Abstract
Background
Campylobacter jejuni can cause a spectrum of diseases in humans, ranging from enteritis and diarrhoea to severe inflammation, profuse bloody diarrhoea and chronic relapsing infection. Norepinephrine (NE) levels in the intestine increase under conditions of stress and trauma, and are thought to result in spill over of NE into the intestinal lumen. NE is known to stimulate the growth of a range of bacterial species, and to increase the pathogenicity of Escherichia coli.
Aim
To determine the effects of NE on the pathogenic potential of C jejuni in a model system.
Methods
C jejuni was grown in iron‐replete and iron‐limited media in the presence and absence of 100 μM NE. Several virulence‐associated characteristics, including motility and cell invasion, were measured.
Results
When C jejuni was grown in iron‐limited media in the presence of NE, growth rate, motility and invasion of cultured epithelial cells were increased compared with cultures grown in the absence of NE. Bacteria exposed to NE during growth also caused greater subsequent disruption of cultured epithelial cell monolayers, inducing widespread breakdown of tight junctions.
Conclusion
Exposure to NE causes an increase in the virulence‐associated properties of Campylobacter. Stress and concomitant infection could therefore be contributory factors to the variable presentation of this disease.
Campylobacter spp are estimated to infect approximately 1% of the population of Europe each year1 and are responsible for more consultations with general practitioners and hospital admissions in England and Wales than any other cause of foodborne disease.2 In humans, C jejuni can cause a spectrum of diseases, ranging from enteritis and diarrhoea to severe inflammation, profuse bloody diarrhoea and chronic relapsing infection.3 Invasion of the intestinal mucosa by Campylobacter causes many changes, including superficial ulceration and neutrophil infiltration of the epithelium,4 which leads to the production of bloody diarrhoea owing to destruction of the epithelial cells. In rare cases, invasive infection can result in extraintestinal infection and neuropathies of the peripheral nervous system, such as Miller–Fisher and Guillain–Barré syndromes.5
The human enteric nervous system contains approximately 100 million neurones of various subtypes, distributed differently depending on segment.6 Norepinephrine (NE) is a major neurotransmitter in this system. The dense innervation of the mesenteric organs is mainly responsible for their production of a large proportion of the body's NE, and high concentrations of noradrenergic neurones are found throughout the intestine, terminating within the submucosal plexus and intestinal mucosa.7 A large amount of NE released by these neurones escapes breakdown at the site of release and spills over into the circulation, where it is spread throughout the mesenteric tissues.8 This high‐tissue concentration is thought to cause NE to further spill over into the intestinal lumen,9 as is known to occur with other neurotransmitters.10,11 Increased NE levels have been correlated with severity of injury in multisystem trauma,12 and trauma‐induced destruction of noradrenergic neurones and subsequent release of NE are known to stimulate the growth of the intestinal flora of mice.9 Sepsis has also been shown to markedly increase NE levels in the gut of rats by increasing expression of the catecholamine biosynthetic enzyme tyrosine hydroxylase.13
Catecholamines stimulate the growth of various Gram‐positive and Gram‐negative bacteria,14 including Pseudomonas aeruginosa, Yersinia enterolitica,15Salmonella enterica,16Listeria spp,17Bordetella18 and Staphylococcus spp.19 NE stimulates the production of Escherichia coli O157 virulence factors in vitro.20 It also increases the adherence of this bacterium to tissue in vivo and stimulates enteritis in a bovine intestinal loop model.21
Although high concentrations of iron can be present in the tissues and fluids of the animal host, levels of free iron are very low. Iron is sequestered in and on the intestinal mucosa by high‐affinity iron‐binding proteins of the host, such as lactoferrin and transferrin. Both pathogenic and commensal bacteria have therefore developed strategies to capture iron in this environment.22 This principally involves secretion of iron chelators (siderophores) by bacteria, which scavenge iron from the environment and present it to cell surface receptor proteins.23 In C jejuni, siderophore production either occurs at very low levels or is absent.24 It does have the ability to utilise siderophores produced by other organisms, and possesses several putative uptake systems for these.25 Host‐secreted factors, such as NE, might also be important, as these can mediate the removal of iron from host transferrins.26
The aim of this work was to determine whether, when exposed to NE, Campylobacter would show an increase in the three key determinants of pathogenicity likely to increase the severity of infection—growth rate, motility and the ability to cross the epithelial barrier.
