Abstract
Background and aims
An algorithm based on a 2 log10 decline in hepatitis C virus (HCV) RNA at week (W) 12 has been proposed in US and European recommendations for the management of patients with chronic hepatitis C treated with pegylated‐interferon and ribavirin.
Methods
We examined rapid virological response (RVR; at W2 and W4 after the initiation of therapy) in HIV/HCV co‐infected patients. Using HCV RNA measurements (Versant HCV RNA 3.0, Cobas Amplicor HCV 2.0), RVR was studied in 323 patients from the ANRS HC02 RIBAVIC trial, comparing interferon α2b 3 MU ×3/week with pegylated interferon α2b 1.5 μg/kg/week, each combined with ribavirin 800 mg/day over 48 weeks.
Results
The best positive and negative predictive values of sustained virological response (SVR) were obtained with an undetectable HCV RNA at W4 (97%) and with more than a 2 log10 decrease at W12 (99%), respectively. Prediction of non‐SVR was obtained in all patients by using HCV RNA cut‐off levels above 460 000 IU/ml at W4 and above 39 000 UI/ml at W12 irrespective of the HCV genotype and arm of treatment.
Conclusion
We propose a new algorithm based on RVR thresholds using HCV RNA that allows for excellent prediction of non‐SVR as early as W4.
Keywords: HIV/HCV co‐infection, hepatitis C therapy, HCV viral load, rapid and early viral decline, prediction of response
As they share the same route of transmission, chronic hepatitis C is frequently associated with HIV infection. The rate of hepatitis C virus (HCV) co‐infection in HIV patients is about 30% overall but can climb to 90% for intravenous drug users.1 Mortality due to AIDS has decreased with the use of highly active antiretroviral treatment (HAART), but complications associated with HCV infection have emerged.2 End‐stage liver disease has become the leading cause of non‐AIDS related death in these patients.3 Spontaneous HCV clearance may occur under HAART containing protease inhibitors, as we first described, but this is a rare event.4 Furthermore, treatment of chronic hepatitis C is complicated in these patients because of the priority of AIDS therapy. Recently, several randomised trials, the “Agence Nationale pour la Recherche sur le SIDA” (ANRS) HC02 RIBAVIC (n = 412), the AIDS Pegasys Ribavirin International Coinfection Trial (APRICOT) (n = 860) and the AIDS Clinical Trial Group (ACTG) 5071 (n = 133) evaluated anti‐HCV treatment combining pegylated interferon (PEG‐IFN) or standard interferon (IFN) and ribavirin for HIV/HCV co‐infected patients with controlled HIV infection.5,6,7 In these studies, rates of sustained virological response (SVR) were significantly higher in the PEG‐IFN group (27%, 40% and 27%, respectively) than in the IFN group (20%, 12% and 12%, respectively). Nevertheless, these studies showed that SVR rates were lower than in mono‐infected patients treated with PEG‐IFN plus ribavirin (SVR rates about 55%).8,9
In the ANRS HC02 RIBAVIC study,5 the viral factors for treatment response were identified as a baseline viraemia below 5.7 log10 IU/ml and genotypes 2, 3 and 5 as reported by others.10,11 However, these factors are weak and predicted SVR in only 31% and 44% of patients with these criteria. Therefore, following large pivotal trials in HCV‐infected patients12,13 it has been recommended that early virological response (EVR) should be evaluated after 12 weeks of treatment, based on a 2 log decline or an undetectable HCV RNA algorithm.14,15,16 This approach allows for the early discontinuation of treatment in those who do not respond (negative predictive value of 97–100%) and the avoidance of side effects and expense for these patients.5,6,7,9,10 However, the general application of this algorithm based on rapid virological response (RVR) at week (W) 2 or W4 has not previously been reported in HIV/HCV co‐infected patients.
Therefore, in this study, we evaluated the usefulness of HCV RNA measurements for therapeutic follow‐up of HIV/HCV co‐infected patients included in the ANRS HC02 RIBAVIC study. We also investigated whether early variations in HCV RNA could accurately predict treatment response to an IFN‐based regimen plus ribavirin using a new algorithm.
