Abstract
Background
Low‐grade inflammation may play a role in the pathogenesis of irritable bowel syndrome (IBS). Although corticosteroids are potent inhibitors of inflammatory processes, only one study with corticosteroids in patients with postinfectious IBS exists, which suggests that prednisolone is not an effective treatment for IBS symptoms.
Aim
To evaluate whether dexamethasone treatment prevents protease‐activated receptor‐2 (PAR‐2) activation‐induced visceral hyperalgesia and increased permeability in rats, and to determine whether the effects involve colonic mast cells.
Methods
Abdominal contractions provoked by rectal distension were recorded in rats equipped with intramuscular electrodes. Changes in visceral hypersensitivity provoked by intracolonic administration of PAR‐2‐activating peptide (SLIGRL; H‐serine‐leucine‐isoleucine‐glycine‐arginine‐leucine‐OH), changes in colonic mucosal rat mast cell protease‐II (RMCP‐II) content, mast cell count and PAR‐2 expression were measured after a 4‐day treatment with dexamethasone (1 mg/day/rat intraperitoneally) or its vehicle (water). The effect of mast cell stabiliser (doxantrazole, 1 mg/kg intraperitoneally, 2 h before and 6 h after intracolonic infusion of SLIGRL) on SLIGRL‐induced visceral hyperalgesia was also assessed. The effects of SLIGRL and a mast cell degranulator (compound 48/80) on the permeability of colonic strips from vehicle‐ or dexamethasone‐treated rats were investigated in Ussing chambers.
Results
4 days of dexamethasone as well as doxantrazole diminished the SLIGRL‐induced hyperalgesia for all volumes of distension. This effect of dexamethasone was accompanied by a reduced responsiveness of colonic permeability to compound 48/80, and decreased RMCP‐II content and mast cell number. Dexamethasone treatment did not influence colonic mucosal PAR‐2 expression and permeability responsiveness to SLIGRL.
Conclusions
Dexamethasone treatment improves PAR‐2 agonist‐induced visceral hypersensitivity but does not prevent PAR‐2 agonist‐induced increase in colonic permeability in rats. This effect is coupled with a reduction of colonic mast cell number and RMCP‐II contents.
Irritable bowel syndrome (IBS) is a chronic gastrointestinal disorder characterised by continuous or remittent abdominal pain, bloating and altered defecation. The pathogenesis of IBS remains only partly understood, and no specific and universally effective treatment has been developed. Altered colonic motor function, visceral hypersensitivity, changes in neural transmission within the gut, alterations of spinal and supraspinal sensory afferent system, and low‐grade inflammation of the intestinal mucosa may play a role in the development of IBS.1 There is growing evidence that this low‐grade inflammation plays a role in the pathogenesis of IBS, particularly in initiating symptoms developed after gastrointestinal infection.2,3 Although corticosteroids are potent inhibitors of inflammatory processes, and are activated in the treatment of inflammatory bowel disease, only one study with corticosteroids in patients with postinfectious IBS exists, which suggests that prednisolone is not likely to be an effective treatment for IBS symptoms.4
Protease‐activated receptors (PARs) belong to a family of seven transmembrane domain G‐protein‐coupled receptors that are activated by cleavage of their N‐terminal domain by proteolytic enzymes.5 In rats, the intracolonic infusion of a PAR‐2 agonist activated spinal afferent neurons and produced a delayed rectal hyperalgesia.6 PAR‐2 activation from colonic lumen also caused delayed facilitation of the capsaicin‐evoked visceral nociception,7 and activation of PAR‐2 located in enteric nerves by mast cell tryptase caused neuronal hyperexcitability.8 Our recent work demonstrated elevated faecal protease activity in patients with diarrhoea‐predominant IBS, which could be a potent activator of colonic PAR‐2.9 This elevated level of serine protease was able to alter colonic permeability in mice.10 The origin of the elevated protease activity in the stool of patients with IBS has not yet been identified. We did not observe any change in mast cell tryptase activity and pancreatic digestive enzyme concentration in faecal samples of these patients. Since colonic bacteria release proteases, we can speculate that perturbed bacterial flora may be one of the sources of elevated faecal protease activity. Thus, PARs are potential receptors involved in the development of visceral hypersensitivity in IBS. Therefore, therapeutic modification of PAR function may be beneficial for the relief of IBS symptoms. However, the lack of PAR‐2 antagonists had not permitted until now confirmation of a beneficial effect of blocking PAR‐2 activation in the therapy of inflammatory bowel disease or IBS.
The present study was aimed at (1) evaluating whether dexamethasone treatment prevented PAR‐2 agonist‐induced visceral hyperalgesia in rats and (2) determining more specifically the role of PAR‐2 and colonic mast cells in the effect of corticosteroid therapy on visceral hypersensitivity.
Methods
Animals
Male Wistar rats weighing 200–250 g were obtained from Janvier (Le Genest St‐Isle, France). Rats were housed in polycarbonate cages in a light‐controlled (12 h/12 h cycle) and temperature‐controlled room (20–22°C) and were fed standard pellets. Water was provided ad libitum. The experimental protocols described in this study were approved by the local Institutional Animal Care and Use Committee.