Materials and methods
Bacterial strains and preparation of cultures
C jejuni National Collection of Type Cultures (NCTC) 11168, originally isolated from a human infection,27 was cultured on Columbia agar plates supplemented with 5% citrated blood agar (BA) at 37°C for 48 h in a microaerobic atmosphere. All reagents were obtained from Sigma Aldrich, Poole, Dorset, UK, unless otherwise stated. Three base media were used to prepare bacterial cultures for experiments: Mueller–Hinton (MH) broth (Oxoid, Basingstoke, UK), MH treated with 5% weight/volume chelex resin (Biorad, Hemel Hempstead, UK) for 30 min to deplete iron (CMH), and Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Paisley, UK) supplemented with 2 mM glutamine and 1% non‐essential amino acids (Invitrogen). All media were supplemented with 10% foetal bovine serum (PAA Labs, Pasching, Germany), and cultures were grown in microaerobic conditions.
Bacterial growth assays
C jejuni NCTC 11168 grown on BA was inoculated, at 1×104 cfu/ml, into 1.5 ml volumes of CMH and DMEM with or without 100 μM (−)‐NE. Additionally, 100 μM concentrations of the adrenoreceptor antagonists phenoxybenzamine hydrochloride, (±)‐propranolol hydrochloride and (±)‐metoprolol bitartrate were added in conjunction with NE to determine whether or not bacterial growth responses were adrenoreceptor mediated. Cultures were grown in 200 μl volumes in 100‐well honeycomb plates at 37°C for 48 h in a microaerobic atmosphere, using a Bioscreen plate reader (Thermo labsystems, Basingstoke, UK), and optical density at 600 nm was measured hourly after shaking. All assays were performed in triplicate on three separate occasions. The significance of differences in growth rate and yield was determined using the paired Student's t –test, to account for variation in the bacterial population used as the original inoculum.
Measurement of motility
Bacterial motility was determined by light microscopy using a Leica Microsystems (Milton Keynes, UK) DMR microscope at ×100 magnification, a charge‐coupled device camera and ImageProPlus software (Media Cybernetics, Silver Springs, Maryland, USA) to record 10 s films at 10 s time intervals in 10 fields of view. The proportion of motile cells in each culture was determined from these films, which were viewed randomly and without the culture type being identified to the observer. Differences were examined using the paired Student's t test.
Invasion of Caco‐2 cells
Caco‐2 human colon adenocarcinoma cells were maintained in DMEM containing 2 mM glutamine, 1% non‐essential amino acids and 10% fetal bovine serum. Penicillin/streptomycin (Invitrogen) was used at a concentration of 100 U/ml, until 3 days before cells were used for infection studies. Cells were seeded into 24‐well plates at 3×105 cells/well, and incubated for 12 days at 37°C in a 5% CO2 atmosphere, to give a final density of 1×106 cells/well. The culture medium was changed every 2–3 days. Before bacteria were added, the monolayers were washed with phosphate‐buffered saline (PBS), and 1 ml of fresh DMEM was added.
Bacterial cultures were incubated in 1.5 ml of medium at 37°C for 48 h in a microaerobic atmosphere, and then centrifuged at 7000 g for 5 min. The pellet was resuspended in 10 ml of PBS and recentrifuged at 7000 g for 5 min to remove culture medium and NE. The supernatant was removed, and cells were resuspended to a final optical density of 0.1 at 600 nm in DMEM. A 25 μl volume of Campylobacter cells in DMEM was added to each well to give a multiplicity of infection (MOI) of 25:1, and plates were incubated at 37°C for 2 h. Monolayers were then washed with PBS, and gentamicin (Invitrogen) was added to each well to a final concentration of 100 μg/ml. Monolayers were reincubated for 2 h in 5% CO2 and washed with PBS, and cells were lysed by the addition of 1% Triton X‐100 in PBS and incubation at 37°C for 5 min. Cell lysates were serially diluted 1:10 in PBS and invasive bacteria enumerated on BA. Differences between levels of invasion were examined using the Student's t test.
Cytotoxicity
Cytotoxic effects on the Caco‐2 cells were determined by incubating C jejuni with the cells at an MOI of 25:1, and then, using the Cytotox 96 non‐radioactive cytotoxicity assay kit (Promega, Southampton, UK), as specified in the manufacturer's instructions. Differences between levels of cytotoxicity were examined using the Student's t test.