Methods
Patients study: the ANRS HC02 RIBAVIC protocol
We tested 1500 samples during follow‐up of 323 HIV/HCV co‐infected patients. We selected patients who had completed at least one course of treatment. These patients were assessed at W4 and W12 for HCV RNA measurements according to the ANRS HC02 RIBAVIC protocol (323/412, 78% of included patients). Patients gave written consent at inclusion and received either IFN α2b 3 MU 3×/week (n = 207) or PEG‐IFN α2b 1.5 μg/kg/week (n = 205). Both of these arms included ribavirin 800 mg/day.5 Treatment duration was 48 weeks for all patients, irrespective of genotype and no stopping rules have been proposed in the RIBAVIC trial (started before the 2 log decline rules). Serum samples for viral follow‐up were collected at day (D) 0 and at W2, W4, W12, W24, W36 and W48 and were stored at –80°C. Follow‐up evaluations were carried out 4 (W52) and 24 (W72) weeks after the end of treatment. The main end‐point was a sustained virological response assessed by undetectable HCV RNA at W72. Patients without the W72 end‐point and/or without early monitoring (W4 and W12) were excluded from analysis (n = 79). Four groups of patients were considered according to their response to treatment: group 1, non‐responders (NR) with persistent HCV RNA during treatment (n = 182, 56.7%); group 2, sustained virological responders (SVR) with undetectable HCV RNA at the end of treatment and 6 months after stopping treatment (n = 88, 27.2%); group 3, responders with relapse or relapsers (RR) with undetectable HCV RNA at the end of treatment and detectable HCV RNA 6 months after the end of treatment (n = 26, 8.1%); and group 4, responders with a breakthrough (RB) with undetectable HCV RNA during treatment and reappearance of HCV RNA before the end of treatment despite continuation of therapy (n = 27, 8.4%). Patients from groups 1, 3 and 4 were considered as non‐SVR for comparison with group 2 (SVR) in this study.
Qualitative HCV RNA detection
Qualitative HCV RNA detection was performed using a gene amplification based method (PCR) with the Cobas Amplicor HCV v2.0 system according to the manufacturer's instructions (Roche Diagnostics, Meylan, France). The lower limit of HCV RNA detection is 50 IU/ml for 200 μl of serum. Analysis was carried out for HCV RNA at D0, W2, W4, W12, W24, W36, W48 and W72. For patients in group 3 who relapsed after treatment (negative PCR at W48 and positive at W72), serum analysis at W52 was also performed.
HCV RNA quantification
Quantification of HCV RNA was carried for HCV RNA‐positive samples (50 μl) using the Versant HCV RNA v3.0 assay according to the manufacturer's instructions (Bayer Diagnostics, Eragny, France). The results were expressed in log10 IU/ml. The range of quantification by branched DNA (bDNA) technology is from 2.79 log10 IU/ml to 6.89 log10 IU/ml.
HCV genotype determination
HCV was genotyped at D0 for all patients using the Versant HCV Genotype Assay (LiPA) (Bayer Diagnostics) according to the manufacturer's instructions. The LiPA was carried out with 20 μl of amplicons from Cobas Amplicor technology.
Statistical analysis
Statistical analysis was performed with SPSS software V.10.1 for Windows (SPSS, Chicago, IL, USA). When HCV RNA quantification results were less than the cut‐off value, we used the value with the maximum of likelihood, ie, the logarithm of half the cut‐off value (2.49 log10 IU/ml). The optimal thresholds of HCV RNA at D0, W2, W4 and W12 were measured using univariate receiver operating characteristics (ROC) curves on SPSS showing the highest sensitivity and specificity. Qualitative data (genotypes, more than 80% of doses, undetectable HCV RNA, more than a 2 log reduction) were evaluated for SVR or non‐SVR using the two factors cross tables with calculation of sensitivity, specificity, negative and positive predictive values and odds ratios. We used t tests and Mann Whitney tests for independent samples to examine whether there was an observable difference between quantitative factors and χ2 and Fisher's exact test for qualitative factors. All tests were two‐sided and statistical significance was assessed at the p⩽0.05 level.
Results
Characteristics of study patients before treatment
The patients in both arms of treatment (PEG‐IFN+ribavirin and IFN+ribavirin) showed similar baseline virological characteristics (table 1). Baseline characteristics, such as sex, age and fibrosis score, were similar to those reported for all patients included in the ANRS HC02 RIBAVIC study.5
Table 1 Characteristics of virological responses by group of treatment and genotypes in treated HIV/HCV co‐infected patients.
| IFN group | PEG‐IFN group | Total | p Value | |
|---|---|---|---|---|
| Number of patients, n (%) | 164 (50.8) | 159 (49.2) | 323 | |
| Viral load (log IU/ml) | 5.87±0.70 | 5.88±0.71 | 5.87±0.70 | 0.95 |
| More than 80% of doses, n (%)† | 107 (65.2) | 102 (64.2) | 209 (64.7) | 0.75 |
| Sustained responders, n (%) | 39 (23.8) | 49 (30.8) | 88 (27.2) | 0.041 |
| Responders with relapse, n (%) | 8 (4.9) | 18 (11.3) | 26 (8.1) | 0.032 |
| Responders with breakthrough, n (%) | 14 (8.5) | 13 (8.2) | 27 (8.4) | 0.85 |
| Non‐responders, n (%) | 103 (62.8) | 79 (49.7) | 182 (56.7) | 0.075 |
| Genotype 1 or 4, n (%) | 100 (61.0) | 98 (61.6) | 198 (61.3) | 0.91 |
| Sustained response for genotype 1 or 4, n (%) | 7 (7.2) | 19 (19.4) | 26 (13.3) | 0.007 |
| Sustained response for genotype 2 or 3, n (%) | 30 (48.4) | 30 (49.2) | 60 (48.8) | 1.00 |
*p value for comparison of both arms of treatment (not significant if p>0.05); †percentage of patients receiving more than 80% of their treatment with IFN α2b plus ribavirin or PEG‐IFN α2b plus ribavirin.
