Heart failure is a major public health problem, with a patient population of at least 10 million in Europe and approximately 5 million in North America.1,2,3 Because of its age‐dependent increase in incidence and prevalence, heart failure is one of the leading causes of death and hospitalisation among the elderly. As a consequence of the worldwide increase in life expectancy, and due to improvements in the treatment of heart failure in recent years, the proportion of patients that reach an advanced phase of the disease, so‐called end stage, refractory or terminal heart failure, is steadily growing. Patients with end stage heart failure fall into stage D of the ABCD classification of the American College of Cardiology (ACC)/American Heart Association (AHA), and class III–IV of the New York Heart Association (NYHA) functional classification; they are characterised by advanced structural heart disease and pronounced symptoms of heart failure at rest or upon minimal physical exertion, despite maximal medical treatment according to current guidelines.1,2,3 This patient population has a 1‐year mortality rate of approximately 50% and requires special therapeutic interventions.4 Every attempt should be made to identify and correct reversible causes for a worsening of heart failure, such as poor patient compliance, myocardial ischaemia, tachy‐ or bradyarrhythmias, valvular regurgitation, pulmonary embolism, infection, or renal dysfunction. In this article, we describe current strategies for the treatment of end stage heart failure.
PHARMACOLOGICAL MANAGEMENT OF END STAGE HEART FAILURE
Current recommendations for the pharmacological treatment of heart failure patients with NYHA class III–IV are summarised in table 1, while table 2 gives an overview of the drugs discussed in this article.1,2,3,5 Angiotensin‐converting enzyme (ACE) inhibitors are recommended as first‐line treatment in all patients with reduced left ventricular (LV) systolic function (ejection fraction (EF) ⩽35–40%) independent of clinical symptoms (NYHA I–IV), unless there are contraindications.1,2,3 In several large clinical heart failure trials ACE inhibitors have been shown to improve symptoms and functional capacity while decreasing the rate of hospitalisations and mortality.1,2,3,6 Moreover, ACE inhibitors are indicated in patients who develop heart failure after the acute phase of myocardial infarction, and have been shown to improve survival and reduce reinfarctions and hospitalisations in this patient group. ACE inhibitors should not be titrated based on symptomatic improvement but should be up‐titrated to the target dosages shown to be effective in the large, placebo‐controlled heart failure trials, or to the maximal dose that is tolerated. Treatment should be closely monitored by assessing blood pressure (supine and standing), renal function, and serum electrolytes (especially potassium) at regular intervals. In patients with symptomatic chronic heart failure who do not tolerate ACE inhibitors, angiotensin II type I receptor blockers (ARBs) can be used as an alternative to improve morbidity and mortality.7,8 In heart failure patients remaining symptomatic despite optimal medical treatment including ACE inhibitors, administration of ARBs on top of ACE inhibitors leads to an additive reduction in cardiovascular morbidity and mortality.8,9 However, the higher rate of hypotension, renal dysfunction, and hyperkalaemia with such a combination therapy warrants close monitoring of these parameters.
Table 1 Pharmacological management of end stage heart failure1,2,3,4,5.
| • Goal 1: Improvement of morbidity and mortality |
| ACE inhibitors |
| ARBs (if ACE inhibitor intolerant or plus ACE inhibitors if still symptomatic) |
| Selected β‐blockers |
| Aldosterone antagonists |
| • Goal 2: Control of symptoms |
| Diuretics (eventually thiazide plus loop diuretic) |
| Digitalis (low‐dose) |
| Consider temporary inotropics |
| Selected antiarrhythmics |
| • Goal 3: Palliation |
| Opioids, antidepressants, anxiolytics |
| Oxygen |
| Consider continuous inotropics |
ACE, angiotensin‐converting enzyme; ARBs, angiotensin II type I receptor blockers.
