Rapid progress in interventional cardiology has recently seen the rate of percutaneous coronary intervention overtake that of coronary artery bypass surgery. Now attention is directed towards the treatment of valvular heart disease, with exciting developments in balloon and stent technology having the potential to transform the management of many common heart conditions.
RATIONALE FOR A PERCUTANEOUS APPROACH
Valvular heart disease is an important cause of cardiovascular morbidity and mortality worldwide. In many countries, though improved living conditions and better access to antibiotics and healthcare have seen a decline in rheumatic heart disease, the prevalence of degenerative valve disease has escalated with ageing of the population. In addition, the number of long‐term survivors of surgery for congenital cardiac malformations is growing, with these patients frequently affected by valve dysfunction in later life. Increasing availability of cardiopulmonary bypass, surgical expertise and intensive care facilities has seen valve repair and replacement widely performed to relieve symptoms and improve prognosis, despite associated morbidity and mortality. Many patients, however, particularly the elderly with major co‐morbidities, still do not undergo potentially beneficial interventions because of their high surgical risk.1 Development of a transcatheter alternative could significantly reduce morbidity and mortality, thus extending treatment to those who are not currently considered for surgery. Conversely, a less invasive therapy may also permit treatment of valvular heart disease at an earlier stage in its natural history and avoid the onset of progressive ventricular dysfunction. Patient preference for minimally invasive therapies is strong, particularly in those who have often undergone many operations. Furthermore, the success of these technologies could have an important economic impact due to associated reduction in intensive care and hospital stay.
PERCUTANEOUS MITRAL VALVE REPAIR
Normal mitral valve performance requires thin, mobile leaflets with unrestricted commissures and chordae tendinae and papillary muscles of appropriate size and position. Dysfunction may arise when there is a primary defect in these structures, but can also occur as a consequence of dilatation of the left heart chambers. Symptoms result from pulmonary venous congestion, atrial arrhythmia and reduced cardiac output.
For mitral stenosis, catheter‐based balloon valvuloplasty has become established as the primary therapeutic option, while regurgitant valves have until recently only been amenable to surgical treatment. Valve repair is increasingly preferred as it is associated with better survival and avoids the need for anticoagulation. Moreover, it conserves the papillary muscle architecture and its vital role in overall ventricular function.2 Myxomatous disease and functional regurgitation, which are the most common mechanisms of mitral valve dysfunction, are also the most amenable to valve repair. Recently, various techniques have been developed to reduce mitral regurgitation by a catheter‐based approach.
Edge‐to‐edge repair
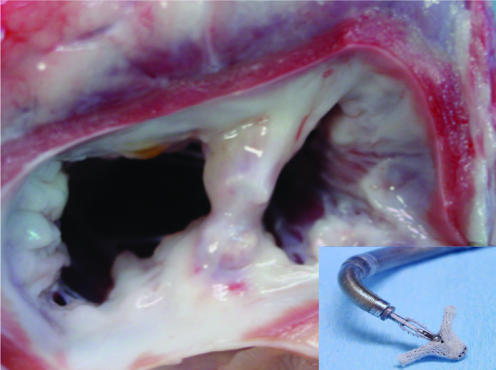
The concept of edge‐to‐edge repair developed as a surgical strategy to treat mitral regurgitation caused by anterior leaflet prolapse. The technique, described by Alfieri, opposes the central portion of the anterior and posterior mitral valve leaflets to create a double‐orifice valve with reduced leaflet excursion and less regurgitation.3 This technique, initially performed as a rescue operation, later became more widely utilised because of its simplicity and encouraging results. Furthermore, its uncomplicated nature attracted interventionalists to develop an analogous transcatheter based technique. In surgery, however, the repair is usually combined with an annuloplasty, which is not currently the case in the catheter approach. Two techniques that can be used percutaneously have recently been introduced, the MitraClip (Evalve, California, USA) and the Mobius II (Edwards Lifesciences, California, USA).4,5 Delivery requires a transseptal puncture followed by deployment of a clip or suture to create a bridge between the anterior and posterior valve leaflets. Currently the largest clinical experience is with the clip device (Evalve, fig 1). If the location of the clip is suboptimal, the clip can be reopened and repositioned; only once the final position is confirmed is the delivery system detached. In some cases deployment of two devices has been necessary to achieve adequate reduction in mitral regurgitation. At 6 months, the device is completely incorporated into the tissue bridge and mimics the pathological result of surgery. The procedure is performed under general anaesthesia with fluoroscopic and transoesophageal echocardiographic guidance; it is technically demanding and is likely to be limited to specialist centres with experienced interventionalists and echocardiologists who work closely together.