Surgery and electromyography
Rats were surgically prepared for electromyographic recording and intracolonic injections as described previously.9 Briefly, a polyethylene catheter (outer diameter 0.7 mm, inner diameter 0.3 mm, length 60 cm) was implanted into the proximal colon, 1 cm from the caecocolonic junction, attached to the abdominal muscle wall. This catheter was used to perform intracolonic injections of PAR‐2‐activating peptide. Rats were also equipped with three groups of three electrodes of NiCr wire (60 cm length, 80 μm diameter), implanted bilaterally in the abdominal external oblique musculature superior to the inguinal ligament. The catheter and electrodes were exteriorised on the back of the neck and protected by a glass tube attached to the skin. Electromyographic recordings began 5 days after surgery. The electrical activities of abdominal striated muscles were recorded with an electromyograph (Mini VIII, Alvar, Paris, France) using a short time constant (0.03 s) to remove low‐frequency signals (<3 Hz) and at a paper speed of 3.6 cm/min.
Distension
Rectal distension was used as a noxious stimulus to evaluate visceral hyperalgesia. Rats were placed in plastic tunnels (6 cm diameter, 25 cm length) and were trained to stay in tunnels and undergo rectal distension during the 3 days preceding the experiments. This training period minimalised stress reactions during experiments. The balloon of an arterial embolectomy catheter (2 mm diameter, 2 cm length, Fogarthy, Edwards Laboratories, Santa Ana, California, USA) was inserted into the rectum at 1 cm from the anus. The balloon was then inflated with water progressively, in 0.4 ml steps, from 0 to 1.2 ml; each step of inflation lasted 5 min. To detect possible leakage, the volume of water introduced into the balloon was checked by complete removal with a syringe at the end of the distension period.11
Experimental protocol
Sensitivity studies
The effect of dexamethasone on PAR‐2‐induced visceral hyperalgesia was assessed in four groups of eight rats surgically prepared for electromyography and intracolonic injections. Rectal distension and abdominal contraction recording was performed 24 h after intracolonic infusion of SLIGRL (H‐serine‐leucine‐isoleucine‐glycine‐arginine‐leucine‐OH) (200 μg) or distilled water (vehicle) at a rate of 0.25 ml/20 min in rats previously injected intraperitoneally daily with saline (groups 1 and 2) or dexamethasone (1 mg/kg) (groups 3 and 4) for 4 days. In two other groups of rats, a mast cell stabiliser, doxantrazole, was injected intraperitoneally (1 mg/kg) 2 h before and 6 h after intracolonic infusion of SLIGRL; rectal sensitivity was tested 18 h after the last injection of doxantrazole.
Permeability studies
In two other groups of rats (n = 8) after 4 days of daily intraperitoneal treatment with dexamethasone or vehicle, the effect of SLIGRL and the mast cell degranulator (compound 48/80) on the paracellular permeability of colonic strips was measured in Ussing chambers.
Finally, in two other groups of rats (n = 8) after 4 days of intraperitoneal treatment with dexamethasone or vehicle, colonic samples were collected for mucosal rat mast cell protease‐II (RMCP‐II) assay, immunohistochemical analysis for PAR‐2 and mast cell count.
In vitro permeability study
The proximal colon was removed and two colonic strips from each rat were mounted in Ussing‐type chambers (Easymount, Physiologic Instruments, San Diego, USA) having a flux area of 0.5 cm2. Both sides of each colonic strip were bathed in 5 ml of Krebs solution maintained at 37°C and continuously oxygenated with 5% CO2 in O2. Permeability was assessed by measuring mucosal to serosal fluxes of fluorescein isothiocyanate (FITC)‐dextran across the colonic strip. For assessment of PAR‐2 responsiveness, the FITC‐dextran flux was determined 1 h after administration of SLIGRL or its vehicle (saline). For measurement of mast cell degranulation, the FITC‐dextran flux was evaluated 1 h after administration of compound 48/80 or its vehicle (distilled water). In brief, after a 20 min equilibrium period, 550 μl of buffer solution on the mucosal side was replaced by 500 μl of FITC‐dextran (10 000 MW, 0.022 g) and 50 μl of SLIGRL (25 μM), saline, compound 48/80 (10 μg/ml) or water. After 30 and 60 min, 800 μl of solution from the serosal side was removed and fluorescence was measured on a fluorimeter (Luminescence Spectrometer LS 50 B, Perkin Elmer, Waltham, Massachusetts, USA). The FITC‐dextran flux was expressed as the amount of FITC‐dextran that crossed 1 cm2 in 1 h (nmol/h/cm2).
Colonic mucosal RMCP‐II assay
The colonic mucosa was carefully removed with a dissector. Mucosal samples were homogenised in RIPA buffer and centrifuged at 10 000 g at 4°C. Supernatants were used for the RMCP‐II and total protein assays. RMCP‐II enzyme‐linked immunosorbent assays (ELISAs) were performed with a Rat Mast Cell Protease‐II ELISA Kit (Moredun, Midlothian, UK). Briefly, coated plates were incubated with monoclonal antibody against RMCP‐II raised in mouse at 4°C for 24 h before use. A 30 min incubation with 4% (w/v) bovine serum albumin (BSA) was carried out before loading standard and unknown samples. The sample incubation was carried out for 1.5 h at 37°C. A sheep anti‐RMCP‐II and horseradish peroxidase conjugate was added afterwards and incubated for 1 h. Plates were developed using o‐phenylenediamine as substrate and read at 492 nm after the reaction was stopped. RMCP‐II concentration was quantified against the RMCP‐II standard curve. Protein concentration was determined with a commercial kit (BCA‐200 Protein Assay Kit, Pierce, Rockford, Illinois, USA). The RMCP‐II content of colonic mucosa was expressed as nanograms of RMCP‐II per milligram of total proteins.
