Abstract
Background
Quantitative information on regional left ventricular volumes from real‐time three‐dimensional echocardiographic (RT3DE) images has significant clinical potential but needs validation.
Aim
To validate these measurements against cardiac magnetic resonance (CMR) and test the feasibility of automated detection of regional wall motion (RWM) abnormalities from RT3DE data.
Methods
RT3DE (Philips) and CMR (Siemens) images were obtained from 31 patients and analysed by using prototype software to semiautomatically calculate indices of regional left ventricular function, which were compared between RT3DE and CMR (linear regression, Bland–Altman). Additionally, CMR images were reviewed by an expert, whose RWM grades were used as a reference for automated classification of segments as normal or abnormal from RT3DE and from CMR images. For each modality, normal regional ejection fraction (REF) values were obtained from 15 patients with normal wall motion. In the remaining 16 patients, REFs were compared with thresholds that were derived from patients with normal wall motion and optimised using receiver operating characteristic analysis.
Results
RT3DE measurements resulted in good agreement with CMR. Regional indices calculated in patients with normal wall motion varied between segments, but overall were similar between modalities. In patients with abnormal wall motion, RWM was graded as abnormal in 74% segments. CMR and RT3DE thresholds were similar (16‐segment average 55 (10)% and 56 (7)%, respectively). Automated interpretation resulted in good agreement with expert interpretation, similar for CMR and RT3DE (sensitivity 0.85, 0.84; specificity 0.81, 0.78; accuracy 0.84, 0.84, respectively).
Conclusion
Analysis of RT3DE data provides accurate quantification of regional left ventricular function and allows semiautomated detection of RWM abnormalities, which is as accurate as the same algorithm applied to CMR images.
The limitations of conventional echocardiographic evaluation of left ventricular function have been long recognised and partially attributed to its two‐dimensional nature, which gives only partial information about cardiac anatomy and function contained in specific cross‐sectional planes. Alternative techniques based on three‐dimensional reconstruction of the left ventricle from multiple planes, albeit time‐consuming and prone to artefacts, have shown promise for more accurate assessment of left ventricular function.1,2,3 More recently, transthoracic real‐time three‐dimensional echocardiographic (RT3DE) technology, which allows fast acquisition of dynamic pyramidal data structures that encompass the entire left ventricle, has become widely available. Several studies have since shown potential improvements in the evaluation of the global left ventricular function by using RT3DE data,4,5,6 despite the reliance on endocardial boundary identification on selected planes.7 Furthermore, information on regional wall motion (RWM) contained in RT3DE datasets has been studied only using visual interpretation of selected planes8,9 or by measurements performed on model‐based, interpolated endocardial surfaces.10
To circumvent these limitations, we recently developed and tested a novel approach for objective evaluation of regional left ventricular wall motion from RT3DE datasets, based on the detection of dynamic endocardial surface in three‐dimensional space11 and direct quantification of regional surface displacement throughout the cardiac cycle. In a recent study, we hypothesised that by fully exploiting the volumetric information contained in RT3DE datasets, without the need for either plane selection or geometric modelling, this technique promises to allow objective evaluation of the magnitude and temporal aspects of left ventricular function.12 However, in this study, RT3DE measurements of regional left ventricular function were validated against cardiac magnetic resonance (CMR) values obtained from stacks of short‐axis slices. This CMR method is increasingly recognised as a less than ideal reference because of several limitations that include poor endocardial definition near the apex owing to partial‐volume artefacts and the use of spatially fixed slices that disregards left ventricular systolic shortening. Analysis of radial long‐axis CMR images can potentially overcome these limiting factors and thus provide a better reference. Indeed, such analysis was recently shown to provide more accurate and reproducible measurements.13
In view of these findings, volumetric quantification of RWM was recently implemented in prototype software for analysis of both radial long‐axis CMR images and pyramidal RT3DE datasets. This method is based on tracking changes in segmental left ventricular volume throughout the cardiac cycle and calculating regional ejection fraction (REF) from both modalities, and thus allows direct intermodality comparisons while eliminating analysis‐related differences as a source of error. Although REF is readily available from the volumetric RT3DE data and seems like the natural choice for a quantitative index of regional left ventricular function, it has not been validated against CMR reference because commercial software for multi‐plane two‐dimensional analysis of CMR images does not report this parameter. The prototype software used in this study offers an opportunity for such validation, which is essential for clinical use of REF.
In the same recent study,12 we also tested the feasibility of objective automated interpretation of RWM from RT3DE data by comparing regional shortening fractions with reference values obtained in normal subjects. If found accurate and reliable, this method could constitute an important clinical application of RT3DE imaging. Indeed, wall motion abnormalities were correctly identified in a small group of patients, as confirmed by visual interpretation of two‐dimensional echocardiographic images by an expert reader. However, the accuracy of the automated interpretation using REF values calculated from RT3DE data was neither tested nor compared with other imaging modalities such as CMR, which is important in view of the relatively low resolution of the RT3DE data.