Figure 1 Main picture: Chronic healing (at 6 months) following Evalve clip deployment in a porcine model. Inset: the Evalve clip. Courtesy of Evalve Inc., California, USA.
Recently, the initial results of the Phase I EVEREST (Endovascular Valve Edge‐to‐Edge REpair STudy) trial of this device were published. Inclusion required moderate to severe mitral regurgitation, due to degenerative disease without annular dilatation, in patients who were candidates for surgery. Clips were implanted in 24 out of 27 patients recruited, with no procedural complications and four major adverse events: three patients experienced partial clip detachment, subsequently requiring surgery, while one patient experienced a limited neurological event. Three further patients required surgery later for significant residual mitral regurgitation. Eighteen patients remained free from surgery and in 13, mitral regurgitation remained mild or less so at 6 months. The majority of those requiring surgery were able to undergo valve repair rather than replacement. Retrospective analysis has shown that leaflet prolapse <10 mm was most likely to result in a successful outcome. The encouraging nature of these early results has led to design and implementation of a pivotal phase II trial in which patients are currently being randomised to the Evalve clip or conventional surgery (EVEREST II). To date, over 70 patients have undergone treatment with this device, within either study, with no reported mortality (T Feldman, personal communication, May 2006).
Annuloplasty
Annuloplasty is a fundamental component of most mitral valve surgery and can sometimes be used alone to treat functional mitral regurgitation. The intention is to rectify annular dilation while reducing the anteroposterior diameter of the valve in order to promote better leaflet co‐aptation and reduce regurgitation. Optimal surgical results are usually achieved with insertion of a prosthetic band or ring, though other techniques using pericardium, Dacron or sutures alone are described. The central role of annular dilatation in the progression of mitral regurgitation makes it an important target for catheter‐based treatments. The coronary sinus, which has already been the target for interventions such as biventricular pacing, has been considered for this application because of its close relationship with the atrioventricular groove. Shortening or re‐shaping the annulus by insertion of a device into the coronary sinus has the potential to mimic the annuloplasty that is performed surgically. Proof of concept has been demonstrated experimentally6 with recent publication of an initial human feasibility study using the Viking device (Edwards Lifesciences, California, USA).7 This approach is, however, limited by the substantial variations in normal coronary sinus anatomy and by the possibility of circumflex artery compromise. Detailed angiography and venography form an important aspect of the “decision to proceed” algorithm. Other concerns include late dilatation of the anterior annulus, leaflet tethering due to papillary muscle displacement, and annular calcification. Finally, the long‐term consequence of leaving such an implant in the coronary sinus is unknown, though experience with pacing wires is reassuring.
An alternative technique of annular remodelling uses a cinching device, composed of a bridge and strut system placed in the atrium rather than the ventricle, to decrease the septal–lateral annular diameter. The aim of this innovative design is to preserve dynamic annular and leaflet function. There is in fact not true septal–lateral tightening because of the angle of placement; however, this may prove beneficial in the setting of ischaemic mitral regurgitation where the ventricle often dilates asymmetrically and regurgitation is eccentric. Feasibility of this concept has been clearly demonstrated in the surgical setting, but recent experimental work has indicated that transcatheter device delivery is also possible.8
WHAT IS THE FUTURE FOR PERCUTANEOUS MITRAL VALVE REPAIR?
Catheter‐based repair of mitral regurgitation is still in its infancy; early results seem positive and progress to larger trials of these devices in humans is underway. These techniques, however, only offer limited therapeutic options in comparison to surgery, which can more easily be adapted to the broad spectrum of mitral valve pathology encountered. Ultimately, combinations of percutaneous therapies might be required to treat mitral valve regurgitation adequately, although earlier intervention, if shown to be safe, could prevent disease progression and delay the need for surgery.