Colonic PAR‐2 immunohistochemistry
Colonic samples were fixed for 12 h in 4% formalin, dehydrated through graded ethanol and embedded in paraffin. Sections (5 μm) were rehydrated and submerged in antigen retrieval solution (citrate buffer, 10 mM, pH 6, 95°C, 3 min). After inhibition of endogenous peroxidases with 0.6% H2O2 in 100% methanol for 30 min and incubation in blocking solution (phosphate‐buffered saline (PBS) containing 1% BSA and 2% goat normal serum), sections were incubated with rabbit PAR‐2 antibody (supplied by MD Hollenberg, Calgary, Canada; overnight, 4°C), followed by biotinylated goat anti‐rabbit IgG immune serum (30 min, 22°C) and subsequently with ABC complexes coupled to peroxidase (Vectastain Elite ABC kit, AbCys, Paris, France). Antigen–antibody complexes were revealed using 3‐3′ diaminobenzidine (DAB kit, ICN Pharmaceuticals, Costa Mesa, California, USA). Haemalum was used as a counterstain. As negative controls, sections were treated with the same procedure, except for the absence of primary antibody.
Immunohistochemical analysis was performed in a blinded fashion using a Nikon 90i microscope. PAR‐2 expression was quantified with the software LUCIA G V.4.8 (Nikon, France) measuring the mean density per square micrometre of colonic mucosa. All analyses were carried out on 10 fields per sample.
Colonic mucosal mast cell count
Specimens were fixed in buffered 10% formalin and incubated for 24 h in 30% sucrose at 4°C. Samples were embedded in Tissue Tek medium (Euromedex, Souffelweyersheim, France) and frozen in isopentane at –45°C. Cryostat sections (7 μm) were postfixed with acetone (10 min, –20°C) and hydrated in PBS‐Tween. After incubation in blocking solution (PBS containing 1% BSA and 2% donkey serum), sections were incubated (overnight, 4°C) with sheep anti‐RMCP‐II antibodies (1/500; Moredun, Midlothian, UK), followed by incubation for 1.5 h at room temperature with Alexa fluor 594‐conjugated IgG donkey anti‐sheep (1/2000). After each incubation, sections were rinsed in PBS‐Tween. Sections were mounted in fluorostab medium (MP Biomedicals, Vannes, France) and examined under a Nikon 90i fluorescence microscope.
The number of mast cells was determined with the software LUCIA G V.4.8. Results were expressed per square millimetre of colon mucosa. All analyses were performed on three fields of six control rats and six treated rats.
Chemicals
SLIGRL‐NH2 prepared by solid‐phase synthesis was obtained from Neosystem (Strasbourg, France). The composition and purity of peptides were confirmed by high‐performance liquid chromatography analysis. SLIGRL was dissolved in saline. Dexamethasone and compound 48/80 were obtained from Sigma (St Quentin Fallavier, France) and were dissolved in distilled water.
Statistical analysis
Data are presented as means (SEM). Analyses were carried out by running the GraphPad Prism 4.0 software (GraphPad, San Diego, California, USA). All data were normally distributed. Between‐group comparisons were performed by Student's unpaired t test. Multiple comparisons within groups were performed by repeated‐measures one‐way analysis of variance, followed by Student's t test. Statistical significance was accepted at p<0.05.
Results
Effect of dexamethasone and doxantrazole on SLIGRL‐induced visceral hyperalgesia: involvement of mast cells
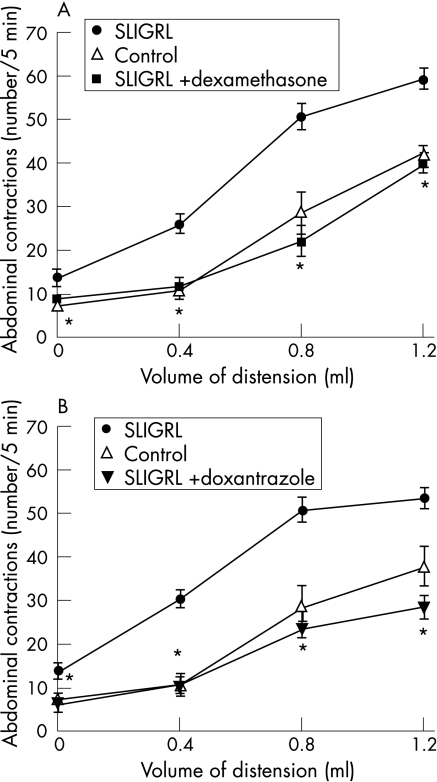
In untreated rats (control groups), gradual rectal distension increased the frequency of abdominal contractions in a volume‐dependent manner, and this increase became significant when the volume of distension reached 0.8 ml. SLIGRL (200 μg/kg intracolonic) infused intracolonically increased significantly the number of abdominal contractions obtained for all volumes of distension compared with control rats (1.2 ml: 60.1 (9.0) vs 37.8 (4.1); p<0.01; fig 1A,B). Dexamethasone treatment (1 mg/kg intraperitoneally, 4 days) suppressed the effect of SLIGRL on abdominal contractions for all volumes of distension (1.2 ml: .25.3 (7.2) vs 60.1 (9.0); fig 1A). Similarly, the mast cell stabiliser doxantrazole (10 mg/kg intraperitoneally) reduced SLIGRL‐induced enhancement of abdominal contraction in response to rectal distension (1.2 ml: 40.0 (4.1) vs 60.1 (9.0); fig 1B). Dexamethasone and doxantrazole treatment in itself had no effect on abdominal response to rectal distension (data not shown).