Accordingly, the primary goal of this study was to validate the RT3DE‐derived indices of magnitude and timing of regional left ventricular function, including REF, against radial long‐axis CMR reference values obtained with the same analysis software. Our secondary goal was to test the feasibility and determine the accuracy of the semiautomated interpretation of RWM based on RT3DE‐derived REF values and compare it with the accuracy of the same algorithm when applied to CMR images. Accordingly, this study included two separate protocols designed to achieve these goals.
Methods
Population
In all, 31 patients (17 men, 14 women, age 60 (15) years) were studied. These 31 patients were selected from 33 who were scanned for transthoracic two‐dimensional acoustic windows that allowed adequate endocardial visualisation. Exclusion criteria were dyspnoea precluding a 10–15‐s breath‐hold, atrial fibrillation or other cardiac arrhythmias, pacemaker or defibrillator implantation, claustrophobia and other known contraindications to CMR imaging. Of these 31 patients, 9 had normal hearts, 14 had suspected coronary artery disease, 7 dilated and 1 apical hypertrophic cardiomyopathy. RT3DE and CMR imaging were performed on the same day. Written informed consent was obtained from all the patients.
RT3DE imaging
RT3DE imaging was performed from the apical window with the patient in the left lateral decubitus position using a commercial scanner (SONOS 7500, Philips Medical Systems, Andover, Massachusetts, USA) equipped with a fully sampled matrix array transducer (×4) in the harmonic mode. To include the entire left ventricular cavity within the pyramidal scan volume, datasets were acquired using the wide‐angled acquisition mode, wherein four wedge‐shaped subvolumes (93°×21°) were acquired over eight consecutive cardiac cycles during a breath‐hold. Acquisition of each sub‐volume (total of 4) was triggered to the ECG R‐wave of every other heartbeat (total of 8 heartbeats) to allow sufficient time for the probe to be recalibrated and each subvolume stored.5 RT3DE datasets were stored digitally for off‐line analysis.
CMR imaging
CMR images were obtained using a 1.5‐T scanner (Sonata, Siemens, Forchheim, Germany) with a phased‐array cardiac coil. Electrocardiogram‐gated localising spin‐echo sequences were used to identify the long axis of the heart. Steady‐state free precession (true FISP) dynamic gradient‐echo mode was then used to acquire images during 10–15‐s breath‐holds. Imaging was performed at a temporal resolution of 20 frames per cardiac cycle in six planes, rotated around the long axis of the left ventricle at 30° steps, resulting in dynamic cine loops of radial long‐axis views of the ventricle. Images were stored digitally for off‐line analysis.
Image analysis
Both pyramidal RT3DE datasets and radial long‐axis CMR images were analysed using prototype 4D‐LV Analysis software (TomTec Imaging Systems, Unterschleisheim, Germany). In every CMR slice and in six long‐axis planes automatically selected from the RT3DE dataset (30° apart), left ventricular endocardial contours were traced semiautomatically frame‐by‐frame, with the papillary muscles included in the left ventricular cavity, and manually corrected when necessary to optimise the boundary position. All measurements were performed by an investigator experienced in the interpretation of cardiac images, who was blinded to results of all prior measurements. After segmentation (16‐segment model14), regional volumes were calculated throughout the cardiac cycle. Each volume curve was analysed (Microsoft Excel) to obtain regional end‐diastolic and end‐systolic volumes (REDV, RESV) that were identified as the maximum and minimum values. REF was then computed as the difference between REDV and RESV in percentage of REDV, as well as regional volumes at half‐ejection and half‐filling times (RV(½et), RV(½ft)).
Protocol 1: validation
All RT3DE‐derived indices of regional left ventricular function were compared with the CMR reference values. The comparisons included linear regression with Pearson's correlation coefficients and Bland–Altman analyses. To avoid clustering by patients as a result of multiple measurements in each patient, these analyses were performed separately for each of the 16 endocardial surface segments (one measurement per patient). The calculated correlation coefficients, biases and limits of agreements were then averaged over the 16 segments. Subsequently, these indices were averaged separately for the basal, mid and apical segments to elucidate possible dependency on the left ventricular level.