Influence of treatment and genotype
The rate of SVR among the 323 patients from the ANRS HC02 RIBAVIC study who were assessed for very early response under treatment (RVR with undetectable HCV RNA at W4) was similar to that of the global study (27% vs 23%, respectively; table 1). The PEG‐IFN+ribavirin treatment was more efficient than standard IFN+ribavirin, with SVR rates of 30.8% (49/159) and 23.8% (39/164), respectively (p = 0.041), mostly for patients with genotype 1 or 4 (19.4 vs 7.2%, respectively, p = 0.007). Most of the included patients received more than 80% of treatment doses, with a similar percentage in each group (about 65%), and their SVR rate was increased (34% vs 14% if less than 80% of treatment doses, p<0.001).
Viral kinetics sorted by response to treatment
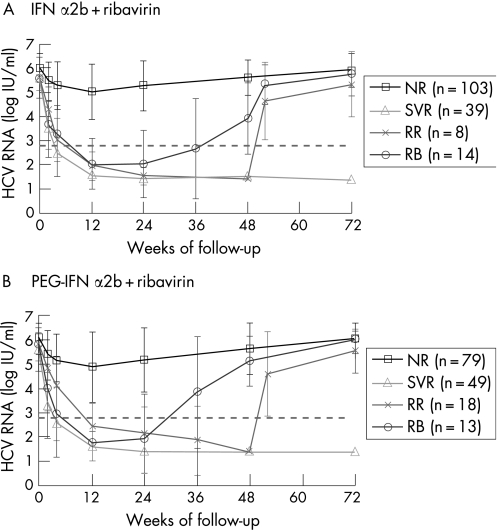
In sustained responders to PEG‐IFN+ribavirin, the rapid decrease (W2) in HCV RNA (2.26±1.11 log10) was significantly different from that of relapsers (1.05±0.70 log10, p<0.01); the latter was also significantly different from that of non‐responders (0.73±0.94 log10, p = 0.03) (table 2). This kinetic difference was also observed at W4. HCV RNA kinetics in non‐responders showed a mean transient decrease between D0 and W12 (1.21±1.38 log10) but an increase was always observed from W12 to W72 (return to baseline value) (fig 1). Patients with a breakthrough showed a rapid viral decrease, with the reduction being between that of responders and relapsers (table 2). Similar kinetics were observed in the standard IFN arm.
Table 2 Rapid and early decrease of HCV RNA by response and group of treatment (n = 323).
| IFN α2b+ribavirin group | PEG‐IFN α2b+ribavirin group | |||||||
|---|---|---|---|---|---|---|---|---|
| n | ΔD0–W2 (SD) | ΔD0–W4 (SD) | ΔD0–W12 (SD) | n | ΔD0–W2 (SD) | ΔD0–W4 (SD) | ΔD0–W12 (SD) | |
| SVR | 39 | 2.13 (1.02) | 3.18 (0.94) | 4.10 (0.95) | 49 | 2.26 (1.11) | 2.99 (1.32) | 3.97 (0.95) |
| *p = 0.24 | *p = 0.012 | *p = 0.09 | *p = 0. 09 | *p = 0.59 | *p = 0.63 | |||
| RR | 8 | 1.35 (0.42) | 2.60 (0.53) | 3.69 (0.70) | 18 | 1.05 (0.70) | 1.78 (1.27) | 3.41 (1.02) |
| †p = 0.03 | †p = 0.05 | †p = 0.16 | †p<0.01 | †p<0.01 | †p = 0.04 | |||
| RB | 14 | 1.74 (0.72) | 2.26 (1.15) | 3.52 (1.15) | 13 | 1.75 (0.79) | 2.83 (1.23) | 4.05 (1.08) |
| ‡p = 0.25 | ‡p = 0.77 | ‡p = 0.83 | ‡p = 0.018 | ‡p = 0.037 | ‡p = 0.11 | |||
| NR | 103 | 0.55 (0.55) | 0.74 (0.79) | 1.00 (1.00) | 79 | 0.73 (0.94) | 0.95 (0.96) | 1.21 (1.38) |
| §p<0.01 | §p<0.01 | §p<0.01 | §p = 0.03 | §p<0.01 | §p<0.01 | |||
Data are expressed as log HCV RNA load decline in serum samples at W2, W4 and W12 compared to viral load at baseline (day 0), with mean value and 95% confidence intervals for each treatment group and response.
