google Scholar]
- 3. Increased use of colorectal cancer tests—United States, 2002 and 2004. MMWR Morb Mortal Wkly Rep 200655308–311. [PubMed] [Google Scholar]
- 4.Geenen R W, Hussain S M, Cademartiri F.et al CT and MR colonography: scanning techniques, postprocessing, and emphasis on polyp detection. Radiographics 200424e18. [DOI] [PubMed] [Google Scholar]
- 5.Pickhardt P J, Choi J R, Hwang I.et al Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 20033492191–2200. [DOI] [PubMed] [Google Scholar]
- 6.Brenner D J, Elliston C D. Estimated radiation risks potentially associated with full‐body CT screening. Radiology 2004232735–738. [DOI] [PubMed] [Google Scholar]
- 7.Debatin J F, Luboldt W, Bauerfeind P. Virtual colonoscopy in 1999: computed tomography or magnetic resonance imaging? Endoscopy 199931174–179. [DOI] [PubMed] [Google Scholar]
- 8.Ajaj W, Pelster G, Treichel U.et al Dark lumen magnetic resonance colonography: comparison with conventional colonoscopy for the detection of colorectal pathology. Gut 2003521738–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartmann D, Bassler B, Schilling D.et al Colorectal polyps: detection with dark‐lumen MR colonography versus conventional colonoscopy. Radiology 2006238143–149. [DOI] [PubMed] [Google Scholar]
- 10.Pappalardo G, Polettini E, Frattaroli F M.et al Magnetic resonance colonography versus conventional colonoscopy for the detection of colonic endoluminal lesions. Gastroenterology 2000119300–304. [DOI] [PubMed] [Google Scholar]
- 11.Tangka F K, Molinari N A, Chattopadhyay S K.et al Market for colorectal cancer screening by endoscopy in the United States. Am J Prev Med 20052954–60. [DOI] [PubMed] [Google Scholar]
- 12.Thomeer M, Bielen D, Vanbeckevoort D.et al Patient acceptance for CT colonography: what is the real issue? Eur Radiol 2002121410–1415. [DOI] [PubMed] [Google Scholar]
- 13.Dafnis G, Granath F, Pahlman L.et al Patient factors influencing the completion rate in colonoscopy. Dig Liver Dis 200537113–118. [DOI] [PubMed] [Google Scholar]
- 14.Nelson D B, McQuaid K R, Bond J H.et al Procedural success and complications of large‐scale screening colonoscopy. Gastrointest Endosc 200255307–314. [DOI] [PubMed] [Google Scholar]
- 15.Rex D K, Cutler C S, Lemmel G T.et al Colonoscopic miss rates of adenomas determined by back‐to‐back colonoscopies. Gastroenterology 199711224–28. [DOI] [PubMed] [Google Scholar]
- 16.Ajaj W, Lauenstein T C, Pelster G.et al MR colonography in patients with incomplete conventional colonoscopy. Radiology 2005234452–459. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann D, Bassler B, Schilling D.et al Incomplete conventional colonoscopy: magnetic resonance colonography in the evaluation of the proximal colon. Endoscopy 200537816–820. [DOI] [PubMed] [Google Scholar]
- 18.Cotton P B, Durkalski V L, Pineau B C.et al Computed tomographic colonography (virtual colonoscopy): a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA 20042911713–1719. [DOI] [PubMed] [Google Scholar]
- 19.Rockey D C, Paulson E, Niedzwiecki D.et al Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005365305–311. [DOI] [PubMed] [Google Scholar]
- 20.Rogalla P, Meiri N, Hoksch B.et al Low‐dose spiral computed tomography for measuring abdominal fat volume and distribution in a clinical setting. Eur J Clin Nutr 199852597–602. [DOI] [PubMed] [Google Scholar]
- 21.Bond J H. Clinical relevance of the small colorectal polyp. Endoscopy 200133454–457. [DOI] [PubMed] [Google Scholar]
- 22.Ferrucci J T. Colon cancer screening with virtual colonoscopy: promise, polyps, politics. AJR Am J Roentgenol 2001177975–988. [DOI] [PubMed] [Google Scholar]