Figure 1 Comparative effect of dexamethasone (A) and doxantrazole (B) on SLIGRL (H‐serine‐leucine‐isoleucine‐glycine‐arginine‐leucine‐OH)‐induced increase of abdominal contractions in response to rectal distension in male rats. Values are means (SEM). *p<0.01 significantly different from SLIGRL‐treated groups.
Permeability responsiveness of colonic mucosa to PAR‐2 agonist
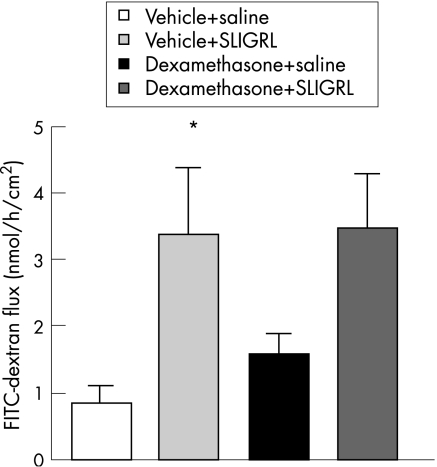
In Ussing chambers, values of FITC‐dextran flux significantly increased after SLIGRL administration (3.39 (0.98) vs 0.86 (0.20) nmol/h/cm2; p<0.05) compared with vehicle‐treated rats. Colonic strips collected from rats treated with dexamethasone exhibited slightly, but not significantly, increased FITC‐dextran flux as compared with strips collected from controls (1.53 (0.33) nmol/h/cm2). However, 4‐day dexamethasone treatment did not influence the effect of PAR‐2 agonist on colonic permeability (3.44 (0.85) nmol/h/cm2; fig 2).
Figure 2 Basal fluorescein isothiocyanate (FITC)‐dextran (10 000 MW) flux and the effect of SLIGRL (H‐serine‐leucine‐isoleucine‐glycine‐arginine‐leucine‐OH) on colonic permeability in vehicle‐ and dexamethasone‐treated rats. Values are means (SEM). *p<0.05, significantly different from basal value in vehicle‐treated control.
Colonic PAR‐2 immunohistochemistry
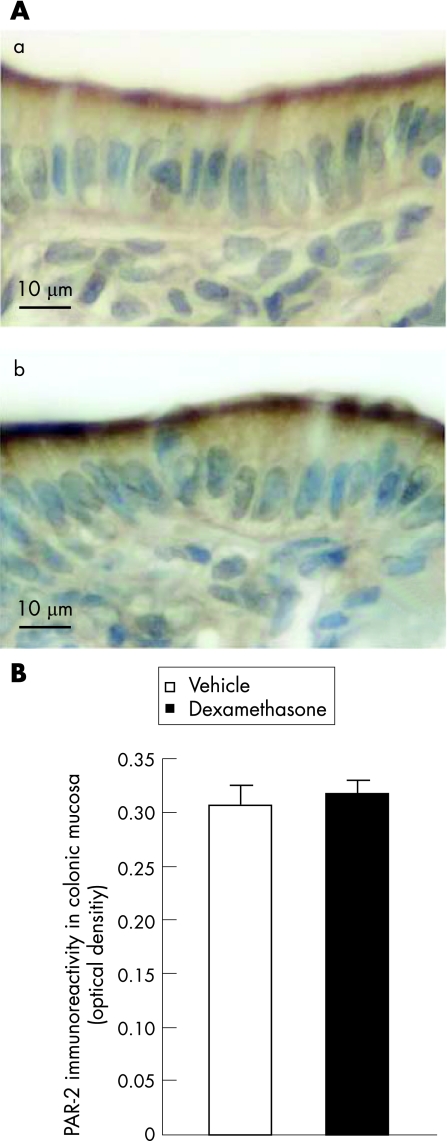
Four days of dexamethasone treatment did not influence the colonic mucosal PAR‐2 expression as compared with vehicle‐treated rats (fig 3A). No significant difference was observed in PAR‐2 immunoreactivity (optical density) between the vehicle‐treated controls (0.30 (0.01)) and dexamethasone‐treated animals (0.31 (0.01) fig 3B).