*Responders with breakthrough (RB) compared to sustained virological responders (SVR); †relapsers (RR) compared to SVR; ‡RB compared to RR; §non‐responders (NR) compared to RR. p Value is not significant if p>0.05.
Figure 1 Viral kinetics for non‐responders (NR), sustained virological responders (SVR), responders with relapse (RR) and responders with a breakthrough under treatment (RB). HIV/HCV co‐infected patients were treated with standard interferon α2b (A) or pegylated‐interferon α2b (B) plus ribavirin therapy for 48 weeks. The broken grey line represents the HCV RNA threshold at 2.79 log IU/ml. Note the similarity between the HCV RNA kinetics in each arm of treatment, with a two phases decay before week (W) 12 as described in HCV mono‐infected treated patients.17 The viral decline was rapid for SVR, slower for RR and RB, and slight for NR with a viral rebound. Return to viral baseline value for RR, RB and NR was observed at the end of follow‐up (W72).
Predictive values of response, relapse and non‐response
The negative predictive value (NPV) and the positive predictive value (PPV) of response to treatment were calculated according to undetectable HCV RNA or to a decrease in HCV RNA greater than 2 log10 under treatment. NPV and PPV were studied at W2, W4 and W12 for RVR or EVR (table 3). The best PPV was obtained using qualitative detection of HCV RNA (undetectable HCV RNA) at W4 (97%) compared to W12 (81%), but most responder patients had undetectable HCV RNA at W12 (sensitivity of 87%) compared to those at W4 (sensitivity of 40%). Conversely, nearly all the non‐SVR patients had detectable HCV RNA at W4 (specificity of 98%). The best NPV was obtained at W12 using a 2 log reduction for HCV RNA or an undetectable HCV RNA (NPV = 99%), allowing detection of 65% (specificity) of the non‐SVR (table 3). PPV at W4 and NPV at W12 were similar in the PEG‐IFN and standard IFN arms (PPV at 98.5% and 96.5%, respectively, with odds ratios (95% CI) of 12.74 (8.29 to 16.82) and 8.41 (4.88 to 11.75), and NPV at 98.8% and 100%, with odds ratios of 1.97 (1.61 to 2.41) and 2.05 (1.65 to 2.59)).
Table 3 Positive (PPV) and negative (NPV) predictive values of sustained virological response (SVR).
| n* | Se | Sp | PPV | NPV | OR† (SVR) | OR† (non‐SVR) | ||
|---|---|---|---|---|---|---|---|---|
| W2 | Und.HCV | 310 | 10.8 | 99.6 | 90.0 | 75.3 | 3.65 (2.74 to 4.85) | 0.13 (0.02 to 0.85) |
| ΔHCV>2 log | 288 | 62.5 | 91.3 | 73.5 | 86.4 | 0.18 (0.13 to 0.27) | 3.26 (2.18 to 4.85) | |
| ΔHCV>2 log or Und.HCV | 288 | 62.5 | 90.9 | 72.5 | 86.3 | 0.19 (0.13 to 0.27) | 3.13 (2.13 to 4.61) | |
| W4 | Und.HCV | 323 | 39.8 | 97.9 | 97.5 | 81.3 | 9.67 (6.57 to 14.11) | 0.15 (0.07 to 0.35) |
| ΔHCV>2 log | 320 | 86.2 | 77.7 | 59.1 | 93.8 | 0.10 (0.06 to 0.18) | 2.29 (1.85 to 2.83) | |
| ΔHCV>2 log or Und.HCV | 320 | 87.4 | 77.3 | 67.9 | 94.2 | 0.10 (0.05 to 0.18) | 2.29 (1.85 to 2.83) | |
| W12 | Und.HCV | 323 | 86.6 | 86.4 | 80.9 | 95.3 | 15.10 (8.15 to 27.99) | 0.30 (0.23 to 0.40) |
| ΔHCV>2 log | 323 | 97.7 | 64.6 | 50.6 | 98.7 | 0.03 (0.01 to 0.10) | 1.99 (1.71 to 2.33) | |
| ΔHCV>2 log or Und.HCV | 323 | 98.9 | 64.8 | 60.3 | 99.3 | 0.01 (0.01 to 0.09) | 2.01 (1.73 to 2.34) | |
Predictive values of response to IFN α2b+ribavirin or PEG‐IFN α2b+ribavirin therapy were assessed with an undetectable HCV RNA (Und.HCV), a greater than 2 log HCV RNA decline (ΔHCV>2 log) or both, evaluated at W2, W4 and W12 of treatment.
*Number of tested patients; †odds ratios and 95% confidence intervals.
SE, sensitivity; Sp, specificity.