Protocol 2: semiautomated interpretation of wall motion
Initially, dynamic CMR images were reviewed by an expert, who classified RWM in each left ventricular segment (16‐segment model14) as normal or abnormal. Based on this interpretation, study patients were divided into two groups: 16 patients with RWM abnormalities (10 men, 6 women, mean (standard deviation (SD)) age 62 (13) years) and 15 patients whose RWM was classified as normal in all segments (7 men, 8 women, age 59 (14) years). RT3DE and CMR data were used separately for automated detection of RWM abnormalities, which was performed as follows: data obtained from the 15 patients with normal RWM were averaged to obtain normal values for each segment. In the remaining 16 patients, RWM was evaluated in each segment by comparing the calculated REF against a predetermined threshold value, which was initially set arbitrarily as the mean REF −1 SD of the normal group. Assuming that REF value below the threshold could be used to classify wall motion in a segment as abnormal, these automated classifications were compared with the expert interpretations. Receiver operating characteristic (ROC) analysis15 was then used to optimise the REF thresholds for the automated detection of RWM abnormalities, as described below.
Statistical analysis
In protocol 1, for each calculated index of magnitude and timing of RWM, the significance of the biases between the RT3DE and CMR measurements was tested using paired t tests. p Values <0.05 were considered significant. In protocol 2, for each patient, the level of agreement between the semiautomated technique and the expert interpretation was assessed by counting concordant grades (true positive and true negative) as well as discordant grades (false positive and false negative) assigned by the automated technique. The counts of concordant and discordant grades were used to calculate the sensitivity, specificity, positive and negative predictive values and the overall accuracy, which were used for optimisation of REF thresholds.
ROC analysis
REF thresholds for automated detection of RWM abnormalities varied in each segment on varying the number of standard deviation below the mean REF of the normal group from 0 to 2 in 0.1 increments. For each increment, the sensitivity, specificity, positive and negative predictive values and overall accuracy of the semi‐automated interpretation were calculated against the expert interpretation. The ROC curve was then constructed as a plot of sensitivity versus (1−specificity) and used to determine the optimal REF thresholds.15
Results
RT3DE imaging and analysis were feasible in all study subjects, including the cardiomyopathic patients despite their enlarged ventricles (CMR‐derived end‐diastolic volume ranged from 90 to 526 ml and end‐systolic volume ranged from 25 to 441 ml). The time required for image analysis, including data retrieval, surface detection and segmentation, and the computation of all indices of RWM, was approximately 30 min on a Pentium 4 personal computer. Manual corrections were necessary to optimise the position of the endocardial boundaries in 19 of 31 study subjects.
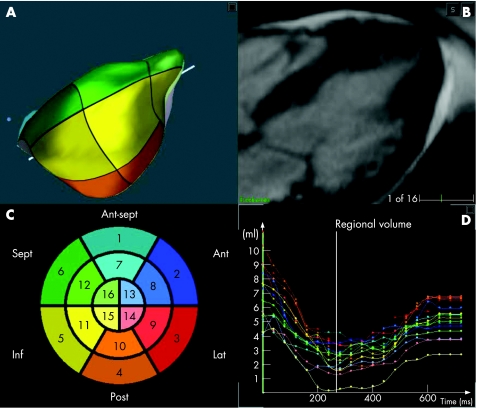
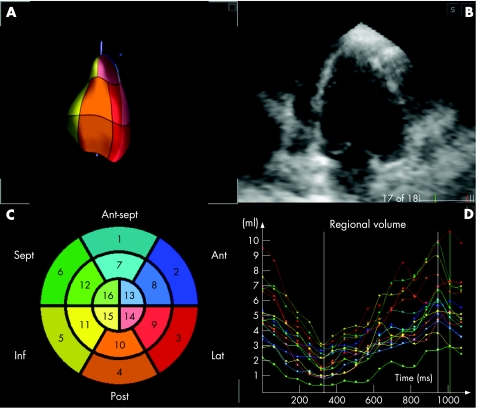
Figure 1B shows an example of a radial long‐axis CMR image obtained at end diastole in a patient with apical hypertrophic cardiomyopathy. The detected endocardial surface (A) shows in three dimensions the typical left ventricular morphology for this condition, in close correspondence with the two‐dimensional image (B). Regional left ventricular volume curves (D) show the expected ejection and filling phases of the cardiac cycle. Figure 2 shows, in the same format, the RT3DE data obtained in the same patient. The RT3DE‐derived regional volume curves (D) had similar shape, despite the less detailed endocardial delineation, compared with the CMR images (fig 1B).
Figure 1 Example of radial long‐axis cardiac magnetic resonance image of the left ventricle obtained at end diastole in a patient with apical hypertrophic cardiomyopathy (B) and the detected endocardial surface (A). Schematic “bull's eye” representation of the three‐dimensional segmentation (C), with the colour notation used for the different segments in both the endocardial surface (A) and the regional left ventricular volume‐time curves (D). Ant, anterior; ant sept, antero‐septal; inf, inferior; lat, lateral; post, posterior; sept, septal.