Table 2 Overview of the drugs used for the pharmacological management of end stage heart failure1,2,3,4,5.
| Drug class | Mode of action | Selected drugs | Concentrations | Selected side effects |
|---|---|---|---|---|
| ACE inhibitors | Blockade of theangiotensin‐convertingenzyme | Ramipril | 1.25–10 mg/day | Cough, hyperkalaemia, renal insufficiency, angioedema |
| Enalapril | 2.5–20 mg/day | |||
| Trandolapril | 1–4 mg/day | |||
| ARBs | Blockade of theangiotensin II type Ireceptor | Losartan | 12.5–100 mg/day | Similar to ACE inhibitors |
| Candesartan | 4–32 mg/day | |||
| Valsartan | 80–320 mg/day | |||
| β‐blockers | Blockade of the β1‐adrenergic receptor | Metoprolol succinate | 12.5–200 mg/day | Bradycardia, hypotension |
| Bisoprolol | 1.25–10 mg/day | |||
| Carvedilol | 3.125–50 mg/day | |||
| Aldosterone antagonists | Blockade of the aldosterone receptor | Spironolactone | 12.5–50 mg/day | Hyperkalaemia, gynaecomastia (spironolactone) |
| Eplerenone | 25–50 mg/day | |||
| Thiazides | Blockade of the Na+/Cl− co‐transporter | Hydrochlorothiazide | 12.5–75 mg/day | Electrolyte disturbance, glucose intolerance,hyperuricaemia |
| Xipamide | 10–80 mg/day | |||
| Loop diuretics | Blockade of the Na+/2Cl−/K+ co‐transporter | Furosemide | 20–500 mg/day | Similar to thiazides,ototoxicity |
| Torasemide | 5–200 mg/day | |||
| Digitalis | Blockade of theNa+/K+ ATPase | Digoxin | 0.0625–0.25 mg/day | Brady‐ and tachyarrhythmias |
| Digitoxin | 0.05–0.1 mg/day |
As patients with end stage heart failure frequently show signs of fluid retention or have a history of such, inhibitors of the renin‐angiotensin system should be co‐administered with diuretics, which usually leads to rapid symptomatic improvement of dyspnoea and exercise tolerance while lacking significant effects on survival. End stage heart failure usually requires the use of loop diuretics, which may be effectively used in combination with thiazides in case of treatment refractory fluid overload due to a synergistic mechanism of action (sequential nephron blockade).
In addition to standard treatment with ACE inhibitors and diuretics, patients with symptomatic stable systolic heart failure (NYHA II–IV) should be treated with β‐adrenergic receptor blockers unless there are contraindications.1,2,3 Results from several large clinical trials show that the β‐adrenergic receptor blockers carvedilol, bisoprolol, and metoprolol succinate decrease the rate of hospitalisations and mortality and lead to improvements of symptoms and functional class (fig 1).1,2,3,10 β‐adrenergic receptor blocker treatment should be initiated in stable heart failure patients showing no signs of fluid retention at very small doses, and up‐titrated to the target doses used in the large clinical heart failure trials, or to the maximal dose that is tolerated. Patients should be closely monitored for evidence of heart failure symptoms, fluid retention, hypotension, and bradycardia.
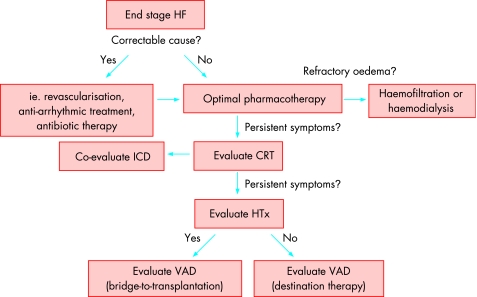
Figure 1 Suggested treatment algorithm for the management of end stage heart failure.1,2,3 CRT, cardiac resynchronisation therapy; HF, heart failure; HTx, heart transplantation; ICD, implantable cardioverter‐defibrillator; VAD, ventricular assist device.
In patients with advanced heart failure (NYHA III–IV), aldosterone receptor antagonists are recommended in addition to ACE inhibitors, β‐adrenergic receptor blockers, and diuretics, unless contraindicated, and have been shown in the RALES and the EPHESUS trials to improve survival and morbidity.11,12 Treatment should be monitored by assessing serum potassium values, renal function, and fluid status, as well as gynaecomastia in the case of spironolactone.