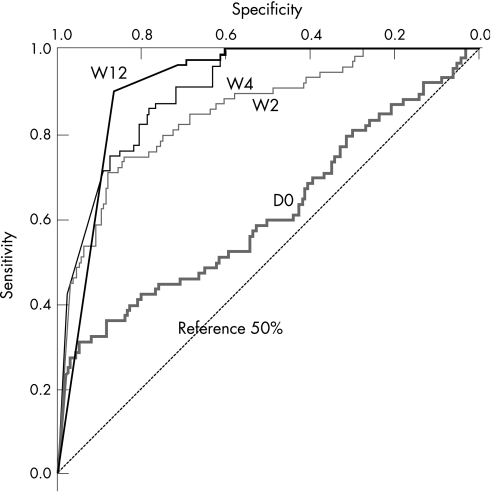
The optimal threshold of HCV RNA for NPV response prediction, evaluated by ROC curves (fig 2), was determined to be 8×106 IU/ml (6.9 log) at D0, 600 000 IU/ml (5.8 log) at W2, 460 000 IU/ml (5.6 log) at W4 and at 39 000 IU/ml (4.6 log) at W12 allowing for detection of 2.5%, 18%, 32% and 61% of the non‐SVR, respectively (NPV = 100%). At W24, the best NPV was defined by a detectable HCV RNA (99%) allowing the detection of 93% of the non‐SVR.
Figure 2 Receiver operating characteristics (ROC) curves for HCV RNA quantification in HIV/HCV co‐infected patients at baseline (D0) and at week (W) 2, W4 and W12 of pegylated‐interferon α2b plus ribavirin therapy. The optimal cut‐off levels at W4 and W12 for non‐sustained response were calculated to be 460 000 IU/ml (5.6 log) and 39 000 IU/ml (4.6 log), respectively. There was not enough information at D0 and W2 for practical treatment follow‐up. Intra‐assay variation using the branched DNA assay for viral load measures is very low (about 3%) as we reported previously.18 Cut‐off levels would thus vary from 453 000 to 467 000 IU/ml at W4 and from 38 400 to 39 600 IU/ml at W12, within the 95% confident intervals for cut‐off calculation from the ROC curves (310 000 to 610 000 IU/ml at W4 and 30 000 to 48 000 IU/ml at W12). The reference 50% line corresponds to 50% specificity and 50% sensitivity, showing no advantage of the test.
Discussion
Treatment of chronic hepatitis C using IFN‐based regimens has been increasingly effective over the last decade. About half of the patients using PEG‐IFN combined with ribavirin will eliminate the virus, as identified by an SVR.8,9 However, it has been recently demonstrated that this treatment was less effective in HIV/HCV co‐infected patients, with only approximately one third having an SVR.5,6,7 This two‐drug therapy is made more difficult by the concomitant use of antiretroviral treatment for HIV infection, which usually contains three other drugs. Side effects are very frequent (about 20%) and regular medical supervision and dose adjustment of PEG‐IFN and/or ribavirin is required. Indeed, dose reductions during the first 12 weeks, to less than 80% of the prescribed dose, are frequently observed in HCV mono‐infected patients (20.5%), resulting in a marked reduction in EVR (33% vs 80% for those who received more than 80% of the dose).13 Comparatively, we found that among HIV/HCV co‐infected patients treated with less or more than 80% of the dose, 39% and 60% of SVR exhibited EVR, respectively (p<0.001).
Therefore, it is important to define the optimal management of PEG‐IFN+ribavirin therapy for these patients in order to preferably treat those with reasonable chances of sustained response.
Thus, HCV RNA could be used for EVR evaluation. EVR was defined at the European and American consensus conferences in 2002 as either undetectable HCV RNA, or a 2 log decrease from baseline HCV RNA level at W12 of treatment, for both HIV/HCV co‐infected patients and HCV mono‐infected patients.14,15,16 This end‐point has been applied mostly to patients who should discontinue treatment at W12 if they have not reached this point (prediction of non‐response, ie, RR and NR). Interestingly, in our study, an RVR was obtained at W4 of therapy; undetectable HCV RNA was the best positive predictive factor of SVR (PPV of 97%). Patients who reached this RVR should be motivated to complete the prescribed course of full‐dose treatment. However, only 40% of SVR reached this end‐point, similarly to the study of Davis et al in HCV mono‐infected patients (48.7%, PPV of 88.7%).10 No benefit was obtained at week 2 in our study (PPV of 90%). We also observed, as noted in previous reports using branched DNA or PCR‐based methods,11,19 that patients with more than 460 000 IU/ml (5.66 log) at week 4 have no chance of achieving a sustained response (NPV of 100%). Using that cut‐off level, as proposed by Berg for HCV mono‐infected patients treated with PEG‐IFN+ribavirin therapy (cut‐off level at 450 000 IU/ml or 5.65 log),11 treatment could be discontinued earlier in a more non‐responder patients in the ANRS HC02 RIBAVIC study (33% vs 14.6% in Berg's report).