Interaction with polarised cell monolayers
Caco‐2 cells were cultured in Transwell polycarbonate inserts (12 mm diameter, 3 μm pore size; Corning, New York, USA) in a 12‐well tissue‐culture plate. A 1.5 ml volume of DMEM was added to each basal well of the plate, and 3×105 cells in 0.5 ml DMEM added to the apical chamber of the insert. Plates were incubated at 37°C in 5% CO2 for 10 days (with changes of culture medium every 2–3 days), by which time cells were polarised, as assessed by measurement of transepithelial electrical resistance (TEER) using an epithelial voltmeter. Campylobacter, prepared as above, were added to the apical chamber to an MOI of 25:1 and incubated with the monolayer for 24 h. TEER was measured at intervals of 2 h and differences evaluated statistically at each time point using the Student's t test. After 24 h, the medium was removed, and the Transwell inserts placed in fresh wells containing 1.5 ml 4% paraformaldehyde in PBS, 0.5 ml paraformaldehyde was added to the apical chamber and the cells fixed at 4°C for 45 min. Inserts were washed in a beaker containing PBS, the cells were permeabilised in 0.1% Triton X‐100 for 10 min and washed again. The permeabilised cells were blocked for 1 h with 5% human and goat serum and 200 µl mouse antioccludin primary monoclonal antibody (Zymed, San Francisco, California, USA), diluted 1:200, added to the apical chamber and the inserts incubated overnight at 4°C. Inserts were washed in PBS and 200 µl fluorescein isothiocyanate‐goat anti‐mouse IgG1 isotype‐specific secondary conjugate (Southern Biotech Associates, Birmingham, Alabama, USA), diluted 1:100, added to the apical chamber and the inserts incubated for a further 1 h at room temperature in the dark. Inserts were washed, placed in PBS and the membrane cut out with a scalpel. Membranes were mounted on plain glass slides with Vectashield (Vector Laboratories, Bilingame, California, USA) and coverslips sealed with nail varnish. All antibodies were titrated to optimal‐working concentrations.
Distribution of anti‐occludin labelling was examined by confocal microscopy, using a Leica true confocal scanner SP2 acoustico–optical beam splitter imaging system attached to a Leica DM IRE2 inverted microscope, and equipped with an argon laser for 488 nm excitation of fluorescein isothiocyanate. An oil‐immersion objective lens (×63, NA 1.4) was used and imaging parameters standardised to allow direct comparison between images. Image acquisition was performed in a blind fashion by concealing the identity of each sample. Stacks of images at 0.5 μm intervals were acquired and maximum projections of these created with Leica confocal software.
Bacterial uptake of 55Fe and [3H]NE
The growth medium was labelled by incubating MH and CMH containing serum with 5 μCi/ml 55FeCl3 (Amersham Biosciences, Little Chalfont, UK) for 3 h at 37°C. This corresponded to a concentration of 10 μM of 55Fe in the media. A calcein fluorescence assay28 determined that the total iron binding capacity of both MH and CMH containing serum was ⩾30 μM, which indicated that the concentration of 55Fe added to the growth medium would be fully sequestered by the serum. Triplicate Campylobacter cultures of 1.5 ml medium were incubated for 48 h at 37°C in 55Fe‐loaded MH and CMH containing 100 μM NE. Cells were harvested by centrifugation, washed with 1 ml of ice‐cold PBS and resuspended in 50 μl PBS. Suspensions were added to 500 μl of OptiScint scintillation fluid (Perkin‐Elmer, Beaconsfield, UK), and counts were determined using 1219 RackBeta liquid scintillation counter (LKB Wallac, St Albans, UK). Cell number was calculated by total viable count, and results were normalised to counts per minute (cpm) per 1×109 cfu/ml. To investigate the internalisation of NE by C jejuni, the above experiment was repeated with cells grown in MH and CMH containing 100 μM NE and 2 μCi [3H]NE (Perkin‐Elmer).