Unless there are contraindications, cardiac glycosides are indicated for heart rate control in symptomatic heart failure patients (NYHA I–IV) with tachyarrhythmia due to atrial fibrillation (AF) already treated with adequate dosages of β‐blockers.1,2,3,13 In that respect, a combination therapy of cardiac glycosides with β‐adrenergic receptor blockers seems to be more effective than either agent alone. In patients with systolic LV dysfunction (EF ⩽35–40%) and sinus rhythm remaining symptomatic under treatment with ACE inhibitors, β‐adrenergic receptor blockers, diuretics, and aldosterone receptor antagonists, additional treatment with cardiac glycosides at low serum concentrations (digoxin 0.5–0.8 ng/ml) may improve symptoms and reduce hospitalisations without having an effect on mortality.1,2,3,14 Treatment should be monitored by assessing heart rate, atrioventricular conduction, and serum values of potassium and the cardiac glycoside administered, as well as renal function in the case of digoxin, which in contrast to the primarily hepatically metabolised digitoxin is eliminated by renal excretion.
Most supraventricular and ventricular arrhythmias in heart failure patients can be effectively treated with the class III antiarrhythmic amiodarone, which may restore and maintain sinus rhythm or improve the success of electrical cardioversion in heart failure patients with AF.1,2,3,15 Amiodarone treatment is neutral with respect to mortality and is not indicated for primary prophylaxis of ventricular arrhythmias. Its benefits should be carefully weighed against potentially serious side effects including hyper‐ and hypothyroidism, corneal deposition, dermal photosensibility, hepatitis, and pulmonary fibrosis. Dofetilide is a new class III antiarrhythmic without negative effects on mortality in heart failure patients, whose potential benefits should be weighed against an increased incidence of torsades de pointes tachycardias.16 Anticoagulation is indicated in heart failure patients with AF, a previous thromboembolic event, a mobile LV thrombus or following myocardial infarction.2
While repeated or prolonged treatment with positive inotropic agents such as β‐adrenergic agonists (dobutamine) and phosphodiesterase inhibitors (milrinone, enoximone) increases mortality and is not recommended for the treatment of chronic heart failure, intermittent intravenous inotropic treatment may be used in cases of severe cardiac decompensation with pulmonary congestion and peripheral hypoperfusion, or as a bridge to heart transplantation.1,2,3,17 However, treatment‐related complications such as proarrhythmia or myocardial ischaemia may occur and the effect on prognosis is unclear. The new calcium sensitiser levosimendan has been shown to improve symptoms with fewer side effects than dobutamine in patients with severe low‐output LV dysfunction.1,2,3,18 However, data from the REVIVE‐II and the SURVIVE studies presented at the AHA meeting in November 2005 were conflicting so that the definitive role of levosimendan in heart failure treatment needs to be further clarified.19
For palliation of symptoms in patients with refractory end stage heart failure, data from a recently published study indicate that continuous outpatient support with inotropes may be an acceptable treatment option.20 While there is no specific role for direct‐acting vasodilators in the treatment of systolic heart failure, a combination treatment of hydralazine and isosorbide dinitrate may improve symptoms and survival in heart failure patients intolerant of both ACE inhibitors and ARBs, as well as in an African‐American subpopulation of heart failure patients treated with ACE inhibitors and β‐adrenergic receptor blockers.1,2,3,21,22 In addition to baseline heart failure treatment, nitrates may improve angina and dyspnoea, and the calcium antagonists amlodipine and felodipine may be used to treat refractory arterial hypertension and angina.1,2,3 Opioids may be used to ameliorate symptoms in symptomatic patients with end stage heart failure in end‐of‐life situations where no further therapeutic options are available.3
MECHANICAL AND SURGICAL MANAGEMENT OF END STAGE HEART FAILURE
Fig 1 presents a suggested algorithm for the treatment of patients with end stage heart failure. In patients with reduced LV function (EF ⩽35%), sinus rhythm, left bundle branch block or echocardiographic signs of ventricular dyssynchrony and QRS width ⩾120 ms, who remain symptomatic (NYHA III–IV) despite optimal medical treatment, cardiac resynchronisation therapy (CRT) using biventricular pacing improves symptoms and exercise capacity while decreasing hospitalisations and mortality.1,2,3,23,24,25 In the COMPANION trial, heart failure patients in NYHA class III–IV with LVEF ⩽35% and QRS width ⩾120 ms were randomised to optimal pharmacological treatment alone or in combination with either CRT or CRT plus implantable cardioverter‐defibrillator (ICD).23 Importantly, while mortality was reduced in both device arms there was no significant difference in mortality between CRT and CRT/ICD. Therefore, currently available data indicate that the use of an ICD in combination with CRT should be based on the indications for ICD therapy.3