Using the consensus algorithm for discontinuation of therapy at W12 based on the 2 log10 decline for HCV mono‐infected patients,14,15,16 we found one patient with a viral decline of less than 2 log10 and detectable HCV RNA at W12 who achieved a sustained response (NPV of 99%). This patient was infected by HCV genotype 1a and included in the PEG‐IFN+ribavirin arm. This patient's HCV RNA was detectable up to W24 but was thereafter undetectable and remained so up to W72. This is in accordance with other reports using an end‐point of W12, where prediction was not 100% for HCV mono‐infected patients or for HIV/HCV co‐infected patients (NPV of 97% and 98%, respectively).6,9 When using the algorithm proposed by Davis10 for HCV mono‐infected patients with an undetectable HCV RNA at W24 as another end‐point, this patient would have been retained for complete therapy, whereas using the 2 log10 decay at W12, he would have incorrectly been discontinued.
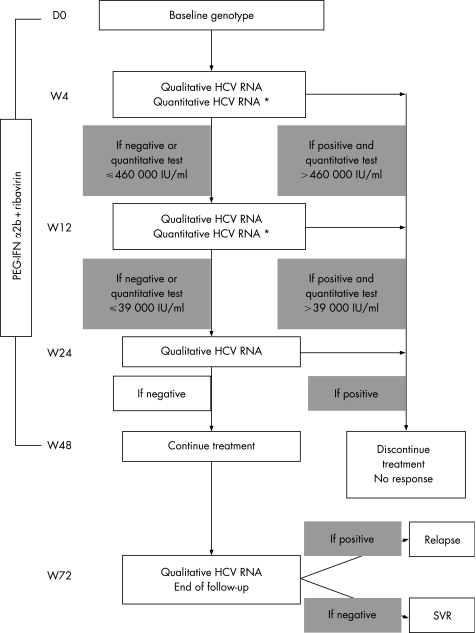
Therefore, Castro et al proposed the use of a 3 log10 decline at W12, predicting 100% of non‐SVR.20 Berg et al proposed another algorithm based on an HCV RNA cut‐off level at W12 of 30 000 IU/ml or 4.48 log.11 Using this cut‐off level, the authors found the highest prediction of non‐SVR at W12 (NPV of 100%), with 53.7% of the non‐SVR patients (defined as RR or NR) correctly defined. We have calculated this cut‐off level in our study and found a value close to that proposed by Berg irrespective of genotype (39 000 IU/ml or 4.59 log); NPV was excellent (100%) with 61% of the non‐SVR patients detected at W12. For patients below this cut‐off level, HCV RNA was assessed at W24 as proposed by Davis.10 Using this algorithm, all patients but one were non‐responders and could have stopped their treatment at W24 (NPV of 99%), with 93% of non‐SVR patients detected at this end‐point. Only one patient infected by HCV genotype 3 and included in the IFN+ribavirin arm still had a detectable HCV RNA at W24, but he had a low HCV RNA load (between 50 and 600 IU/ml). This patient had less than a 2 log10 decline at W12 (1.94 log10) and would have prematurely discontinued his treatment with the 2 log10 decay algorithm proposed by Davis.10 This patient had late viral clearance (undetectable HCV RNA at W36) that lasted to the end of follow‐up and was classified as SVR. Two HIV/HCV co‐infected patients treated by PEG‐IFN (Pegasys) plus ribavirin in the APRICOT study also showed SVR with less than a 2 log10 decline at W12 and late viral clearance (at W24 and W36) as reported by the authors.6 Late viral clearance has also been described by Ebeling et al with a genotype 3 patient presenting detectable HCV RNA at W24 under consensus IFN.21 However, our patient had a very low viral load which might have been undetectable if he had received PEG‐IFN+ribavirin therapy. In this arm, only non‐SVR patients were detected at W24 using our algorithm (fig 3).
Figure 3 Proposed algorithm for management of HIV/HCV co‐infected patients with chronic hepatitis C during pegylated‐interferon α2b plus ribavirin therapy. A total of 93% of the non‐responder patients included in the ANRS HC02 RIBAVIC study would discontinue their treatment with an early 100% prediction of non‐response before W24, with 33% detected at W4, 32% at W12 and 28% at W24. All sustained responder patients would have continued their treatment up to W48 but some of them (17.6%) might relapse after the end of treatment; all relapsers were detected by a qualitative HCV RNA positive test by W52 compared to W72 at end of the follow‐up. Similar results were obtained in the interferon α2b plus ribavirin group. *Quantitative assay for HCV RNA was performed when the qualitative test was positive.