Results
Effect of NE on the growth of Campylobacter
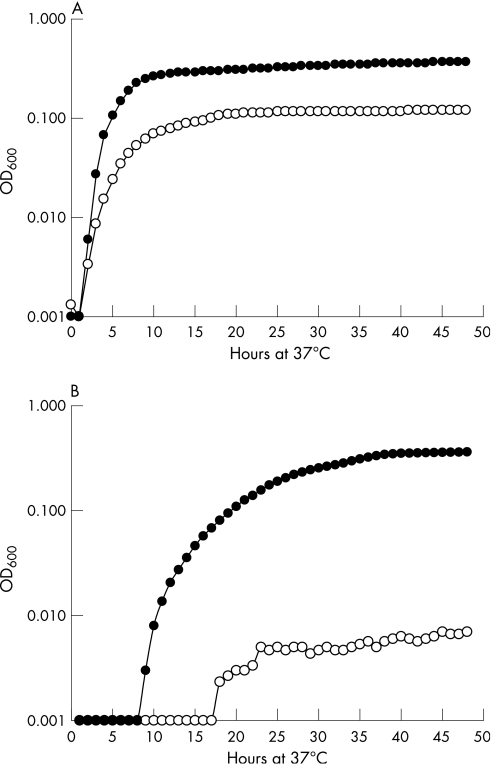
The growth response of C jejuni NCTC 11168 to NE was analysed in DMEM, MH and CMH media, all of which contained serum and had an excess iron‐binding capacity of >30 μM, resulting in very low levels of available iron. In CMH, the addition of 100 μM NE resulted in a threefold increase in final culture density of C jejuni over control cultures (fig 1A). In DMEM, the addition of NE resulted in a 50‐fold increase in final density (fig 1B). There was no significant difference between the final optical density of cultures grown in DMEM and CMH when NE was added, although the growth rate was lower in DMEM than in CMH, where peak cell numbers were reached in 6 h. There was a marked apparent lag phase before growth could be detected when cells were grown in DMEM (fig 1B), which was shorter when NE was present in the medium. The addition of the adrenoreceptor antagonists phenoxybenzamine hydrochloride, (±)‐propranolol hydrochloride and (±)‐metoprolol bitartrate to growth media did not reverse the effects of addition of NE and the growth curves were essentially identical to those when NE alone was present (data not shown).
Figure 1 Growth of Campylobacter jejuni NCTC 11168 at 37°C in (A) Mueller–Hinton broth treated with chelex and (B) Dulbecco's modified Eagle's medium, both containing 10% fetal bovine serum. Cultures were grown with (•) and without (o) 100 μM norepinephrine, with optical density (OD) being measured at hourly intervals. Results are the means of three separate experiments.
Effect of NE on Campylobacter motility in iron‐limited conditions
The greatest numbers of motile C jejuni cells was in CMH containing NE, where 60% (SD 11%) of cells showed motility. In CMH without NE, the percentage of motile cells was significantly lower, at 18% (SD 6%), p<0.05. Growth in MH resulted in significantly more motile cells (52% (SD 12%)) than in CMH (p<0.05), and the numbers were not significantly different from those seen in CMH containing NE.
Effect of NE on the invasion of Caco‐2 cells by C jejuni
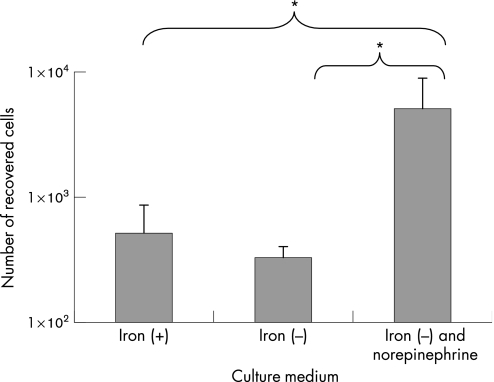
C jejuni cells were grown in MH, CMH and CMH with NE, and used to determine the rate of invasion of Caco‐2 cells. There was no significant difference in the numbers of bacteria recovered from cells when C jejuni was grown in MH or CMH. However, when NE was added to the primary broth culture, 10 times more bacteria were recovered from the Caco‐2 cells, even though the neurotransmitter was not present during this assay (fig 2).
Figure 2 Invasion of Caco‐2 epithelial cells by Campylobacter jejuni NCTC 11168 after 2 h. Campylobacter cells were grown in Mueller–Hinton (MH) broth (iron(+)), MH broth treated with chelex (iron(−)), and this medium containing 100 μM norepinephrine, and added to cell monolayers at a multiplicity of infection of 25:1. Results represent the mean plus SD of three separate experiments. *p<0.05.