Figure 2 Example of cross‐sectional view of the left ventricle extracted from the real‐time three‐dimensional echocardiographic dataset obtained in the same patient. Data are presented in the same format as in fig 1. Ant, anterior; ant sept, antero‐septal; inf, inferior; lat, lateral; post, posterior; sept, septal.
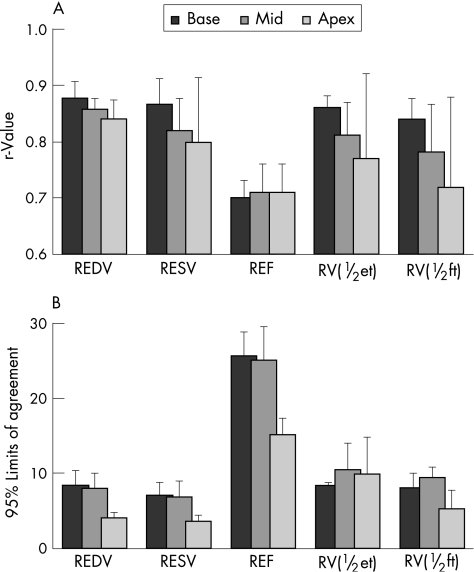
Table 1 shows the results of the comparisons between the RT3DE‐derived indices of regional left ventricular function and CMR reference values in protocol 1. The intertechnique agreement was reflected by the high correlation coefficients, essentially zero biases and relatively wide limits of agreement. Figure 3 shows the break‐up of these data by left ventricular level, obtained by separating basal, mid and apical segments. Of note, there was a clear trend towards lower correlation with CMR (fig 3A) in more distal segments for all measured indices, with the exception of REF. The calculated biases were near zero in all segments, with no significant differences between the three left ventricular levels (not presented in a graphic format). Interestingly, the limits of agreement were tighter for the apical than for the non‐apical segments (fig 3B).
Table 1 Comparison of real‐time three‐dimensional echocardiographic derived indices of regional left ventricular function against cardiac magnetic resonance reference values.
| RESV | REDV | REF | RV(½et) | RV(½ft) | |
|---|---|---|---|---|---|
| r | 0.82 (0.08) | 0.85 (0.04) | 0.71 (0.05) | 0.82 (0.09) | 0.79 (0.10) |
| Bias | 0.2 (2.3) ml | 0 (2.3) ml | −1.3 (9.5)% | −0.3 (2.7) ml | 0.5 (2.7) ml |
| Limits of agreement | 6 (2) ml | 7 (3) ml | 23 (6)% | 9 (3) ml | 8 (2) ml |
r, Pearson's correlation coefficient obtained by linear regression analysis for each index; REDV, regional end‐diastolic volumes; REF, regional ejection fraction; RESV, regional end‐systolic volumes; RV(½et), regional volume at half ejection times; RV(½ft), regional volume at half filling times.
Bias and limits of agreement obtained by Bland–Altman analysis.
Each index was calculated from measurements obtained in the 31 patients in each of the 16 endocardial surface segments.
Values are mean (SD) of the 16 segments.
Figure 3 Comparisons between the real‐time three‐dimensional echocardiography derived indices of regional left ventricular function against cardiac magnetic resonance (CMR) reference values calculated for basal, mid and apical segments: (A) Pearson's correlation coefficients and (B) limits of agreement. Bars represent segmental data averaged over the 31 study patients. REDV, regional end‐diastolic volumes; REF, regional ejection fraction; RESV, regional end‐systolic volumes; RV(½et), regional volume at half ejection; RV(½ft), regional volume at half filling times.
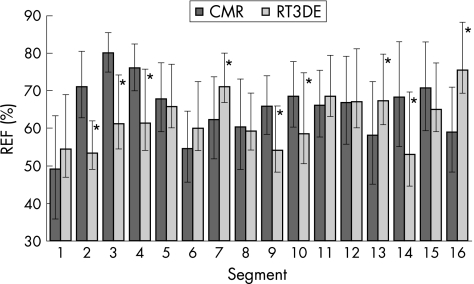
In protocol 2, in the 15 patients with normal wall motion, REF values averaged over the 16 segments were not different between imaging modalities (CMR 65% (11)% vs RT3DE 62% (13)%; NS). Nevertheless, significant differences were found on a regional basis. Figure 4 shows CMR‐derived and RT3DE‐derived mean REF values in each of the 16 segments. RT3DE‐derived REF values were lower in the basal anterior, lateral and posterior segments (2, 3 and 4), the mid lateral and posterior segments (9 and 10), and the apical posterolateral segment (14). Conversely, the CMR‐derived values were lower in the mid anteroseptal and apical anterior segments (7, 13 and 16). In the other segments, REF values were not significantly different between the two imaging modalities.