Figure 3 Effect of 4‐day dexamethasone treatment on colonic protease‐activated receptor (PAR‐2) expression. PAR‐2 immunoreactivity (brown) in colonic sections of (a) vehicle‐treated control animals and (b) dexamethasone‐treated rats (A); quantification of PAR‐2 immunoreactivity in colonic mucosa (B). Data are expressed as optical density, as the total number of grey levels per square micrometre of colonic mucosa.
Permeability responsiveness of colonic mucosa to a mast cell degranulator
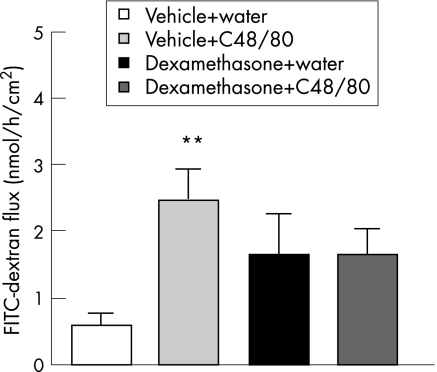
In Ussing chambers, control values of FITC‐dextran flux significantly increased after administration of compound 48/80 to the mucosal site (2.45 (0.46) vs 0.58 (0.16) nmol/h/cm2; p<0.01) of strips collected from vehicle‐treated animals. Dexamethasone treatment resulted in a slight, but not statistically significant, increase in dextran flux (1.64 (0.60) nmol/h/cm2). Four days of corticosteroid treatment diminished the significant effect of a mast cell degranulator on colonic permeability seen in control rats (1.67 (0.97) nmol/h/cm2; fig 4).
Figure 4 Basal fluorescein isothiocyanate (FITC)‐dextran (10 000 MW) flux and the effect of compound 48/80 on colonic permeability in vehicle‐ and dexamethasone‐treated rats. Values are means (SEM). **p<0.01, significantly different from basal value in vehicle‐treated control. C48/80, compound 48/80.
RMCP‐II content in colonic mucosa
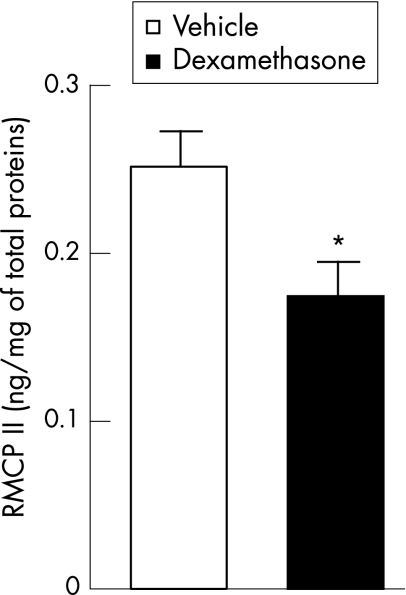
In vehicle‐treated rats, the RMCP‐II content of colonic mucosa was 0.25 (0.02) ng/mg of total protein. After 4 days of dexamethasone treatment, RMCP‐II content was significantly decreased (0.17 (0.01) ng/mg of total proteins; p<0.05) as compared with control values obtained from vehicle‐treated animals (fig 5).
Figure 5 Effect of dexamethasone treatment on colonic mucosal rat mast cell protease‐II (RMCP‐II) content. Values are means (SEM). *p<0.05, significantly different from vehicle‐treated controls.
Colonic mucosal mast cell count
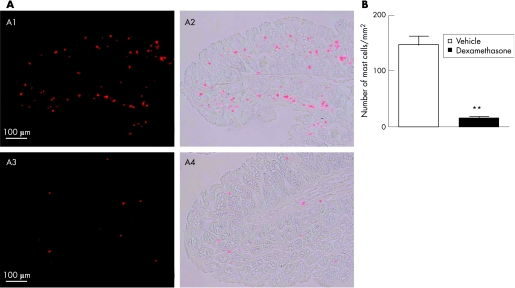
In vehicle‐treated rats, the number of mast cells per mm2 of colonic mucosa was 147.3 (14.4). After 4 days of dexamethasone treatment, the mast cell number in the colonic mucosa was significantly reduced to 15.0 (2.8) mast cells/mm2 (p<0.01; fig 6A,B).
Figure 6 (A) Effect of dexamethasone treatment on colonic mucosal mast cell number. Mast cell immunoreactivity (red) in colonic sections of (A1 and A2) vehicle‐treated control animals and (A3 and A4) dexamethasone‐treated rats. (B) Quantification of mast cell immunoreactivity in colonic mucosa. Data are expressed as number of mast cells per square millimetre of colon mucosa. Values are means (SEM). **p<0.01, significantly different from vehicle‐treated controls.
Discussion
This study provides new evidence that dexamethasone treatment prevents intracolonic PAR‐2 agonist‐provoked delayed rectal hypersensitivity in rats. We also establish that this PAR‐2‐induced rectal hypersensitivity is linked to mast cell degranulation, and that the effect of corticosteroid treatment on visceral hyperalgesia is accompanied by a reduction of colonic mast cell number, tryptase content and mast cell degranulation. However, dexamethasone treatment is unable to prevent the alterations of colonic permeability triggered by intracolonic PAR‐2 activation.