NE‐mediated uptake of iron by C jejuni
Cell pellets from cultures grown in the presence of radiolabelled NE showed very low levels of radioactivity (1 (SD 1) cpm), indicating no uptake of NE, which remained in the growth medium. In all media, radiolabelled iron was taken up by bacterial cells. In the presence of NE, lower levels of 55Fe were detected in cells grown in MH (5744 (SD 1439) cpm) than in those grown in CMH (8723 (SD 2065) cpm), showing uptake of non‐radiolabelled iron from the iron‐replete medium in addition to the added 55Fe. 55Fe levels in bacterial cells were lower in MH (4378 (SD 579) cpm) and CMH (4957 (SD 718) cpm) in the absence of NE than in its presence.
Effects on cells and tight junctions
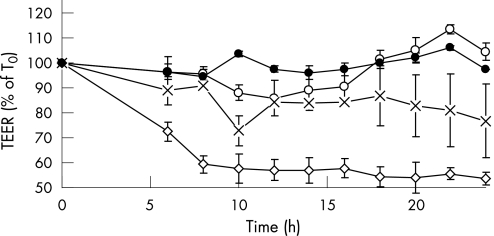
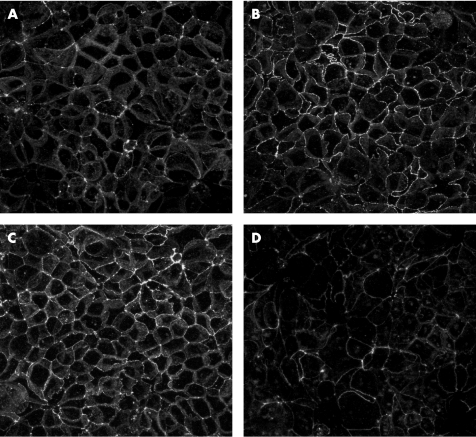
No cytotoxicity was seen when C jejuni cells grown in any of the media were incubated with Caco‐2 cells for 4 h (data not shown). When bacteria grown in MH or CMH were placed on polarised Caco‐2 monolayers for 24 h, TEER was not significantly decreased (p>0.05; fig 3) and no visible breakdown of occludin was observed (fig 4). When the Caco‐2 cells were incubated with Campylobacter previously grown in CMH containing NE for 24 h, TEER was significantly decreased (p<0.05; fig 3) and occludin staining was fragmented, indicating disruption of the tight junctions between cells (fig 4).
Figure 3 Change of transepithelial resistance of polarised Caco‐2 monolayers exposed to Campylobacter jejuni NCTC 11168 grown in Mueller–Hinton (MH) broth (o), MH broth treated with chelex (×) and this medium containing 100 μM norepinephrine (◊). The growth medium was removed by washing bacteria with phosphate‐buffered saline before these were added to the monolayers. Resistance of cell monolayer not exposed to bacteria is also shown (control, •). Results represent the mean plus/minus SD of three separate experiments. TEER, transepithelial electrical resistance.
Figure 4 Effect of Campylobacter jejuni on integrity of polarised Caco‐2 monolayers. (A) The monolayer not exposed to Campylobacter is shown as control. (B) Cell monolayers were coincubated with Campylobacter cells grown in Mueller–Hinton (MH) broth, (C) MH broth treated with chelex and (D) the same medium containing 100 μM norepinephrine for 24 h. Monolayers were fixed and stained for occludin, and analysed by confocal microscopy. Images are projected confocal images and show a 119×119 μm field of view.
Discussion
The aim of this investigation was to determine whether NE, which is released into the lumen of the intestine during conditions of sepsis and stress, would increase the pathogenic potential of C jejuni, the most common cause of foodborne disease in the UK.
In iron‐restricted media, the presence of NE increased bacterial growth rate and resulted in higher numbers of C jejuni cells per unit volume of medium (fig 1). Neither α‐ nor β‐adrenoreceptor antagonists blocked the effect of NE, suggesting that Campylobacter does not possess NE receptors similar to those found on eukaryotic cells. The growth enhancement effect is similar to that seen with a range of other bacterial species.16,29 In Salmonella spp, the effect is thought to be mediated via iron uptake, and involves cell surface siderophore‐receptor proteins.30 Similar siderophore receptors are expressed by C jejuni,25 and it is thus possible that the interaction with NE involves these iron‐uptake receptors. Radiolabelled NE was not internalised by Campylobacter, suggesting that it facilitates the transfer of iron between serum proteins and the bacterial‐cell surface. This was supported by the fact that, in the presence of NE, radiolabelled iron was internalised by the bacterial cells during growth in serum‐containing media. Lower levels of radiolabelled iron were seen in cells grown in the absence of NE. This mechanism might thus be dissimilar to that proposed to operate in E coli by Freestone et al,31 who found that radiolabelled NE was internalised by bacteria concomitantly with the uptake of iron.