For secondary prevention of sudden cardiac death (SCD), ICD implantation has been shown to reduce mortality in cardiac arrest survivors and in patients with sustained symptomatic ventricular tachyarrhythmias.1,2,3,26 For primary prevention of SCD in heart failure patients with optimal pharmacological treatment, ICD therapy is indicated in selected patients with LVEF ⩽30% after myocardial infarction (>40 days) and in patients with ischaemic and non‐ischaemic heart failure (NYHA class II–III) with LVEF ⩽35% to reduce mortality.2,3,23,27,28 Importantly, effectiveness of ICD therapy is time‐dependent as there was no survival benefit following ICD implantation until after the first year in the MADIT II and SCD‐HeFT trials.27,28 Thus, the decision for ICD implantation in stage D heart failure patients, which have a poor prognosis and a high frequency of ventricular arrhythmias, is particularly complex and must be made on an individual basis; this is especially so because of the possibility that ICD therapy might not alter total mortality but shift the mode of death from SCD to progressive haemodynamic failure and might impair the quality of life by frequent shocks. Importantly, ICDs or conventional pacemakers with right ventricular pacing have the potential for worsening of heart failure and LV function as well as increasing hospitalisations.1,2,3 However, ICD therapy associated with CRT in patients with severe heart failure (NYHA class III–IV) with LVEF ⩽35% and QRS duration ⩾120 ms clearly improves morbidity and mortality.1,2,3,23
Heart transplantation is a firmly established surgical approach for the treatment of end stage heart failure and has been shown to improve exercise capacity, quality of life, and survival compared with conventional treatment.1,2,3,29 Selection criteria for heart transplantation are presented in table 3,3 while contraindications include current drug or alcohol abuse, lack of compliance, serious uncontrollable mental disease, severe comorbidity (that is, treated malignancy with remission and <5 years follow‐up, systemic infection, significant renal or hepatic failure), and fixed pulmonary hypertension.1,2 Cardiac allograft rejection is a significant problem during the first year after heart transplantation while the long‐term prognosis is mainly limited by complications of immunosuppression (infections, hypertension, renal failure, malignancies, and transplant vasculopathy).1,2,3 Overall, 5‐year survival is 70–80% in heart transplantation patients receiving triple immunosuppressive therapy.29
Table 3 Absolute and relative indications for heart transplantation (modified according to Hunt3).
| • Absolute indications in appropriate patients |
| For haemodynamic compromise due to heart failure |
| Refractory cardiogenic shock |
| Documented dependence on iv inotropic support to maintain adequate organ perfusion |
| Peak Vo2 <10 ml/kg/min with achievement of anaerobic metabolism |
| Severe symptoms of ischaemia that constantly limit routine activity and are not amenable to coronary artery bypass surgery or percutaneous coronary intervention |
| Recurrent symptomatic ventricular arrhythmias refractory to all therapeutic modalities |
| • Relative indications |
| Peak Vo2 11–14 ml/kg/min (or 55% of predicted) and major limitation of patient's daily activities |
| Recurrent unstable ischaemia not amenable to other intervention |
| Recurrent instability of fluid balance/renal function not due to patient non‐compliance with medical regimen |
| • Insufficient indications |
| Low left ventricular ejection fraction |
| History of functional class III or IV symptoms of heart failure |
| Peak Vo2 >15 ml/kg/min (and >55% of predicted) without other indications |
The availability of heart transplantation for patients who could benefit from the procedure is limited by the continuing shortage of donor hearts and the increasing number of transplant candidates. Intra‐aortic balloon counterpulsation (IABP) can provide short‐term haemodynamic support. In patients with end stage heart failure considered too unstable to await a suitable donor organ, biventricular or LV assist devices (LVAD) as well as total artificial hearts can be employed as bridge‐to‐transplantation therapy and have been shown to improve quality of life, survival‐to‐transplantation rates, and post‐transplant survival.1,2,3,30,31 In patients with end stage heart failure who are ineligible for heart transplantation, a recently conducted landmark clinical trial has shown that implantation of an LVAD improves survival and quality of life.1,2,3,32 These data have led to the use of ventricular assist devices as an alternative to transplantation—so‐called destination therapy. Complications of assist devices include infections, bleeding, thromboembolism, and device failure. In regard to the timing of assist device therapy, a recent report found that survival of patients undergoing bridge‐to‐transplantation therapy was best when assist devices were implanted electively, as compared to implantations for urgent or emergency indications.33