Figure 4 Cardiac magnetic resonance (CMR) derived and real‐time three‐dimensional echocardiography (RT3DE) derived mean regional ejection fraction (REF) values in 16 segments obtained in 15 patients with normal wall motion. The upward error bars represent the SD, whereas the tips of the downward error bars point at the optimised REF threshold values for each imaging modality (*p<0.05 vs CMR).
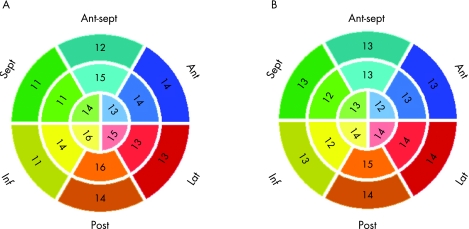
In the remaining 16 patients, RWM abnormalities were noted in 206 of 256 (80%) segments based on expert interpretation of CMR images. In 7 of 16 patients, left ventricular dysfunction was global (abnormal wall motion in all segments), whereas in the remaining 9 patients left ventricular dysfunction had more localised character (a total of 94/144 (65%) abnormal segments). Computer analysis resulted in automated classification of these abnormalities that reached maximum accuracy with thresholds at 0.9 SD below the mean value for the CMR images and 0.5 SD below the mean for the RT3DE images. These values corresponded to a 16‐segment mean threshold of 56% (7)% for CMR images and 55% (10)% for RT3DE images. Figure 4 also shows the optimal segment‐by‐segment thresholds for the automated classification as abnormal (downward error bars). These threshold REF values allowed correct automated classification of 215 of 256 (84%) segments as normal or abnormal in the remaining 16 patients by analysis of either RT3DE or CMR images, in agreement with the expert wall motion scores based on visual interpretation of the CMR images. Importantly, the semiautomated interpretation of wall motion from RT3DE data was as accurate as it was from the CMR data (table 2). This finding was not affected by isolating patients with global left ventricular dysfunction from those with localised wall motion abnormalities, despite the reduction in the accuracy. Figure 5 shows the rates of agreement of both imaging modalities with the expert grades for each individual endocardial surface segment. Of note, for both imaging modalities, the highest rates of agreement were noted in the posterior wall segments, whereas the lowest rates of agreement were noted in the septal and inferior walls.
Table 2 Performance of the algorithm for semiautomated detection of wall motion abnormalities from cardiomagnetic resonance (CMR) and real‐time three‐dimensional echocardiography images assessed against conventional visual interpretation of regional wall motion by an expert reader of CMR images.
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| RT3DE | 0.84 (0.72) | 0.78 (0.86) | 0.95 (0.95) | 0.47 (0.47) | 0.84 (0.78) |
| CMR | 0.85 (0.73) | 0.81 (0.85) | 0.96 (0.94) | 0.52 (0.52) | 0.84 (0.75) |
NPV, negative predictive values; PPV, positive predictive values.
Numbers in brackets show results obtained in nine patients with localised wall motion abnormalities.
Figure 5 Rates of agreement with the expert grades for each individual endocardial surface segment calculated in 16 patients with wall motion abnormalities for the automated interpretation of wall motion from cardiac magnetic resonance images (A) and real‐time three‐dimensional echocardiography images (B). Ant, anterior; ant sept, antero‐septal; inf, inferior; lat, lateral; post, posterior; sept, septal.
Discussion
The recent development of three‐dimensional echocardiographic imaging has resolved many of the limitations associated with the evaluation of global left ventricular function from two‐dimensional images and considerably improved the accuracy of these measurements. However, clinical assessment of regional left ventricular function remains subjective as, even for two‐dimensional images, there is no widely accepted technique for quantitative analysis of RWM. The new software we used in this study for volumetric quantification of regional left ventricular function from RT3DE data provides frame‐by‐frame values of regional left ventricular volumes, which have not been validated against a reliable independent reference technique. This study is the first to validate these measurements against those obtained from radial long‐axis CMR images that offer significant advantages over the conventional short‐axis images, including more uniform endocardial definition from base to apex and optimal visualisation of left ventricular systolic shortening within the imaging plane. The use of the same software to analyse both the RT3DE and the CMR images allowed us to eliminate the possible analysis‐related intermodality differences as a source of error. In addition to validating volumetric indices of regional left ventricular function against these improved CMR reference values, we successfully tested the feasibility of using regional ejection fraction values as a basis for objective semiautomated interpretation of regional left ventricular wall motion.