In the present study, we also showed that a longer period of viral clearance (related to RVR) was significantly related to SVR and that prolonging therapy for at least 12 weeks for patients showing slower viral clearance associated with further relapse might increase SVR irrespective of genotype (undetectable HCV RNA for a period of 36 versus 24 weeks in SVR and RR, respectively, p<0.001). Such an approach comparing SVR after therapy has been evaluated in the TERAVIC‐4 study showing a benefit in HCV genotype 1 infected patients after 72 weeks compared to 48 weeks of treatment (SVR 46% vs 32% and lower relapse of 14% vs 29%).22 Berg et al also showed that extended treatment up to 72 weeks should only be reserved for patients with slow virological response defined as HCV RNA positive at week 12 and negative at week 24 (SVR 29% vs 17% with treatment duration at 48 weeks).23 Further studies on patients infected with other HCV genotypes are required to confirm the benefits of longer treatment in HIV/HCV co‐infected patients who present a late response during treatment.
In conclusion, using results from the ANRS HC02 RIBAVIC study, we have defined a new algorithm for HIV/HCV co‐infected patients treated with PEG‐IFN α2b and ribavirin (fig 3), based on RVR and EVR marker cut‐off levels at W4, W12 and W24 irrespective of HCV genotype. Viral load cut‐off thresholds were predictive of non‐response irrespective of treatment arm, the genotypes involved or the doses of treatment (greater or less than 80%). This algorithm may allow therapy to be discontinued for 93% of the non‐SVR patients with 100% prediction (only 65% of the non‐SVR patients are identified using the 2 log10 decline at W12 with 99% prediction). Early detection of non‐SVR patients at W4, W12 and W24 with early cessation of treatment would in our study allow a reduction of 144 one‐year equivalent treatments for 323 treated patients or 94 treatments for the 159 patients included in the PEG‐IFN+ribavirin arm (at a cost savings of about €1 million, as 1 year of PEG‐IFN+ribavirin therapy costs €16 000 per patient, with extra costs of €14 000 for EVR evaluation for HCV RNA). This strategy would thus allow cost savings of 41% in our study compared to a complete 1‐year PEG‐IFN+ribavirin therapy. Furthermore, this approach should make the treatment of chronic hepatitis C in HIV/HCV co‐infected patients more appealing by providing a limited test period of treatment before commitment to a full course of therapy was required. It could also be a strong goal to motivate adherence for patients and their physicians by providing an earlier marker of response to therapy (RVR at W4) which if reached, is associated with a great likelihood of sustained response. However, this strategy using the proposed algorithm should be confirmed in other trials including HIV/HCV co‐infected patients and using higher doses of ribavirin (1000–1200 mg per day) as proposed by Berg et al for HCV mono‐infected patients.11
Acknowledgements
We wish to thank the scientific committee of the ANRS HC02 RIBAVIC study and all the investigators and virologists involved in this study: F Bani‐Sadr (Groupe Hospitalier Universitaire Est, Université Paris 6, Inserm U444), E Rosenthal (Hôpital de l'Archet, Faculté de Médecine, Nice), A Benzeckri and C Degott (Groupe Hospitalier Universitaire Nord, Université Paris 7), C Goujard (Groupe Hospitalier Universitaire Sud, Université Paris 11), G Pialoux (Groupe Hospitalier Universitaire Est, Université Paris 6), L Piroth (Centre Hospitalier Universitaire, Dijon), D Salmon‐Céron (Groupe Hospitalier Universitaire Ouest, Université Paris 5). Many thanks to Juliette Deshayes for technical assistance, and to Kevin L Erwin, qualified biomedical translator, for proofreading this manuscript.
Abbreviations
EVR - early virological response
HAART - highly active antiretroviral treatment
HCV - hepatitis C virus
IFN - interferon
NPV - negative predictive value
NR - non‐responders
PEG‐IFN - pegylated interferon
PPV - positive predictive value
RB - responders with breakthrough
ROC - receiver operating characteristics
RR - responders with relapse
RVR - rapid virological response
SVR - sustained virological response/responders
Footnotes
Financial support: ANRS (the French National Agency for Research on AIDS and Viral Hepatitis) was the sponsor of the ANRS HC02 RIBAVIC study, which was conducted with the support of Schering Plough (C Lemonnier and A Rimailho).
Competing interests: None.