There is growing evidence for a role of colonic microinflammation in the pathogenesis of IBS. IBS symptoms frequently occur in patients in remission from ulcerative colitis12 and after gastrointestinal infection.13,14 An increased number of inflammatory cells is present in the colonic mucosa of patients with IBS.2,3 Despite the anti‐inflammatory effect of corticosteroids, only a little evidence exists in support of a beneficial effect of steroid treatment. In an experimental study, dexamethasone treatment normalised postinfective intestinal dysmotility and visceral hyperalgesia in mice after intestinal Trichinella spiralis infection.15 On the basis of clinical cohort studies, oral corticosteroid therapy can reduce the risk of a diagnosis of IBS,16 and the risk of IBS is also reduced by the use of oral steroids in patients with asthma.17 To date, only one clinical trial with corticosteroids in a treatment of IBS has been performed. In a randomised, double‐blind, placebo‐controlled trial, 30 mg of prednisolone once daily for 3 weeks did not improve symptoms in postinfectious IBS and did not reduce the number of colonic inflammatory cells.4
Mast cells play a key role in the maintenance of colonic micro‐inflammation and subsequently in the development of IBS symptoms. Increased mast cell number can be detected in coecal and colonic biopsies from patients with IBS18,19,20 and activated mast cells in proximity to colonic nerves correlate with abdominal symptoms in IBS.21 There are contradictory data and also remarkable differences in species regarding the effect of corticosteroids on mast cell function. Dexamethasone treatment abolishes intravenous worm antigen‐mediated intestinal anaphylaxis in Nippostrongylus brasiliensis‐infected rats, and suppression of the response is associated with depletion of RMCP‐II in intestinal mucosa.22 Corticosteroid treatment reduces mast cell number in rectal biopsy specimens of patients with IBD; however, the reduction in mast cell counts is independent of the degree of inflammation.23 Budesonide and dexamethasone had potent inhibitory effects on the release of cytokines from a human mast cell line24; however, in another study, dexamethasone treatment failed to inhibit the release of mast cell mediators from cultures of human airway, skin and intestinal tissues.25
In our study, we show that a 4‐day daily treatment with dexamethasone inhibits PAR‐2 agonist‐induced rectal hyperalgesia in rats. Using doxantrazole, we validate that this hyperalgesia is linked to mast cell degranulation. Treatment with corticosteroid is coupled with a decrease in the colonic mucosal mast cell number, RMCP‐II content and the degree of mast cell degranulation. Therefore, we can hypothesise that the effects of dexamethasone on intracolonic SLIGRL‐induced rectal hyperalgesia are linked to its inhibitory effects on mast cells. Clinical observations indicated a lack of efficacy of prednisolone therapy in postinfectious IBS,4 and IBS symptoms occur progressively, often associated with chronic changes in colonic mucosal cell function and alterations in nerve–mast cell communication in the gastrointestinal tract.21
PAR‐2 are highly expressed on the apical side of colonic epithelial cells,26,27 and intracolonic infusion of PAR‐2 agonists increases colonic paracellular permeability.28 This increase of paracellular permeability is linked to the contraction of the epithelial cell cytoskeleton by phosphorylation of MLC,29 and PAR‐2 activation activates intracellular MLC kinase (MLCK) through a calmodulin‐dependent mechanism.30 Increased intestinal permeability may be an important pathophysiological factor triggering colonic microinflammation and visceral hypersensitivity by enhancing the exposure of the gut mucosal immune system to luminal antigens.31 Indeed, in rats, an increase in colonic paracellular permeability induced by acute stress32 or systemic administration of lipopolysaccharide33 was shown to be responsible for the delayed rectal hypersensitivity to distension. Increased intestinal permeability was also observed in patients with postinfectious IBS.2,34,35 Our results indicate a lack of direct action of corticosteroids on intracolonic PAR‐2 agonist‐induced increase of colonic permeability and subsequent mucosal immune stimulation. This finding is enough to explain the lack of efficacy of corticosteroids in the treatment of patients with IBS. We recently demonstrate that faecal protease activity is highly increased in diarrhoea‐predominant patients with IBS.9 This elevated level of serine protease is able to alter colonic permeability in mice,10 and we can hypothesise a similar action on colonic epithelium in patients with IBS and IBD. Furthermore, our data establish that mast cell tryptase secretion, which was strongly reduced after dexamethasone treatment, has no regulatory effect on PAR‐2 expression on the apical side of colonic epithelial cells. In a previous study in human colonic epithelial cells, it was shown that basolateral administration of a mast cell degranulator, compound 48/80, had an increasing effect on paracellular permeability mediated by mast cell tryptase, which cleaved PAR‐2 on the basolateral part of colonocytes.36 Since in our experiments we did not observe any changes in colonic mucosal PAR‐2 expression and paracellular permeability coupled with a significant decrease in the mucosal mast cell number, tryptase content and degranulation after dexamethasone treatment, we could speculate different interactions between mast cells and PAR‐2 in the luminal and serosal parts of colonic epithelial cells. Our last finding reinforces the hypothesis of a crucial role of luminal proteases in the regulation of epithelial cell PAR‐2 expression and function, already reported in a previous study.37
Finally, an increased number of mast cells in colonic or rectal biopsies of patients with IBS has been recently confirmed,38,39,40 particularly in patients developing IBS after severe gastroenteritis.41 Therefore, one may speculate that there is a beneficial effect of corticoids as well as mast cell tryptase inhibitors.42 5‐Aminosalicylic acid is an effective inhibitor of IgE‐induced histamine and PGD2 release from human intestinal mast cells,43 and mast cell stabilisers used in allergic diseases, such as sodium cromolyn, could also have an attenuating effect on symptoms in patients with IBS.44,45
The lack of effect of dexamethasone on PAR‐2 agonist‐induced increased colonic permeability suggests that corticoids may be unable to prevent the alterations of intestinal and/or colonic permeability that underpin the development of IBS symptoms. However, the only existing clinical study with prednisolone suggesting the inefficiency of corticosteroids in the treatment of postinfectious IBS symptoms was performed in a small population and only in one subgroup of patients with IBS. Therefore, further studies in a larger patient population and in other subtypes of IBS are required to define the possible importance of corticosteroids in the treatment of IBS symptoms.