- 23.Ferrucci J T. Colonoscopy: virtual and optical—another look, another view. Radiology 200523513–16. [DOI] [PubMed] [Google Scholar]
- 24.Fletcher J G, Johnson C D, Krueger W R.et al Contrast‐enhanced CT colonography in recurrent colorectal carcinoma: feasibility of simultaneous evaluation for metastatic disease, local recurrence, and metachronous neoplasia in colorectal carcinoma. AJR Am J Roentgenol 2002178283–290. [DOI] [PubMed] [Google Scholar]
- 25.Laghi A, Iannaccone R, Bria E.et al Contrast‐enhanced computed tomographic colonography in the follow‐up of colorectal cancer patients: a feasibility study. Eur Radiol 200313883–889. [DOI] [PubMed] [Google Scholar]
- 26.Kirchin M A, Runge V M. Contrast agents for magnetic resonance imaging: safety update. Top Magn Reson Imaging 200314426–435. [DOI] [PubMed] [Google Scholar]
- 27.Shellock F G, Kanal E. Safety of magnetic resonance imaging contrast agents. J Magn Reson Imaging 199910477–484. [DOI] [PubMed] [Google Scholar]
- 28.Luboldt W, Bauerfeind P, Steiner P.et al Preliminary assessment of three‐dimensional magnetic resonance imaging for various colonic disorders. Lancet 19973491288–1291. [DOI] [PubMed] [Google Scholar]
- 29.Lauenstein T C, Herborn C U, Vogt F M.et al Dark lumen MR‐colonography: initial experience. Rofo 2001173785–789. [DOI] [PubMed] [Google Scholar]
- 30.Lauenstein T C, Ajaj W, Kuehle C A.et al Magnetic resonance colonography: comparison of contrast‐enhanced three‐dimensional vibe with two‐dimensional FISP sequences: preliminary experience. Invest Radiol 20054089–96. [DOI] [PubMed] [Google Scholar]
- 31.Bruzzi J F, Moss A C, Brennan D D.et al Colonic surveillance by CT colonography using axial images only. Eur Radiol 200414763–767. [DOI] [PubMed] [Google Scholar]
- 32.Bond J H. Clinical evidence for the adenoma–carcinoma sequence, and the management of patients with colorectal adenomas. Semin Gastrointest Dis 200011176–184. [PubMed] [Google Scholar]
- 33.Winawer S J, Zauber A G. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am 2002121–9v. [DOI] [PubMed] [Google Scholar]
- 34.Mysliwiec P A, Brown M L, Klabunde C N.et al Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med 2004141264–271. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman D A, Weiss D G, Bond J H.et al Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med 2000343162–168. [DOI] [PubMed] [Google Scholar]
- 36.Wardle J, Sutton S, Williamson S.et al Psychosocial influences on older adults' interest in participating in bowel cancer screening. Prev Med 200031323–334. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention Trends in screening for colorectal cancer—United States, 1997 and 1999. JAMA 20012851570–1571. [PubMed] [Google Scholar]
- 38.Mant D, Fuller A, Northover J.et al Patient compliance with colorectal cancer screening in general practice. Br J Gen Pract 19924218–20. [PMC free article] [PubMed] [Google Scholar]
- 39.Akerkar G A, Yee J, Hung R.et al Patient experience and preferences toward colon cancer screening: a comparison of virtual colonoscopy and conventional colonoscopy. Gastrointest Endosc 200154310–315. [DOI] [PubMed] [Google Scholar]
- 40.van Gelder R E, Birnie E, Florie J.et al CT colonography and colonoscopy: assessment of patient preference in a 5‐week follow‐up study. Radiology 2004233328–337. [DOI] [PubMed] [Google Scholar]
- 41.Ristvedt S L, McFarland E G, Weinstock L B.et al Patient preferences for CT colonography, conventional colonoscopy, and bowel preparation. Am J Gastroenterol 200398578–585. [DOI] [PubMed] [Google Scholar]