References
- 1.Lauer G, Walker B. Hepatitis C virus infection. N Engl J Med 200134541–52. [DOI] [PubMed] [Google Scholar]
- 2.Pol S, Vallet‐Pichard A, Fontaine H. Hepatitis C and HIV co‐infection at the era of HAART. J Viral Hepat 200291–8. [DOI] [PubMed] [Google Scholar]
- 3.Cacoub P, Geffray L, Rosenthal E, for the Joint Study Group on Hepatitis C Virus of the French National Society of Internal Medicine and the French Society of Infectious Diseases (GERMIVIC Study Group) et al Mortality among HIV‐infected patients with cirrhosis or hepatocellular carcinoma due to HCV in French departments of internal medicine/infectious diseases, in 1995 and 1997. Clin Infect Dis 2001321207–1214. [DOI] [PubMed] [Google Scholar]
- 4.Fialaire P, Payan C, Vitour D.et al Sustained disappearance of Hepatitis C viremia in patients receiving protease inhibitor treatment for human immunodeficiency virus infection. J Infect Dis 1999180574–575. [DOI] [PubMed] [Google Scholar]
- 5.Carrat F, Bani‐Sadr F, Pol S.et al Pegylated interferon alfa‐2b versus standard interferon alfa‐2b, plus ribavirin, for chronic hepatitis C in HIV‐infected patients: a randomized controlled trial. JAMA 20042922839–2848. [DOI] [PubMed] [Google Scholar]
- 6.Torriani F J, Rodriguez‐Torres M, Rockstroh J.et al PEG‐interferon alfa‐2a plus ribavirin for chronic HCV infection in HIV‐infected patients. N Engl J Med 2004351438–450. [DOI] [PubMed] [Google Scholar]
- 7.Chung R, Andersen J, Volberding P.et al Pegylated interferon alfa‐2a plus ribavirin versus interferon alfa‐2a plus ribavirin for chronic hepatitis C in HIV co‐infected persons. N Engl J Med 2004351451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manns M P, McHutchison J, Gordon S C.et al Peginterferon α2b plus ribavirin compared with interferon α2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001358958–965. [DOI] [PubMed] [Google Scholar]
- 9.Fried M, Shiffman M, Reddy R.et al Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002347975–982. [DOI] [PubMed] [Google Scholar]
- 10.Davis G, Wong J, McHutchison J.et al Early virologic response to treatment with peginterferon alfa‐2b plus ribavirin in patients with chronic hepatitis C. Hepatology 200338645–652. [DOI] [PubMed] [Google Scholar]
- 11.Berg T, Sarrazin C, Herrman E.et al Prediction of treatment outcome in patients with chronic hepatitis C: significance of baseline parameters and viral dynamics during therapy. Hepatology 200337600–609. [DOI] [PubMed] [Google Scholar]
- 12.McHutchison J, Blatt L, Sedghi‐Vaziri A.et al Is there an optimal time to measure quantitative HCV RNA to predict non‐response following interferon treatment for chronic HCV infection? J Hepatol 199829362–368. [DOI] [PubMed] [Google Scholar]
- 13.Poynard T, Marcellin P, Lee S.et al Randomised trial of interferon α2b plus ribavirin for 48 weeks or for 24 weeks versus interferon α2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 19983521426–1432. [DOI] [PubMed] [Google Scholar]
- 14.Dhumeaux D, Marcellin P, Lerebours E. Treatment of hepatitis C. The 2002 French consensus. Gut 2003521784–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health National Institutes of Health Consensus Development Conference Statement: management of hepatitis C. Hepatology 200236S3–20. [DOI] [PubMed] [Google Scholar]
- 16.Alberti A, Clumeck N, Collins S.et al Short statement of the first European Consensus Conference on the treatment of chronic hepatitis B and C in HIV co‐infected patients. J Hepatol 200542615–624. [DOI] [PubMed] [Google Scholar]
- 17.Zeuzem S, Schmidt J, Lee J H.et al Hepatitis C virus dynamics in vivo: effect of ribavirin and interferon alpha on viral turnover. Hepatology 199828245–252. [DOI] [PubMed] [Google Scholar]
- 18.Lunel F, Cresta P, Vitour D.et al Comparative evaluation of hepatitis C virus RNA quantitation by branched DNA, NASBA, and Monitor assays. Hepatology 199929528–535. [DOI] [PubMed] [Google Scholar]
- 19.Poynard T, McHutchison J, Goodman Z.et al Is an «à la carte» combination interferon α2b plus ribavirin regimen possible for the first line treatment in patients with chronic hepatitis? Hepatology 200031211–218. [DOI] [PubMed] [Google Scholar]
- 20.Castro F, Esteban J, Juarez A.et al Early detection of non response to interferon plus ribavirin combination treatment of chronic hepatitis C. J Viral Hepat 20029202–207. [DOI] [PubMed] [Google Scholar]
- 21.Ebeling F, Lappalainen M, Vuoristo M.et al Factors predicting interferon treatment response in patients with chronic hepatitis C: late viral clearance does not preclude a sustained response. Am J Gastroenterol 2001961237–1242. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez‐Tapias J, Diago M, Escartin M.et al Peginterferon‐alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology 2006131451–460. [DOI] [PubMed] [Google Scholar]
- 23.Berg T, von Wagner M, Nasser S.et al Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon‐alfa‐2a plus ribavirin. Gastroenterology 20061301086–1097. [DOI] [PubMed] [Google Scholar]