Abbreviations
BSA - bovine serum albumin
FITC - fluorescein isothiocyanate
IBD - inflammatory bowel disease
IBS - irritable bowel syndrome
PAR - protease‐activated receptor
PBS - phosphate‐buffered saline
RMCP‐II - rat mast cell protease‐II
Footnotes
Competing interests: None.
References
- 1.Barbara G, De Giorgio R, Stanghellini V.et al New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther 2004201–9. [DOI] [PubMed] [Google Scholar]
- 2.Spiller R C, Jenkins D, Thornley J P.et al Increased rectal mucosal endocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post‐dysenteric irritable bowel syndrome. Gut 200047804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunlop S P, Jenkins D, Nela K R.et al Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology 20031251651–1659. [DOI] [PubMed] [Google Scholar]
- 4.Dunlop S P, Jenkins D, Neal K R.et al Randomized, double‐bind, placebo‐controlled trial of prednisolone in post‐infectious irritable bowel syndrome. Aliment Pharmacol Ther 20031877–84. [DOI] [PubMed] [Google Scholar]
- 5.Nystedt S, Lanson A K, Aberg H.et al The mouse proteinase‐activated receptor‐2 cDNA and gene. Molecular cloning and functional expression. J Biol Chem 19952705950–5955. [DOI] [PubMed] [Google Scholar]
- 6.Coelho A M, Vergnolle N, Guiard B.et al Proteinases and proteinase‐activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology 20021221035–1047. [DOI] [PubMed] [Google Scholar]
- 7.Kawao N, Ikeda H, Kitano T.et al Modulation of capsaicin‐evoked visceral pain and referred hyperalgesia by protease‐activated receptors 1 and 2. J Pharmacol Sci 200494277–285. [DOI] [PubMed] [Google Scholar]
- 8.Reed D E, Barajas‐Lopez C, Cottrell G.et al Mast cell tryptase and proteinase‐activated receptor 2 induce hyperexcitability of guinea‐pig submucosal neurons. J Physiol 2003547531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roka R, Rosztoczy A, Leveque M.et al Fecal serine–protease activity: a patholological factor in diarrhea‐predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007 (Epub ahead of print.) [DOI] [PubMed]
- 10.Gecse K, Roka R, Rosztoczy A.et al Fecal serine–protease activity: a pathophysiological factor and a possible diagnostic marker for diarrhoea‐predominant irritable bowel syndrome [abstract]. Gut 200657(Suppl I)A67 [Google Scholar]
- 11.Morteau O, Hachet T, Causette M.et al Experimental colitis alters visceromotor response to colorectal distension in awake rats. Dig Dis Sci 1994391239–1248. [DOI] [PubMed] [Google Scholar]
- 12.Isgar B, Herman M, Kaye M D.et al Symptoms of irritable bowel syndrome in ulcerative colitis in remission. Gut 198324190–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gwee K A, Graham J C, McKendrick M W.et al Psychometris scores and persistence of irritable bowel after infectious diarrhoea. Lancet 1996347150–153. [DOI] [PubMed] [Google Scholar]
- 14.Neal K R, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of irritable bowel syndrome: postal survey of patients. BMJ 1997314779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bercik P, Wang L, Verdu E F.et al Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology 2004127179–187. [DOI] [PubMed] [Google Scholar]
- 16.Huerta C, Garcia Rodriguez L A, Wallander M A.et al Users of oral steroids are at a reduced risk of developing irritable bowel syndrome. Pharmacoepidemiol Drug Saf 200312583–588. [DOI] [PubMed] [Google Scholar]
- 17.Huerta C, Garcia Rodriguez L A, Wallander M A.et al Risk of irritable bowel syndrome among asthma patients. Pharmacoepidemiol Drug Saf 20021131–35. [DOI] [PubMed] [Google Scholar]
- 18.O'Sullivan M, Clayton N, Breslin N P.et al Increased mast cell in the irritable bowel syndrome. Neurogastroenterol Mot 200012449–457. [DOI] [PubMed] [Google Scholar]
- 19.Park C H, Joo Y E, Choi S K.et al Activated mast cells infiltrate in close proximity to enteric nerves in diarrhea‐predominant irritable bowel syndrome. J Korean Med Sci 200318204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gwee K A, Collins S M, Read N W.et al Increased rectal mucosal expression of interleukin 1beta in recently acquired post‐infectious irritable bowel syndrome. Gut 200352523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbara G, Stanghellini V, DiGiorgio R.et al Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004126693–702. [DOI] [PubMed] [Google Scholar]