PERCUTANEOUS AORTIC VALVE REPLACEMENT
Aortic valve disease is a frequent clinical finding that is increasing in incidence due to the rate of calcific degenerative disease in the growing elderly population. Furthermore, congenital abnormalities of this valve are common with bicuspid valves found in 1–2% of the population. Calcific stenosis of the valve often presents late in its natural history with patients experiencing angina, exertional dyspnoea or syncope. Common risk factors, such as hypertension and hypercholesterolaemia, mean it is often accompanied by co‐existent coronary artery disease. Surgical valve replacement, with or without coronary artery bypass surgery, is currently the treatment of choice, though in‐hospital mortality is around 3% with significant associated morbidity. The first transcatheter approach to treatment of this condition was balloon aortic valvuloplasty. While often effective for the treatment of congenital disease, it failed to demonstrate a sustained benefit in patients with degenerative disease and has almost been abandoned altogether. In selected cases, it may retain a palliative role or provide a bridge to surgical valve replacement.
Attempts to implant a valve via a less invasive approach date back to the 1950s, before the era of cardiopulmonary bypass, when Hufnagel successfully placed a prosthetic valve in the descending aorta of a patient with severe aortic insufficiency.9 Development of a balloon‐expandable valved stent, first proposed by Anderson in the early 1990s,10 subsequently led to a transcatheter approach to native aortic valve replacement.11 The complex anatomy of the aortic valve and root presents a substantial challenge to development of a safe and successful strategy for percutaneous valve replacement. Many approaches are under development, that notably differ in terms of stent technology, and in 2002 the first human implantation was performed.12
Clinical experience
The balloon‐expandable Cribier‐Edwards Valve (Edwards Lifesciences, California, USA), with which there is greatest clinical experience, is composed of equine pericardial valve leaflets sutured into a short (14 mm) stainless steel stent (fig 2). Delivery can be either via an antegrade approach, requiring transseptal puncture and negotiation of the mitral valve, or a retrograde approach from the femoral artery. The antegrade approach, while combining the lower risk of femoral venous access with the ability to cross more heavily calcified valves, may jeopardise the anterior leaflet of the mitral valve. The retrograde approach, while less technically demanding, may be difficult in patients with heavily diseased descending aortas. The choice of approach depends on operator experience and the individual case, though lately the retrograde approach has become more popular. To facilitate device implantation, pre‐dilatation of the diseased valve is carried out with a balloon. Stability of the implantable valve during deployment is critical, in view of the proximity of the coronary artery orifices and the fibrous continuity between the aortic and mitral valves. To create a stable haemodynamic environment, rapid right ventricular pacing is performed (200–220 beats/min) resulting in a transient but immediate fall in left ventricular pressure. The heart valve is deployed, normal cardiac rhythm restored and the delivery system withdrawn.
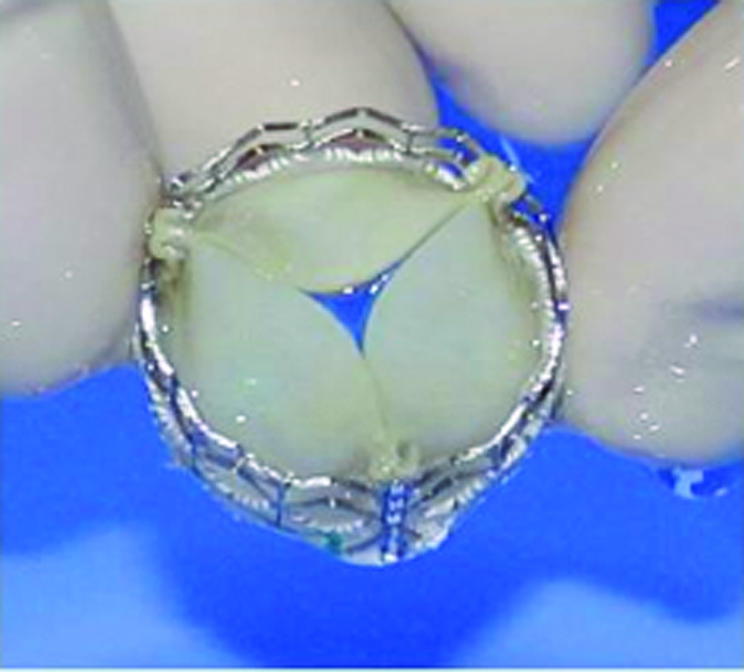
Figure 2 The Cribier‐Edwards percutaneous heart valve. Courtesy of Dr Alain Cribier, Edwards LifeScience, New Jersey, USA.