In protocol 1, despite the use of improved CMR reference values, not surprisingly, the levels of agreement between RT3DE‐derived and CMR‐derived regional volumes were lower than those previously reported for global left ventricular volumes and related indices.6,7,9,12 This finding could be explained by volume subdivision as a result of potential segmentation differences between imaging modalities. In particular, one may notice that the limits of agreement shown in table 1 are of the same order of magnitude as the measured volumes. However, it is important to remember that 95% limits of agreement are nothing but ±2SD of the differences between the RT3DE measurements and the reference technique. Thus, wide limits of agreement could be caused by wide variability in these differences, which would render the RT3DE technique inaccurate. On the other hand, a large SD could also be caused even by a single outlier whereas the rest of the measurements could be in perfect agreement. Thus, using the limits of agreement as an isolated factor by which the level of agreement is judged would be a mistake. That is why it is customary to use several indices, including the correlation coefficient r that measures to what extent the two techniques are related to each other in a linear manner, and the combination of the two Bland–Altman parameters (bias and limits of agreement) that gives quantitative expression to the level of agreement. Therefore, the accuracy of our technique should be judged by the values of all three parameters: (1) r values between 0.71 and 0.85 for all measured volumes, which is good, (2) essentially zero biases, which is excellent, and (3) the relatively wide limits of agreement, which is not ideal. Keeping this difficulty with the interpretation in mind, one might understand why it was important to test the value of the volumetric analysis of RWM in the context of a specific clinical application, such as semiautomated interpretation of wall motion in protocol 2.
Interestingly, the results of the separate measurements performed for segments grouped by left ventricular level (figure 3) showed that the limits of agreement were tighter for the apical than for the non‐apical segments, although this finding is not surprising as regional volumes in the former segments are typically smaller than in the latter segments. Nevertheless, it shows that the previously reported poor agreement with CMR in the apical segments12 was improved by the use of long‐axis radial CMR images. This finding therefore indicates that short‐axis CMR is a less than perfectly accurate reference for left ventricular function measurements near the apex.
The intermodality differences in regional ejection fractions (figure 4) could also be caused by the semiautomated endocardial border identification, which depends for each modality on its ability to visualise boundary details and include or exclude trabeculae from the left ventricular cavity. Another reason for these differences in REF could be potential segmentation differences, as for both modalities, the segmentation requires the knowledge of the position of the junction between the right ventricular free wall and the interventricular septum.8,12,16 This anatomical landmark may shift at different levels of the ventricle and may also be difficult to identify accurately on radial CMR images, as it may be located between imaging planes. The intersegmental differences in the level of agreement with the CMR reference (fig 5) may also be explained by the differences between the physics underlying the two imaging modalities, and how different factors, such as fibre orientation, affect the endocardial definition in different regions of the ventricle.
Protocol 2 was designed as a step for clinical testing of the volumetric echocardiographic assessment of regional left ventricular function. The feasibility of semiautomated identification of RWM abnormalities was tested to establish the clinical usefulness of segmental analysis of RT3DE data in patients with CAD who are the ultimate target population of this methodology. Compared with our previous study,12 this protocol tested the clinical feasibility of this approach in a relatively large group of patients. Also, in this study, CMR images that offer better endocardial border definition than ultrasound images were used for expert visual interpretation of RWM that served as a standard for comparisons against the quantitative analysis of both CMR and RT3DE. Importantly, the goal of this protocol was achieved as reflected by the high levels of agreement with the reference technique.
One of the limitations of the technique we tested in this study is the relatively significant time commitment it requires. However, time requirements of this procedure are likely to decrease as computational power continues to grow. Another limitation of this methodology is the relatively low temporal resolution of the RT3DE imaging, currently 40–50 ms, which may impede more accurate determination of the timing of events within the cardiac cycle. In an attempt to address this issue, the analysis software uses interpolated volume data at 1000 time points per cardiac cycle. Yet another limitation is the use of a relatively small number of imaging planes (6 planes every 30°), which might have limited the accuracy of regional volume measurements. Although using a larger number of planes might result in even better agreement with the CMR reference, this hypothesis was not tested in this study, because the number of CMR planes was predetermined.
Additionally, one might suggest that larger patient groups should have been used in protocol 2, to obtain more reliable normal REF values, assuming that this parameter may be age‐dependent. However, previous studies have shown using two‐dimensional echocardiography that, unlike growth‐related changes in myocardial contractile function in children,17,18 left ventricular contractile function remains essentially unchanged throughout adulthood.19 Therefore, in the context of the feasibility study in protocol 2, age did not seem to be a major factor that would require establishing normal values over a wide age range in a large group of subjects.