C jejuni can multiply only at temperatures above 30°C, unlike many enteric bacteria, which have a much wider range of growth temperatures. In temperate zones, this restricts its multiplication to host, rather than environmental, niches. The fact that C jejuni does not express siderophores could thus be a reflection of its adaptation to the host, where it is able to rely on either siderophores excreted by other bacteria or host‐secreted catecholamines for iron uptake during growth. C jejuni can also take up Fe2+ directly through the Feo system.32 Regulated iron supply is also important in the production of catalase and superoxide dismutase, essential for resistance to oxidative damage caused by the generation of reactive oxygen intermediates by cellular metabolism,33 and for the survival of C jejuni in epithelial cells.34,35 Free iron in the extracellular environment also results in the production of oxygen radicals via Haber–Weiss–Fenton reactions. The production of catalase, superoxide dismutase and alkyl hydroperoxide reductase are controlled in response to environmental iron levels in Campylobacter by the proteins Fur and PerR.36
Motility is an important virulence factor in C jejuni, as it allows penetration and colonisation of the mucin lining the intestine,37 the primary habitat of C jejuni in humans and other animals. It is also required for the invasion of epithelial cells and translocation across epithelial cell monolayers.38 The greater motility exhibited in the presence of NE under iron‐limited conditions suggests that the presence of NE in the lumen would allow better colonisation of the intestine by C jejuni and also increase the number of invasive cells. Both of these factors could potentially exacerbate infection.
Preincubation of C jejuni with NE increased the subsequent invasion of Caco‐2 cells (fig 2), although no cytotoxicity was observed during a 4 h incubation of cells with bacteria (data not shown). Prolonged intracellular survival and multiplication of C jejuni have been reported after invasion.39,40 Intracellular persistence might protect the bacterium from an antibody‐mediated immune response, which is thought to be particularly important in limiting the disease.41 Persistent infection in mice has been shown to stimulate the production of the proinflammatory cytokine interferon γ,42 which is a potent upregulator of major histocompatibility complex class I expression on epithelial cells. Pathological findings suggest that a similar inflammatory response occurs during the course of Campylobactercolitis infection in humans.4,43 This process would result in the destruction of infected epithelial cells by cytotoxic CD8 T cells, which are known to be important in the clearance of Campylobacter disease.44 This could contribute to the epithelial destruction that is characteristic of severe campylobacteriosis. The increased invasion stimulated by NE is thus likely to increase the severity of the disease and the degree of T cell‐mediated tissue destruction, rather than increasing the cytotoxicity of the Campylobacter cells themselves, although this study did not look specifically for the production of cytolethal‐distending toxin. Intriguingly, the level of invasion seen in the presence of NE was significantly greater than that seen in MH or CMH alone, indicating a non‐iron‐associated effect. This will be the subject of further work by our group.
Epithelial degeneration and the entry of blood and immune cells into the intestinal lumen would be further exacerbated by the breakdown of tight junctions similar to that seen in vitro in the presence of C jejuni cells that had been grown in medium containing NE, indicated by the loss of occludin staining (fig 4) and by a decrease in TEER (fig 3). Campylobacter cells in high numbers have been observed to break down occludin, a major component of epithelial tight junctions, over 24 h.45 These authors also found that lower numbers, such as those used in these experiments, affected the distribution of occludin over 5–7 days, but the data presented here suggest that NE stimulates low numbers of C jejuni to do this over 24 h, resulting in a more rapid loss of epithelial‐barrier integrity than would be seen with bacteria alone in these numbers.
The presence of NE in vitro therefore stimulates Campylobacter, leading to greater numbers of more motile, more invasive and potentially more virulent cells. In vivo stress and concomitant infection, both of which cause NE release, could therefore be contributory factors to the variable presentation of Campylobacter disease.
Abbreviations
BA - blood agar
CMH - Mueller–Hinton broth treated with chelex
cpm - counts per minute
DMEM - Dulbecco's modified Eagle's medium
MH - Mueller–Hinton
MOI - multiplicity of infection
NCTC - National Collection of Type Cultures
NE - norepinephrine
PBS - phosphate‐buffered saline
TEER - transepithelial electrical resistance
Footnotes
Funding: This study was funded by the BBSRC, the Food Standards Agency and the Health Protection Agency. The University of Bristol cell imaging facility was established with MRC funding.