In patients with end stage heart failure and volume overload refractory to diuretic treatment, haemofiltration or haemodialysis can provide temporary relief.2 In patients with severe LV systolic dysfunction and significant secondary mitral regurgitation, observational studies indicate that mitral valve surgery may be associated with improvements in quality of life and survival.34 LV aneurysmectomy is indicated in heart failure patients with large discrete LV aneurysms.2 According to current thinking, other surgical procedures such as cardiomyoplasty or partial ventriculotomy (Batista operation) are not indicated for the treatment of heart failure.1,2,3
EXPERIMENTAL APPROACHES
Early clinical studies in patients with heart failure have shown the feasibility of transfer of distinct stem and progenitor cell populations to the heart, and have demonstrated beneficial effects on cardiac function and/or tissue viability.35 However, due to small study sizes, lack of randomised control groups, poor understanding of the mechanisms of action of transplanted cells, lack of information on procedural issues (that is, optimal cell type, cell dosage, timing of cell transfer, optimal route of application), and safety concerns with some progenitors (such as the arrhythmogenicity associated with skeletal myoblast grafts), further basic research and the initiation of large, double‐blind, placebo‐controlled, randomised clinical trials with hard end‐points (including mortality) are warranted before the role of cell‐based therapy of heart failure can be judged. Vasopressin receptor antagonists have been shown in early studies to exert beneficial haemodynamic effects in patients with advanced heart failure; however, results from longer‐term clinical trials underway to determine the role of vasopressin receptor antagonists in heart failure therapy have to be awaited.3 The new vasodilator agent nesiritide (recombinant human brain natriuretic peptide) has recently been shown to improve symptoms in patients with acute heart failure without affecting clinical outcome; however, effects on morbidity and mortality are not clear from available clinical trials.2,3 Ivabradine, a new selective inhibitor of the cardiac pacemaker current If that lowers heart rate without negative inotropic effects, is currently being evaluated in a clinical phase III trial involving patients with stable coronary artery disease and systolic heart failure (the BEAUTIFUL study). External ventricular restoration by surgical devices aiming at preventing further LV remodelling and reducing wall stress, such as the myosplint technique and the Acorn external cardiac support device, show promising early results; their relevance for heart failure treatment is currently being evaluated in clinical trials.2,3,36,37
PALLIATIVE APPROACHES
Before the condition of patients with end stage heart failure deteriorates so much that they can not actively participate in decisions, patients and their families should be educated about options for formulating and implementing advance directives and the role of palliative and hospice care services with re‐evaluation for changing clinical status.3 This may include indication of a preference for whether resuscitation should or should not be performed in the event of a cardiopulmonary arrest, indication of which supportive care measures and interventions should be performed, and discussion of the option to inactivate ICDs at the end of life.3 In the final days of life of heart failure patients with NYHA class IV, aggressive procedures such as intubation or ICD implantation that are not expected to result in clinical improvement are not appropriate.3
For patients with end stage heart failure, it is important to ensure the continuity of medical care between inpatient and outpatient settings.3 Hospice care may provide options to relieve suffering from symptoms such as pain, dyspnoea, depression, fear, and insomnia. Treatment may include psychosocial support, the use of opiates, frequent or continuous administration of intravenous diuretics, oxygen, continuous infusions of positive inotropic agents, anxiolytics, and sleeping medications.3 In caring for patients with end stage heart failure during their final days, it may be particularly difficult for the patients, their families and the physicians to define the time point when the patient's treatment goals shift from improving survival to improving quality of life, thus allowing for a peaceful and dignified death.
CONCLUSIONS AND OUTLOOK
Treatment options for end stage heart failure have improved in recent years and include a combination of drugs, mechanical devices and surgical procedures which may improve symptoms and survival. Ultimately, the progressive course of heart failure leads to death and the treatment of end stage heart failure includes palliation. Future research into heart failure pathophysiology and therapeutic options is warranted.
INTERACTIVE MULTIPLE CHOICE QUESTIONS
This Education in Heart article has an accompanying series of six EBAC accredited multiple choice questions (MCQs).