Moreover, for both CMR and RT3DE techniques, the thresholds for automated detection of wall motion abnormalities were obtained from REF values averaged in the normal group. Obtaining these values required tracing and correcting the endocardial boundaries, which is inevitably associated with intraobserver and interobserver variabilities. As the levels of variability in the evaluation of RWM were studied previously for both RT3DE20 and CMR21,22,23 techniques, we did not perform repeated measurements for variability analysis, which could be viewed as an additional limitation of this study.
Also, subjective visual interpretation of wall motion in protocol 2 may not be the ideal standard for comparison, and other quantitative measurements of regional left ventricular function could have been used. Indeed, in protocol 1, our quantitative measurements were validated against CMR reference values. Having these measurements validated, we designed protocol 2 to test the feasibility of using our technique in the clinical setting. In particular, we compared this technique with the results of visual interpretation of two‐dimensional images by a single expert reader, despite the subjective nature of this method. Indeed, one might suggest that using consensus interpretation by a number of readers24,25 or an independent expert panel decision based on a variety of clinical data23 could have provided a more reliable reference for comparison. Nevertheless, it is important to remember that, despite this limitation, visual interpretation by a single experienced reader represents the most widely used clinical standard for the assessment of ventricular wall motion.
Despite these limitations, our results have shown that in patients with adequate transthoracic two‐dimensional acoustic windows, RT3DE‐derived volumetric indices of regional left ventricular function are relatively accurate, including the readily available but not previously validated regional ejection fraction. Our results have also shown that this quantitative index can be used as a basis for semiautomated interpretation of RWM, which was found to be as accurate as that based on analysis of CMR images, despite the inferior resolution of ultrasound images. These findings may have important implications regarding the use of volumetric analysis of RT3DE data for various clinical applications, even beyond objective diagnosis of RWM abnormalities. Such potential application may include echocardiographic guidance of resynchronisation treatment,10,26,27 as accurate measurement of regional left ventricular volume over time may also allow the diagnosis of asynchrony in the temporal patterns of left ventricular contraction. Witnessing how the RT3DE technology and software for volumetric analysis of RWM are gaining widespread use, we believe that this new methodology will soon become routine in clinical practice.
Acknowledgements
This study was supported by a Grant‐in‐Aid from the American Heart Association (VM‐A principal investigator).
Abbreviations
CMR - cardiac magnetic resonance
REDV - regional end‐diastolic volume
REF - regional ejection fraction
RESV - regional end‐systolic volume
ROC - receiver operating characteristic
RT3DE - real‐time three‐dimensional echocardiographic
RWM - regional wall motion
Footnotes
Competing interests: None declared.
References
- 1.Hozumi T, Yoshikawa J, Yoshida K.et al Three‐dimensional echocardiographic measurement of left ventricular volumes and ejection fraction using a multiplane transesophageal probe in patients. Am J Cardiol 1996781077–1080. [DOI] [PubMed] [Google Scholar]
- 2.Gopal A S, Schnellbaecher M J, Shen Z.et al Freehand three‐dimensional echocardiography for determination of left ventricular volume and mass in patients with abnormal ventricles: comparison with magnetic resonance imaging. J Am Soc Echocardiogr 199710853–861. [DOI] [PubMed] [Google Scholar]
- 3.Mele D, Maehle J, Pedini I.et al Three‐dimensional echocardiographic reconstruction: description and applications of a simplified technique for quantitative assessment of left ventricular size and function. Am J Cardiol 199881107G–10G. [DOI] [PubMed] [Google Scholar]
- 4.Shiota T, Jones M, Chikada M.et al Real‐time three‐dimensional echocardiography for determining right ventricular stroke volume in an animal model of chronic right ventricular volume overload. Circulation 1998971897–1900. [DOI] [PubMed] [Google Scholar]
- 5.Sugeng L, Weinert L, Lang R M. Left ventricular assessment using real time three dimensional echocardiography. Heart 200389(Suppl 3)iii29–iii36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkins C, Bricknell K, Hanekom L.et al Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real‐time three‐dimensional echocardiography. J Am Coll Cardiol 200444878–886. [DOI] [PubMed] [Google Scholar]