Initial clinical applications were associated with significant mortality13 often related to high levels of co‐morbidity in a population required to be in either New York Heart Association (NYHA) functional class IV or cardiogenic shock and with valve areas <0.7 cm2. Further experience, in a similar patient population, where the valved stent was deployed only from the retrograde approach with a specially designed steerable delivery catheter, has been associated with a more favourable outcome.14 In this series, percutaneous aortic valve implantation was successfully performed in 14 out of 18 patients in whom it was attempted. Iliac arterial injury was initially problematic but did not recur after improvements in screening and access site management were made. There were no intraprocedural deaths and at follow‐up of 75 days, 16 patients (89%) remained alive. In patients who have undergone successful valve implantation, an increase in valve area with associated haemodynamic and clinical benefit has been demonstrated.15
The high levels of morbidity and mortality in the early human experience, however, reflect the serious challenge presented. The anatomy of the implantation site is complex, access is difficult, the frequency of paravalvular leak is of concern, the target population is high‐risk, and the long‐term durability of these devices is unknown. Alternative device designs have been proposed including Revalving (Corevalve, California, USA), a self‐expandable nitinol device that returns to a pre‐designated shape and may prove well‐suited to treatment of regurgitant lesions.16 Clinical data are, however, limited and results have to be awaited before superiority of other devices are demonstrated.
WHAT IS THE FUTURE FOR PERCUTANEOUS AORTIC VALVE REPLACEMENT?
A different strategy, supported by the authors, would be to implant percutaneous valves into failed bioprostheses. While this represents a smaller target population, the availability of surgical back‐up along with a definable implantation site is attractive and could allow a more conservative introduction of this technology. For the future, one of the most exciting prospects is an implantable, nano‐synthesised, nitinol valve (fig 3) that could possess the durability of conventional mechanical valves but without the necessity for anticoagulation.
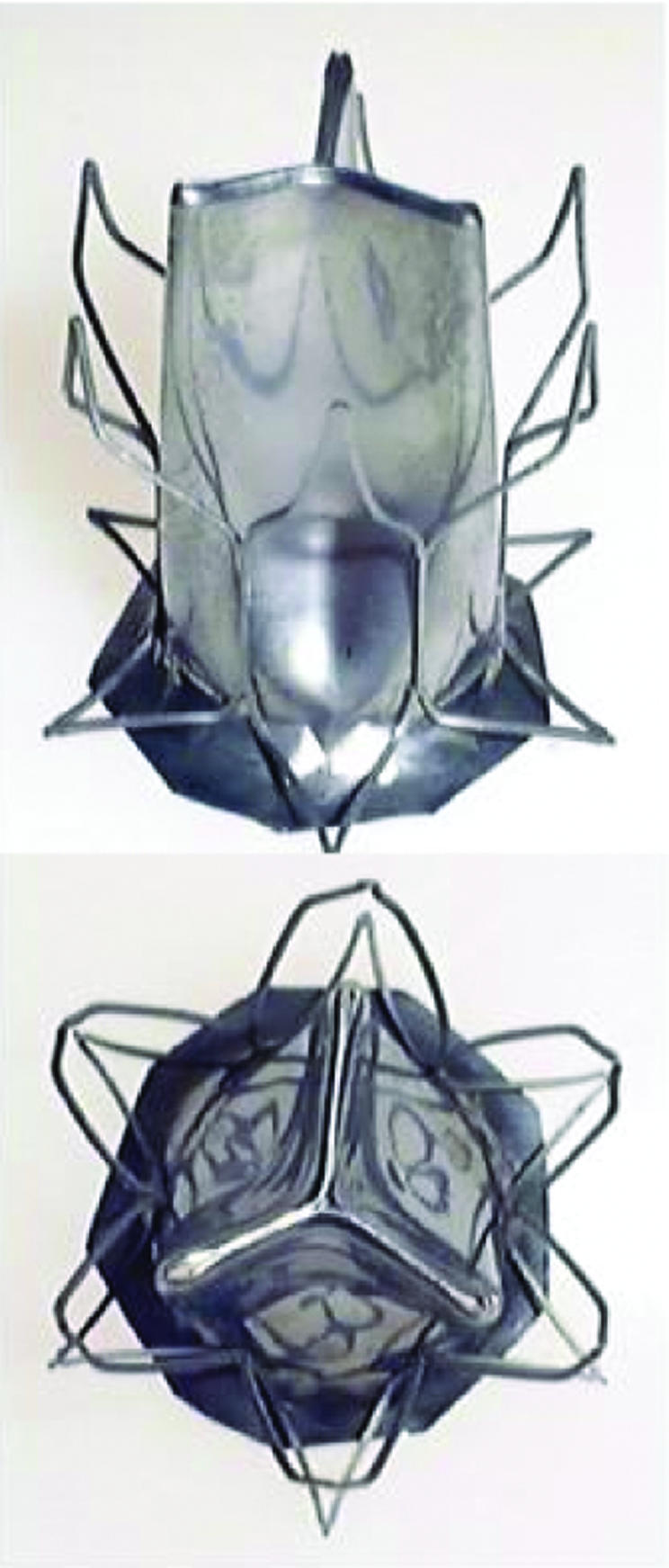
Figure 3 Nano‐synthesised nitinol percutaneously implantable aortic valve. Courtesy of Dr Steven Bailey, University of Texas, USA.