To access the questions, click on BMJ Learning: Take this module on BMJ Learning from the content box at the top right and bottom left of the online article. For more information please go to: http://heart.bmj.com/misc/education.dtl Please note: The MCQs are hosted on BMJ Learning—the best available learning website for medical professionals from the BMJ Group.
If prompted, subscribers must sign into Heart with their journal's username and password. All users must also complete a one‐time registration on BMJ Learning and subsequently log in (with a BMJ Learning username and password) on every visit.
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
References
- 1.Hoppe U C, Böhm M, Dietz R.et al Leitlinien zur Therapie der chronischen Herzinsuffizienz. Z Kardiol 200594488–509.Current recommendations of the German Cardiac Society for the treatment of chronic heart failure. [DOI] [PubMed] [Google Scholar]
- 2.Swedberg K, Cleland J, Dargie H.et al Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 2005261115–1140.Current ESC practice guidelines; compare with reference 1. [DOI] [PubMed] [Google Scholar]
- 3.Hunt S A. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 200546e1–82.Current ACC/AHA practice guidelines including comprehensive overview of the literature until 2005, together with references 1 and 2. [DOI] [PubMed] [Google Scholar]
- 4.Cleland J G, Gemmell I, Khand A.et al Is the prognosis of heart failure improving? Eur J Heart Fail 19991229–241. [DOI] [PubMed] [Google Scholar]
- 5.Böhm M, Werner N, Kindermann M. Drug treatment for chronic heart failure. Clin Res Cardiol 200695(suppl 4)36–56. [DOI] [PubMed] [Google Scholar]
- 6.Flather M, Yusuf S, Kober L.et al Long‐term ACE‐inhibitor therapy in patients with heart failure or left‐ventricular dysfunction: a systematic overview of data from individual patients. ACE‐Inhibitor Myocardial infarction Collaborative Group. Lancet 20003551575–1581.Meta‐analysis of clinical trials on the use of ACE inhibitors in heart failure. [DOI] [PubMed] [Google Scholar]
- 7.Granger C B, McMurray J J, Yusuf S.et al Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function intolerant to angiotensin‐converting‐enzyme inhibitors: the CHARM‐Alternative trial. Lancet 2003362772–776. [DOI] [PubMed] [Google Scholar]
- 8.Cohn J N, Tognoni G. A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med 20013451667–1675. [DOI] [PubMed] [Google Scholar]
- 9.McMurray J J, Ostergren J, Swedberg K.et al Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function taking angiotensin converting‐enzyme inhibitors: the CHARM‐Added trial. Lancet 2003362767–771. [DOI] [PubMed] [Google Scholar]
- 10.Brophy J M, Joseph L, Rouleau J L. Beta‐blockers in congestive heart failure: a Bayesian meta‐analysis. Ann Intern Med 2001134550–560.Meta‐analysis of clinical trials on the use of β‐blockers in heart failure. [DOI] [PubMed] [Google Scholar]
- 11.Pitt B, Zannad F, Remme W J.et al The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999341709–717.Landmark trial showing that aldosterone antagonism improves mortality in heart failure. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B, Remme W, Zannad F.et al Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 20033481309–1321. [DOI] [PubMed] [Google Scholar]
- 13.Khand A U, Rankin A C, Kaye G C.et al Systematic review of the management of atrial fibrillation in patients with heart failure. Eur Heart J 200021614–632. [DOI] [PubMed] [Google Scholar]
- 14.Digitalis Investigation Group The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997336525–533. [DOI] [PubMed] [Google Scholar]
- 15.Levy S, Breithardt G, Campbell R W.et al Atrial‐fibrillation: current knowledge and recommendations for management. Working Group on Arrhythmias of the European Society of Cardiology. Eur Heart J 1998191294–1320. [DOI] [PubMed] [Google Scholar]
- 16.Torp‐Pedersen C, Moller M, Bloch Thomsen P E.et al Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med 1999341857–865. [DOI] [PubMed] [Google Scholar]
- 17.Task Force on A H F. Executive summary of the guidelines on the diagnosis and treatment of AHF: The Task Force on AHF of the European Society of Cardiology. Eur Heart J 200526384–416. [DOI] [PubMed] [Google Scholar]