- 7.Zeidan Z, Erbel R, Barkhausen J.et al Analysis of global systolic and diastolic left ventricular performance using volume‐time curves by real‐time three‐dimensional echocardiography. J Am Soc Echocardiogr 20031629–37. [DOI] [PubMed] [Google Scholar]
- 8.Collins M, Hsieh A, Ohazama C J.et al Assessment of regional wall motion abnormalities with real‐time 3‐dimensional echocardiography. J Am Soc Echocardiogr 1999127–14. [DOI] [PubMed] [Google Scholar]
- 9.Kuhl H P, Schreckenberg M, Rulands D.et al High‐resolution transthoracic real‐time three‐dimensional echocardiography: quantitation of cardiac volumes and function using semi‐automated border detection and comparison with cardiac magnetic resonance imaging. J Am Coll Cardiol 2004432083–2090. [DOI] [PubMed] [Google Scholar]
- 10.Krenning B J, Szili‐Torok T, Voormolen M M.et al Guiding and optimization of resynchronization therapy with dynamic three‐dimensional echocardiography and segmental volume‐‐time curves: a feasibility study. Eur J Heart Fail 20046619–625. [DOI] [PubMed] [Google Scholar]
- 11.Corsi C, Saracino G, Sarti A.et al Left ventricular volume estimation for real‐time three‐dimensional echocardiography. IEEE Trans Med Imaging 2002211202–1208. [DOI] [PubMed] [Google Scholar]
- 12.Corsi C, Lang R M, Veronesi F.et al Volumetric quantification of global and regional left ventricular function from real‐time three‐dimensional echocardiographic images. Circulation 20051121161–1170. [DOI] [PubMed] [Google Scholar]
- 13.Bloomer T N, Plein S, Radjenovic A.et al Cine MRI using steady state free precession in the radial long axis orientation is a fast accurate method for obtaining volumetric data of the left ventricle. J Magn Reson Imaging 200114685–692. [DOI] [PubMed] [Google Scholar]
- 14.Cerqueira M D, Weissman N J, Dilsizian V.et al Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002105539–542. [DOI] [PubMed] [Google Scholar]
- 15.Metz C E. Basic principles of ROC analysis. Semin Nucl Med 19788283–298. [DOI] [PubMed] [Google Scholar]
- 16.Mor‐Avi V, Vignon P, Koch R.et al Segmental analysis of color kinesis images: new method for quantitative assessment of left ventricular contraction and relaxation. Circulation 1997952082–2097. [DOI] [PubMed] [Google Scholar]
- 17.Colan S D, Parness I A, Spevak P J.et al Developmental modulation of myocardial mechanics: age‐ and growth‐related alterations in afterload and contractility. J Am Coll Cardiol 199219619–629. [DOI] [PubMed] [Google Scholar]
- 18.Kimball T R, Daniels S R, Khoury P.et al Age‐related variation in contractility estimate in patients <20 years of age. Am J Cardiol 1991681383–1387. [DOI] [PubMed] [Google Scholar]
- 19.Mor‐Avi V, Spencer K, Gorcsan J.et al Normal values of regional left ventricular endocardial motion: multicenter color kinesis study. Am J Physiol Heart Circ Physiol 2000279H2464–H2476. [DOI] [PubMed] [Google Scholar]
- 20.Corsi C, Coon P, Goonewardena S.et al Quantification of regional left ventricular wall motion from real‐time 3‐dimensional echocardiography in patients with poor acoustic windows: effects of contrast enhancement tested against cardiac magnetic resonance. J Am Soc Echocardiogr 200619886–893. [DOI] [PubMed] [Google Scholar]
- 21.Corsi C, Lamberti C, Catalano O.et al Improved quantification of left ventricular volumes and mass based on endocardial and epicardial surface detection from cardiac MR images using level set models. J Cardiovasc Magn Reson 20057595–602. [DOI] [PubMed] [Google Scholar]
- 22.Caiani E G, Toledo E, MacEneaney P.et al Automated interpretation of regional left ventricular wall motion from cardiac magnetic resonance images. J Cardiovasc Magn Reson 20068427–433. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann R, von B S, Kasprzak J D.et al Analysis of regional left ventricular function by cineventriculography, cardiac magnetic resonance imaging, and unenhanced and contrast‐enhanced echocardiography: a multicenter comparison of methods. J Am Coll Cardiol 200647121–128. [DOI] [PubMed] [Google Scholar]
- 24.Decara J M, Mor‐Avi V, Weinert L.et al Automated quantitative assessment of wall motion in patients with poor acoustic windows. J Am Soc Echocardiogr 200417723–731. [DOI] [PubMed] [Google Scholar]
- 25.Mor‐Avi V, Jacobs L D, Weiss R J.et al Color encoding of endocardial motion improves the interpretation of contrast‐enhanced echocardiographic stress tests by less‐experienced readers. J Am Soc Echocardiogr 20061948–54. [DOI] [PubMed] [Google Scholar]
- 26.Hong T E, Sugeng L, Weinert L.et al Use of real‐time three‐dimensional echocardiography to measure ventricular dyssynchrony and assess cardiac resynchronization in heart failure patients [abstract]. J Am Coll Cardiol 200443309A [Google Scholar]
- 27.Kapetanakis S, Kearney M T, Siva A.et al Real‐time three‐dimensional echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation 2005112992–1000. [DOI] [PubMed] [Google Scholar]