Substantial interest exists among cardiologists, scientists and industry to ensure percutaneous aortic valve replacement succeeds; it is likely that a great deal of progress will occur in this field over the coming years. For now, however, surgical valve replacement remains the “gold standard” of care for treatment of patients with aortic valve disease.
NEW PERCUTANEOUS APPROACHES TO RIGHT SIDED VALVE DISEASE
Acquired pulmonary valve disease in adults is rare, with regurgitation occurring in the setting of pulmonary hypertension being the most frequent aetiology. In individuals with complex congenital heart disease, however, pulmonary valve dysfunction is common and often the principle reason for late complications. Pulmonary regurgitation commonly occurs following patch augmentation of the right ventricular outflow tract as part of tetralogy of Fallot repair and also in those who have right ventricular to pulmonary artery conduits placed that have subsequently degenerated. This is concerning, as evidence clearly indicates that longstanding pulmonary regurgitation has detrimental effects on right ventricular function, exercise capacity and arrhythmia potential. In addition, conduits can calcify and stenose; to date, this has been treated with bare stenting which relieves obstruction but at the cost of residual pulmonary regurgitation. For the rapidly growing population of survivors with repaired congenital heart disease, therefore, a percutaneous approach to pulmonary valve replacement could avoid the morbidity and discomfort of repeated open‐heart surgeries.
Percutaneous pulmonary valve replacement
Implantation of a transcatheter pulmonary valve was first reported by us in a 12‐year‐old boy with a stenosed and regurgitant prosthetic conduit in 2000.17 This procedure has since been performed in over 125 patients with repaired congenital heart disease (age 7–58 years), with results from the first 59 consecutive cases recently described.18
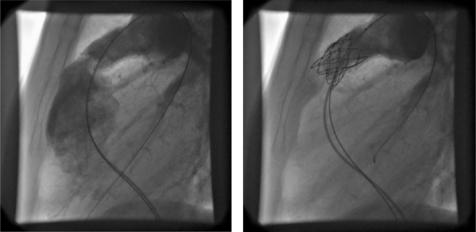
The Melody valve (Medtronic, Minnesota, USA) is composed of a valved bovine jugular vein, thinned down and sutured into a balloon expandable platinum iridium stent (fig 4). The venous valve is well suited to the lower pressures on the right side of the circulation, and with its large co‐aptation surface is able to function at a variety of different final diameters. It is delivered from a femoral or internal jugular approach, under general anaesthesia with the guidance of biplane angiography (fig 4). The size of the delivery system limits the procedure to those older than 5 years and >20 kg in weight.