- 22.King S J, Miller H R P, Newlands G F J.et al Depletion of mucosal mast cell protease by corticosteroids: effect on intestinal anaphylaxis in the rat. Proc Natl Acad Sci USA 1985821214–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldsmith P, McGarity B, Walls A F.et al Corticosteroid treatment reduces mast cell number in inflammatory bowel disease. Dig Dis Sci 1990351409–1413. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, Leung P C, Woo K S.et al Inhibitory effects of budesonide, desloratadine and dexamethasone on cytokine release from human mast cell line (HMC‐1). Inflamm Res 200453664–669. [DOI] [PubMed] [Google Scholar]
- 25.Cohan V L, Undem B J, Fox C C.et al Dexamethasone dose not inhibit the release of mediators from human mast cell residing in airway, intestine, or skin. Rev Respir Dis 1989140951–954. [DOI] [PubMed] [Google Scholar]
- 26.Bohm S K, Kong W, Bromme D.et al Molecular cloning, expression and potential functions of the human proteinase‐activated receptor‐2. Biochem J 19963141009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunzelmann K, Schreiber R, Konig J.et al Ion transport induced by proteinase‐activated receptors (PAR2) in colon and airways. Cell Biochem Biophys 200236209–214. [DOI] [PubMed] [Google Scholar]
- 28.Cenac N, Coelho A M, Nguyen C.et al Induction of intestinal inflammation in mouse by activation of proteinase‐activated receptor‐2. Am J Pathol 20021611903–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cenac N, Garcia‐Villar R, Ferrier L.et al Proteinase‐activated receptor‐2‐induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J Immunol 20031704296–4300. [DOI] [PubMed] [Google Scholar]
- 30.Cenac N, Chin A C, Garcia‐Villar R.et al PAR2 activation alters colonic paracellular permeability in mice via IFN‐γ dependent and ‐independent pathways. J Physiol 200458913–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ait‐Belgnaoui A, Gradesi S, Fioramonti J.et al Acute stress‐induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain 2005113141–147. [DOI] [PubMed] [Google Scholar]
- 32.Santos J, Yang P C, Soderholm J D.et al Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut 200148630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriez R, Salvador‐Cartier C.et al Myosin light chain kinase is involved in lipopolysaccharide‐induced disruption of colonic epithelial barrier and bacterial translocation in rats. Am J Pathol 20051671071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall J K, Thabane M, Garg A X.et al Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther 2004201317–1322. [DOI] [PubMed] [Google Scholar]
- 35.Dunlop S P, Hebden J, Campbell E.et al Abnormal intestinal permeability in subgroups of diarrhea‐predominant irritable bowel syndromes. Am J Gastroenterol 20061011288–1294. [DOI] [PubMed] [Google Scholar]
- 36.Jacob C, Yang P C, Darmoul D.et al Mast cell tryptase controls paracellular permeability of the intestine. Role of protease‐activated receptor 2 and beta‐arrestins. J Biol Chem28031936–31948. [DOI] [PubMed] [Google Scholar]
- 37.Roka R, Demaude J, Cenac N.et al Colonic luminal proteases activate colonocyte proteinase‐activated receptor‐2 and regulate paracellular permeability in mice. Neurogastroenterol Motil 20071957–65. [DOI] [PubMed] [Google Scholar]
- 38.O'Sullivan M, Clayton N, Breslin N P.et al Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil 200012449–457. [DOI] [PubMed] [Google Scholar]
- 39.Barbara G, Stanghellini V, De Giorgio R.et al Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004126693–702. [DOI] [PubMed] [Google Scholar]
- 40.Park J H, Rhee P L, Kim H S.et al Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol 20062171–78. [DOI] [PubMed] [Google Scholar]
- 41.Dunlop S P, Jenkins D, Spiller R C. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol 2003981578–1583. [DOI] [PubMed] [Google Scholar]
- 42.Tremaine W J, Brzezinski A, Katz J A.et al Treatment of mildly to moderate active ulcerative colitis with tryptase inhibitor (APC 2059): an open labelled pilot study. Aliment Pharmacol Ther 200216407–413. [DOI] [PubMed] [Google Scholar]
- 43.Fox C C, Moore W C, Lichtenstein L M. Modulation of mediator release from human intestinal mast cells by sulfasalazyne and 5‐amonisalicylic acid. Dig Dis Sci 199136179–184. [DOI] [PubMed] [Google Scholar]
- 44.Serna H, Porras M, Vergara P. Mast cell stabilizer Ketotifen prevents mucosal mast cell hyperplasia and intestinal dysmotility in experimental T. spiralis inflammation in the rat. J Pharmacol Exp Ther 20063191104–1111. [DOI] [PubMed] [Google Scholar]
- 45.Grazioli I, Melzi G, Balsamo V.et al Food intolerance and irritable bowel syndrome of childhood: clinical efficacy of oral sodium cromoglycate and elimination diet. Minerva Pediatr 199345253–258. [PubMed] [Google Scholar]