- 18.Follath F, Cleland J G, Just H.et al Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low‐output heart failure (the LIDO study): a randomized double‐blind trial. Lancet 2002360196–202. [DOI] [PubMed] [Google Scholar]
- 19.Cleland J G, Freemantle N, Coletta A P.et al Clinical trials update from the American Heart Association: REPAIR‐AMI, ASTAMI, JELIS, MEGA, REVIVE‐II, SURVIVE, and PROACTIVE. Eur J Heart Fail 20068105–110. [DOI] [PubMed] [Google Scholar]
- 20.Hershberger R E, Nauman D, Walker T L.et al Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail 20039188–191. [DOI] [PubMed] [Google Scholar]
- 21.Cohn J N, Archibald D G, Phil M.et al Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration cooperation study. N Engl J Med 19863141547–1552. [DOI] [PubMed] [Google Scholar]
- 22.Taylor A L, Ziesche S, Yancy C.et al Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 20043512049–2057. [DOI] [PubMed] [Google Scholar]
- 23.Bristow M R, Saxon L A, Boehmer J.et al Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac resynchronization therapy with or without implantable defibrillator in advanced chronic heart failure. N Engl J Med 20043502140–2150.First study to show that CRT improves mortality in heart failure. [DOI] [PubMed] [Google Scholar]
- 24.Cleland J G, Daubert J C, Erdmann E.et al The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 20053521539–1549. [DOI] [PubMed] [Google Scholar]
- 25.Götze S, Butter C, Fleck E. Cardiac resynchronization therapy for heart failure—from experimental pacing to evidence‐based therapy. Clin Res Cardiol 200695(suppl 4)18–35. [DOI] [PubMed] [Google Scholar]
- 26.Lee D S, Green L D, Liu P P.et al Effectiveness of implantable defibrillators for preventing arrhythmic events and death. J Am Coll Cardiol 2003411573–1582.Meta‐analysis of trials on secondary prevention of SCD. [DOI] [PubMed] [Google Scholar]
- 27.Moss A J, Zareba W, Hall W J.et al Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002346877–883.First study to implicate ICD implantation for primary prevention of SCD in patients with severe LV dysfunction. [DOI] [PubMed] [Google Scholar]
- 28.Bardy G H, Lee K L, Mark D B.et al Amiodarone or an implantable cardioverter defibrillator for congestive heart failure. N Engl J Med 2005352225–237. [DOI] [PubMed] [Google Scholar]
- 29.Hosenpud J D, Bennett L E, Keck B M.et al The registry of the International Society for Heart and Lung Transplantation: sixteenth official report–1999. J Heart Lung Transplant 199918611–626. [DOI] [PubMed] [Google Scholar]
- 30.El Banayosy A, Deng M, Loisance D Y.et al The European experience of Novacor left ventricular assist (LVAS) therapy as a bridge to transplant: a retrospective multi centre study. Eur J Cardiothorac Surg 199915835–841. [DOI] [PubMed] [Google Scholar]
- 31.Copeland J G, Smith R G, Arabia F A.et al Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med 2004351859–867. [DOI] [PubMed] [Google Scholar]
- 32.Rose E A, Gelijns A C, Moskowitz A J.et al Long‐term mechanical left ventricular assistance for end‐stage heart failure. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. N Engl J Med 20013451435–1443.Landmark study establishing LVADs as destination therapy in end stage heart failure. [DOI] [PubMed] [Google Scholar]
- 33.Deng M C, Weyand M, Hammel D.et al Selection and management of ventricular assist device patients: the Muenster experience. J Heart Lung Transplant 200019(suppl 8)S77–S82. [DOI] [PubMed] [Google Scholar]
- 34.Geha A S, El‐Zein C, Massad M G. Mitral valve surgery in patients with ischemic and nonischemic dilated cardiomyopathy. Cardiology 200410115–20. [DOI] [PubMed] [Google Scholar]
- 35.Wollert K C, Drexler H. Cell‐based therapy for heart failure. Curr Opin Cardiol 200621234–239. [DOI] [PubMed] [Google Scholar]
- 36.Guccione J M, Salahieh A, Moonly S M.et al Myosplint decreases wall stress without depressing function of the failing heart: a finite element model study. Ann Thorac Surg 2003761171–1180. [DOI] [PubMed] [Google Scholar]
- 37.Oz M C, Konertz W F, Kleber F X.et al Global surgical experience with the Acorn cardiac support device. J Thorac Cardiovasc Surg 2003126983–991. [DOI] [PubMed] [Google Scholar]