Figure 4 Lateral angiogram (left) before and (right) after percutaneous pulmonary valve implantation in a regurgitant right ventricular to pulmonary artery homograft conduit.
Procedural complications are unusual with a total of six patients having required early conversion to surgery; two due to device dislodgement, two due to homograft rupture, one due to obstruction of a pulmonary branch and one due to coronary artery compression. Dysfunctional homograft conduits appear to provide the safest environment for implantation. An implantation site >22 mm in diameter risks device stability and patients who have transannular patches, which often become aneurysmal, are usually not suitable for this approach. Patient selection can be optimised by the use of three‐dimensional imaging, balloon sizing at the time of catheterisation and by carefully defining the course of the coronary arteries, which in some cases may be anomalous. Total mortality is 1.6% reflecting two patients, both in cardiogenic shock, who underwent technically successful palliative procedures. One patient died at 24 h due to intractable pulmonary oedema, and the other died at 6 weeks following a chest infection and renal failure. The majority of patients have an excellent clinical outcome with a rapid improvement in symptoms, objective exercise capacity and right ventricular performance, and an average hospital stay of two days.
As with other biological valves, it is likely that, in time, these valves may degenerate and calcify. Longest follow‐up is currently five‐and‐a‐half years and, to date, deterioration of valve function has not been identified outside the context of infective endocarditis. The development of stent fractures resulting, perhaps, from stress on the device or metal fatigue is recognised, with around one in six patients having this finding on x ray, mainly without clinical consequence. However, if widespread fractures occur with evidence of device dysfunction on echocardiography, we have found that implantation of a second device within the first provides an effective solution and avoids surgery.
WHAT IS THE FUTURE FOR PERCUTANEOUS PULMONARY VALVE REPLACEMENT?
Although the greatest experience of transcatheter pulmonary valve replacement comes from a single operator environment, the success of this novel technology means that it is now on the verge of wider clinical use. The timing of optimal pulmonary valve replacement is widely debated, with the desire to preserve right ventricular function and avoid arrhythmia tempered by the morbidity and mortality of repeat cardiopulmonary bypass. A less‐invasive alternative supports the current preference for earlier intervention while also extending treatment to those who may not be well enough for open‐heart surgery. Furthermore, modification of the device is underway to address those patients with aneurysmal outflow tracts.19 The impact of this technology on patients with repaired congenital heart disease is already evident with the expectation that it will transform the management of late pulmonary valve dysfunction for many others over the coming years.
THE TRICUSPID VALVE: BEYOND THE SCOPE OF PERCUTANEOUS TECHNOLOGY
Often neglected or considered to be of limited clinical importance, disease of the tricuspid valve can cause significant morbidity and mortality. Regurgitation, in the setting of annular dilatation, is the most common scenario leading to exacerbation of right heart failure, right atrial dilatation and arrhythmia. Surgical repair usually involves annuloplasty and is often performed alongside surgery of the aortic or mitral valves. In primary valve disease, where valve leaflets are dysplastic and not amenable to repair, replacement with a bioprosthetic or mechanical valve can be performed but is often associated with a suboptimal outcome.
A transcatheter approach to repair of this valve has not been attempted, perhaps in view of its complex tri‐leaflet morphology and the absence of any obvious approach for annuloplasty. In surgery, placement of an “Alfieri” stitch has been reported for treatment of tricuspid regurgitation and may provide one option for development of a percutaneous repair technique. Transcatheter valve replacement, on the other hand, has been successfully demonstrated in the experimental setting but has highlighted some important concerns regarding anchorage and sealing of such devices and the potential for stent fractures precipitated by dynamic annular motion.20 The complex anatomy of the tricuspid valve precludes a simple method of transcatheter treatment and, with the attention of interventionalists focused on the left heart, a percutaneous solution to disease of this valve seems remote.