Competing interests: None.
References
- 1.Notermans S H. Epidemiology and surveillance of Campylobacter infections. Report on a WHO consultation on epidemiology and control of campylobacteriosis. Bilthoven, Netherlands: WHO, 199435–44.
- 2.Adak G K, Long S M, O'Brien S J. Trends in indigenous foodborne disease and deaths, England and Wales: 1992 to 2000. Gut 200251832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butzler J P.Campylobacter, from obscurity to celebrity. Clin Microbiol Infect 200410868–876. [DOI] [PubMed] [Google Scholar]
- 4.van Spreeuwel J P, Duursma G C, Meijer C J.et alCampylobacter colitis: histological immunohistochemical and ultrastructural findings. Gut 198526945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nachamkin I, Allos B M, Ho T.Campylobacter species and Guillain‐Barre syndrome. Clin Microbiol Rev 199811555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen M B. The enteric nervous system I: organisation and classification. Pharmacol Toxicol 200392105–113. [DOI] [PubMed] [Google Scholar]
- 7.Lundgren O. Sympathetic input into the enteric nervous system. Gut 20004733–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Åneman A, Eisenhofer G, Olbe L.et al Sympathetic discharge to the mesenteric organs and the liver. J Clin Invest 1996971640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyte M, Bailey M T. Neuroendocrine‐bacterial interactions in a neurotoxin‐induced model of trauma. J Surg Res 199770195–201. [DOI] [PubMed] [Google Scholar]
- 10.Ahlman H, Bhargava H N, Dahlstrom A.et al On the presence of serotonin in the gut lumen and possible release mechanisms. Acta Physiol Scand 1981112263–269. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhofer G, Aneman A, Friberg P.et al Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab 1997823864–3871. [DOI] [PubMed] [Google Scholar]
- 12.Woolf P D, McDonald J V, Feliciano D V.et al The catecholamine response to multisystem trauma. Arch Surg 1992127899–903. [DOI] [PubMed] [Google Scholar]
- 13.Zhou M, Simms H H, Wang P. Increased gut‐derived noradrenaline release in sepsis: up‐regulation of intestinal tyrosine hydroxylase. Biochim Biophys Acta 20041689212–218. [DOI] [PubMed] [Google Scholar]
- 14.Freestone P P, Haigh R D, Williams P H.et al Stimulation of bacterial growth by heat‐stable, norepinephrine‐induced autoinducers. FEMS Microbiol Lett 199917253–60. [DOI] [PubMed] [Google Scholar]
- 15.Lyte M, Ernst S. Catecholamine induced growth of gram negative bacteria. Life Sci 199250203–212. [DOI] [PubMed] [Google Scholar]
- 16.Bailey M T, Karaszewski J W, Lubach G R.et al In vivo adaptation of attenuated Salmonella typhimurium results in increased growth upon exposure to Noradrenaline. Physiol Behav 199967359–364. [DOI] [PubMed] [Google Scholar]
- 17.Coulanges V, Andre P, Vidon D J. Effect of siderophores, catecholamines, and catechol compounds on Listeria spp. Growth in iron‐complexed medium. Biochem Biophys Res Commun 1998249526–530. [DOI] [PubMed] [Google Scholar]
- 18.Anderson M T, Armstrong S K. The Bordetella bfe system: growth and transcriptional response to siderophores, catechols, and neuroendocrine catecholamines. J Bacteriol 20061885731–5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neal C P, Freestone P P, Maggs A F.et al Catecholamine inotropes as growth factors for Staphylococcus epidermidis and other coagulase‐negative staphylococci. FEMS Microbiol Lett 2001194163–169. [DOI] [PubMed] [Google Scholar]
- 20.Walters M, Sperandio V. Autoinducer 3 and epinephrine signaling in the kinetics of locus of enterocyte effacement gene expression in enterohemorrhagic Escherichia coli. Infect Immun 2006745445–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlisidou I, Lyte M, van Diemen P M.et al The neuroendocrine stress hormone Noradrenaline augments Escherichia coli O157:H7‐induced enteritis and adherence in a bovine ligated ileal loop model of infection. Infect Immun 2004725446–5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews S C, Robinson A K, Rodriguez‐Quinone F. Bacterial iron homeostasis. FEMS Microbiol Rev 200327215–237. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths E. Iron in biological systems. In: Bullen JJ, Griffiths E, eds. Iron and infection: molecular, physiological and clinical aspects Chichester, UK: John Wiley & Sons, 19991–26.