CURRENT LIMITATIONS OF NEW PERCUTANEOUS THERAPIES
At present, introduction of these new technologies into general clinical practice remains relatively distant. Despite 28 companies actively developing devices for percutaneous valve repair or replacement, more data are required regarding feasibility, safety, efficacy and long‐term durability of these techniques. In particular, design of clinical trials requires careful thought in order to provide fair comparison with established therapies and to guarantee patient safety. While patients who have no therapeutic alternative often provide an attractive target population for introduction of new procedures, this can be a high‐risk strategy. Potential benefits may be muted in this group, while high levels of morbidity and mortality, often consequent on significant co‐morbidity, become unavoidably linked with the intervention. Where possible, randomisation against accepted treatment strategies should be favoured; however, it must also be accepted that the goals of percutaneous intervention may be different to those of surgery. Lower efficacy might be acceptable in the context of a safer, less invasive procedure, provided conventional treatment at a later stage is not ruled out.
SUMMARY
Percutaneous treatment of valvular heart disease is currently one of the fastest developing areas of cardiology. The prospect of avoiding cardiopulmonary bypass is a strong incentive with many creative solutions being investigated, including development of hybrid procedures whereby devices are implanted through a minimally‐invasive surgical approach (table 1). Careful trial design is crucial, with clear identification of relevant end points, if transcatheter valvular intervention is to fulfil the promise that so many foresee. Ultimately, success will see a dramatic change in the therapeutic armamentarium with no doubt a growth in the population able to benefit from treatment.
Table 1 Summary of new percutaneous treatments for valve disease.
| Technique | Current status |
| Mitral | |
| Balloon valvuloplasty | Clinical practice. Suitable for most pure stenosis |
| Edge‐to‐edge repair | Positive early human experience. Entering clinical trials |
| Coronary sinus annuloplasty | Experimental, few human cases performed |
| Aortic | |
| Balloon valvuloplasty | Clinical practice. Only suitable for congenital stenosis or as a palliative procedure |
| Percutaneous valve | Early human experience. Further development of device design will improve clinical results |
| Pulmonary | |
| Balloon valvuloplasty | Clinical practice. Suitable for congenital valvar stenosis |
| Bare stent | Clinical practice. Suitable for acquired conduit stenosis |
| Likely to be discarded in favour of percutaneous valve | |
| Percutaneous valve | Positive human experience. Entering clinical trials |
| Likely to become clinical practice in the near future | |
| Tricuspid | |
| Percutaneous valve | Experimental, clinical applications remote |
| Hybrid procedures combining percutaneous techniques with minimally invasive surgery are likely to become more popular | |
Additional references appear on the Heart website—http://heart.bmj.com/supplemental
INTERACTIVE MULTIPLE CHOICE QUESTIONS
This Education in Heart article has an accompanying series of six EBAC accredited multiple choice questions (MCQs)
To access the questions, click on BMJ Learning: Take this module on BMJ Learning from the content box at the top right and bottom left of the online article. For more information please go to: http://heart.bmj.com/misc/education.dtl Please note: The MCQs are hosted on BMJ Learning—the best available learning website for medical professionals from the BMJ Group.
If prompted, subscribers must sign into Heart with their journal's username and password. All users must also complete a one‐time registration on BMJ Learning and subsequently log in (with a BMJ Learning username and password) on every visit.
Additional references appear on the Heart website— http://heart.bmj.com/supplemental
Supplementary Material
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article.
Additional references appear on the Heart website— http://heart.bmj.com/supplemental
References
- 1.Iung B, Baron G, Butchart E G.et al A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003241231–1243. [DOI] [PubMed] [Google Scholar]
- 2.Enriquez‐Sarano M, Schaff H V, Orszulak T A.et al Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation 1995911022–1028. [DOI] [PubMed] [Google Scholar]
- 3.Alfieri O, Maisano F, De Bonis M.et al The double‐orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg 2001122674–681. [DOI] [PubMed] [Google Scholar]
- 4.St Goar F G, Fann J I, Komtebedde J.et al Endovascular edge‐to‐edge mitral valve repair: short‐term results in a porcine model. Circulation 20031081990–1993.This paper describes the first experimental application of the Alfieri technique via a transcatheter approach for mitral valve repair. [DOI] [PubMed] [Google Scholar]
- 5.Feldman T, Wasserman H S, Herrmann H C.et al Percutaneous mitral valve repair using the edge‐to‐edge technique: six‐month results of the EVEREST Phase I Clinical Trial. J Am Coll Cardiol 2005462134–2140.First human case series of transcatheter edge‐to‐edge repair technique demonstrating safety and feasibility. [DOI] [PubMed] [Google Scholar]
- 6.Liddicoat J R, MacNeil B D, Gillinov A M.et al Percutaneous mitral valve repair: a feasibility study in an ovine model of acute ischemic mitral regurgitation. Catheter Cardiovasc Interv 200360410–416.This paper describes the first experimental transcatheter approach to mitral annuloplasty using a coronary sinus implant. [DOI] [PubMed] [Google Scholar]
- 7.Webb J G, Harnek J, Munt B I.et al Percutaneous transvenous mitral annuloplasty: initial human experience with device implantation in the coronary sinus. Circulation 2006113851–855.First human case series of transcatheter mitral annuloplasty demonstrating safety and feasibility. [DOI] [PubMed] [Google Scholar]
- 8.Rogers J H, Macoviak J A, Rahdert D A.et al Percutaneous septal sinus shortening a novel procedure for the treatment of functional mitral regurgitation. Circulation 20061132329–2334. [DOI] [PubMed] [Google Scholar]
- 9.Hufnagel C A, Harvey W P, Rabil P.et al Surgical correction of aortic valve insufficiency. Surgery 195435673–680.Landmark report of the first human attempt to implant a prosthetic valve before the era of cardiopulmonary bypass. [PubMed] [Google Scholar]
- 10.Andersen H R, Knudsen L L, Hasenkam J M. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 199213704–708.Original description of the concept of a balloon‐expandable valved stent that could be implanted successfully by a catheter approach. [DOI] [PubMed] [Google Scholar]
- 11.Boudjemline Y, Bonhoeffer P. Steps toward percutaneous aortic valve replacement. Circulation 2002105775–778. [DOI] [PubMed] [Google Scholar]
- 12.Cribier A, Eltchaninoff H, Bash A.et al Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 20021063006–3008.This paper reports the first human implantation of a percutaneous aortic valve. [DOI] [PubMed] [Google Scholar]
- 13.Cribier A, Eltchaninoff H, Tron C.et al Treatment of calcific aortic stenosis with the percutaneous heart valve: mid‐term follow‐up from the initial feasibility studies: the French experience. J Am Coll Cardiol 2006471214–1223.Original series of percutaneous aortic valve implantations in humans highlighting the challenges and problems of this approach. [DOI] [PubMed] [Google Scholar]
- 14.Webb J G, Chandavimol M, Thompson C R.et al Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 2006113842–850. [DOI] [PubMed] [Google Scholar]
- 15.Bauer F, Eltchaninoff H, Tron C.et al Acute improvement in global and regional left ventricular systolic function after percutaneous heart valve implantation in patients with symptomatic aortic stenosis. Circulation 20041101473–1476. [DOI] [PubMed] [Google Scholar]
- 16.Grube E, Laborde J C, Zickmann B.et al First report on a human percutaneous transluminal implantation of a self‐expanding valve prosthesis for interventional treatment of aortic valve stenosis. Catheter Cardiovasc Interv 200566465–469. [DOI] [PubMed] [Google Scholar]
- 17.Bonhoeffer P, Boudjemline Y, Saliba Z.et al Percutaneous replacement of pulmonary valve in a right‐ventricle to pulmonary‐artery prosthetic conduit with valve dysfunction. Lancet 20003561403–1405.Landmark paper reporting the first successful transcatheter valve implantation in a human. [DOI] [PubMed] [Google Scholar]
- 18.Khambadkone S, Coats L, Taylor A.et al Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation 20051121189–1197.Original series of patients undergoing transcatheter pulmonary valve implantation. [DOI] [PubMed] [Google Scholar]
- 19.Boudjemline Y, Agnoletti G, Bonnet D.et al Percutaneous pulmonary valve replacement in a large right ventricular outflow tract: an experimental study. J Am Coll Cardiol 2004431082–1087. [DOI] [PubMed] [Google Scholar]
- 20.Boudjemline Y, Agnoletti G, Bonnet D.et al Steps toward the percutaneous replacement of atrioventricular valves an experimental study. J Am Coll Cardiol 200546360–365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.