- 24.Palyada K, Threadgill D, Stintzi A. Iron acquisition and regulation in Campylobacter jejuni. J Bacteriol 20041864714–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Vliet A H, Ketley J M, Park S F.et al The role of iron in Campylobacter gene regulation, metabolism and oxidative stress defense. FEMS Microbiol Rev 200226173–186. [DOI] [PubMed] [Google Scholar]
- 26.Freestone P P, Williams P H, Haigh R D.et al Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma induced sepsis. Shock 200218465–470. [DOI] [PubMed] [Google Scholar]
- 27.Parkhill J, Wren B W, Mungall K.et al The genome sequence of the food‐borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 2000403665–668. [DOI] [PubMed] [Google Scholar]
- 28.Pourzand C, Watkin R D, Brown J E.et al Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci USA 1999966751–6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinney K S, Austin C E, Morton D S.et al Norepinephrine as a growth stimulating factor in bacteria‐mechanistic studies. Life Sci 2000673075–3085. [DOI] [PubMed] [Google Scholar]
- 30.Williams P H, Rabsch W, Methner U.et al Catecholate receptor proteins in Salmonella enterica: role in virulence and implications for vaccine development. Vaccine 2006243840–3844. [DOI] [PubMed] [Google Scholar]
- 31.Freestone P P, Lyte M, Neal C P.et al The mammalian neuroendocrine hormone noradrenaline supplies iron for bacterial growth in the presence of transferrin or lactoferrin. J Bacteriol 20001826091–6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naikare H, Palyada K, Panciera R.et al Major role for FeoB in Campylobacter jejuni ferrous iron acquisition, gut colonization, and intracellular survival. Infect Immun 2006745433–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowdre J H, Krieg N R, Hoffman P S.et al Stimulatory effect of dihydroxyphenyl compounds on the aerotolerance of Spirillum volutans and Campylobacter fetus subspecies jejuni. Appl Environ Microbiol 197631127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pesci E C, Cottle D L, Pickett C L. Genetic, enzymatic, and pathogenic studies of the iron superoxide dismutase of Campylobacter jejuni. Infect Immun 1994622687–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Day W A, Jr, Sajecki J L, Pitts T M.et al Role of catalase in Campylobacter jejuni intracellular survival. Infect Immun 2000686337–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Vliet A H, Baillon M L, Penn C W.et alCampylobacter jejuni contains two fur homologs: characterization of iron‐responsive regulation of peroxide stress defense genes by the PerR repressor. J Bacteriol 19991816371–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee A, O'Rourke J L, Barrington P J.et al Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni: a mouse cecal model. Infect Immun 198651536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grant C C, Konkel M E, Cieplak W., Jret al Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect Immun 1993611764–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Black R E, Levine M M, Clements M L.et al Experimental Campylobacter jejuni infection in humans. J Infect Dis 1988157472–479. [DOI] [PubMed] [Google Scholar]
- 40.Konkel M E, Hayes S F, Joens L A.et al Characteristics of the internalization and intracellular survival of Campylobacter jejuni in human epithelial cell cultures. Microb Pathog 199213357–370. [DOI] [PubMed] [Google Scholar]
- 41.Johnson R J, Nolan C, Wang S P.et al Persistent Campylobacter jejuni infection in an immunocompromised patient. Ann Intern Med 1984100832–834. [DOI] [PubMed] [Google Scholar]
- 42.Abram M, Vučković D, Wraber B.et al Plasma cytokine response in mice with bacterial infection. Mediators Inflamm 20009229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skirrow M, Blaser M J. Clinical aspects of Campylobacter infection. In: Nachamkin I, Blaser MJ, eds. Campylobacter. Washington, DC: ASM Press, 200066–88.
- 44.Vuckovic D, Abram M, Bubonja M.et al Host resistance to primary and secondary Campylobacter jejuni infections in C57Bl/6 mice. Microb Pathog 20064035–39. [DOI] [PubMed] [Google Scholar]
- 45.MacCallum A, Hardy S P, Everest P H.Campylobacter jejuni inhibits the absorptive transport functions of Caco‐2 cells and disrupts cellular tight junctions. Microbiology 20051512451–2458. [DOI] [PubMed] [Google Scholar]