Abstract
Aim
To investigate organ culture preservation of cultured limbal epithelial cells in order to enhance the availability of tissue‐engineered epithelia that are used to treat patients with limbal stem cell deficiency.
Methods
Limbal epithelial cells were cultured for 3 weeks on intact amniotic membrane fastened to a polyester membrane carrier. The cultured epithelia were stored for 1 week at 23°C in organ culture medium. The preserved epithelia were then examined using a colorimetric cell viability assay, light microscopy and immunohistochemistry.
Results
The viability of the preserved epithelia was 84% (20%), and no statistically significant difference was found compared with non‐preserved epithelia. In general, the cell borders were maintained, the nuclei showed no sign of degeneration, and the original layered structure was preserved. Mild intercellular oedema was occasionally observed. Expression of p63, K19 and vimentin was maintained.
Conclusions
Cultured limbal epithelial cells can be preserved in organ culture medium for 1 week at room temperature, while maintaining the original layered structure and undifferentiated phenotype.
Limbal stem cell deficiency may be treated by transplanting ex vivo expanded limbal epithelial cells.1 However, the availability of cultured tissue is currently limited owing to logistical and methodological challenges. Firstly, there is a shortage of human donors, which limits the supply of limbal epithelial cells available for tissue engineering. Secondly, the method is expensive and is reserved for eye departments with cell culture facilities. Thirdly, it takes several weeks of cell culture to engineer multilayered epithelial sheets, which makes it difficult to schedule operations. Finally, no efficient method of transporting cultured epithelia has been described to date. The present study aimed to design a preservation method based on organ culture that is technically practical and allows cultured epithelia to be transported between eye departments.
Materials and methods
Cell culture and organ culture preservation of limbal epithelial cells
The research was conducted in accordance with the Declaration of Helsinki, and consent was obtained for the use of donor tissue for research purposes. Human amniotic membranes, preserved as reported previously,2 were attached to the polyester membrane of Netwell culture plate inserts (Costar, Corning, New York, New York, USA) using 6‐0 non‐absorbable sutures. Eyes were enucleated from cadavers, and explant cultures (n = 32) were prepared as described previously by Meller et al.3
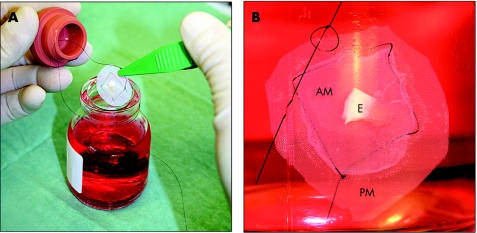
Limbal explants exposed to dispase (Roche Diagnostics, Basel, Switzerland) were incubated with the stromal side facing the amniotic membrane for 21 days at 37°C in a medium consisting of N‐2‐hydroxyethylpiperazine‐N'‐ethane‐sulphonic acid‐buffered Dulbecco's modified Eagle's medium containing sodium bicarbonate and Ham's F12 (Sigma‐Aldrich, St Louis, Missouri, USA) supplemented with 5% fetal bovine serum, 0.5% dimethyl sulphoxide, 2 ng/ml human epidermal growth factor, 5 µg/ml insulin, 5 µg/ml transferrin, 5 ng/ml selenium, 3 ng/ml hydrocortisone, 30 ng/ml cholera toxin (Biomol, Exeter, UK), 50 µg/ml gentamicin and 1.25 µg/ml amphotericin B. The polyester mesh bottom with the cultured epithelium attached was released using a steel blade and suspended in a sterilised 50 ml glass infusion bottle using an Ethicon Ethilon 6‐0 monofilament suture, which was tied to the edge of the polyester membrane (fig 1). The epithelia (n = 16) were incubated for 1 week at 23°C in an organ culture medium containing N‐2‐hydroxyethylpiperazine‐N'‐ethane‐sulphonic acid‐buffered Dulbecco's modified Eagle's medium with 7.5% sodium bicarbonate, 8% fetal bovine serum, 50 µg/ml gentamicin (Garamycin), 100 µg/ml vancomycin (Abbott Laboratories, Abbott Park, Illinois, USA) and 2.5 µg/ml amphotericin B.
Figure 1 Organ culture preservation of cultured epithelium. (A) Experimental design showing the graft attached to the polyester membrane carrier, the rubber stopper and the glass infusion bottle containing the storage medium. (B) Cultured epithelium fully immersed in organ culture medium. The amniotic membrane was fastened to the polyester membrane carrier at four corners using a 6‐0 monofilament suture. AM, amniotic membrane; E, limbal explant; PM, polyester membrane.
Cell viability analysis
Mitochondrial function, an indicator of cell viability, was measured using a colorimetric assay, as reported previously.4,5,6 This technique is based on mitochondrial enzyme reduction of the water‐soluble tetrazolium salt‐8‐(2(2‐methoxy‐4‐nitrophenyl)‐3‐(4‐nitrophenyl)‐5‐(2,4‐disulphophenyl)‐2H‐tetrazolium monosodium salt) and spectrophotometric quantification of the water‐soluble formazan dye generated. Initially, a calibration curve was created to investigate the relationship between the optical density and the number of viable cells in samples from non‐preserved cultured epithelial cells. Disks (n = 12) of cultured epithelium were trephinated using biopsy punches (Kai Industries, Gifu, Japan) of different diameters (2, 3, 4, 5 and 6 mm). They were then incubated in 20 µl CCK‐8 solution (Alexis Corporation, Lausen, Switzerland) and 200 µl organ culture medium for 2 h. The solution was analysed colorimetrically at 450 nm in an automated microplate reader (Kinetic‐QCL, Bio‐Whittaker, Walkersville, Maryland, USA). The discs were subsequently trypsinised, and cell numbers were counted directly using the trypan blue dye exclusion technique. Based on measurements of 3 mm epithelial discs, the optical density after preservation (n = 8) was calculated as the percentage of that before preservation (n = 8).
Light microscopy and immunohistochemistry
Preserved epithelia (n = 8) and non‐preserved epithelia (n = 8) were fixed in neutral buffered 4% formaldehyde and embedded in paraffin. Serial sections of 5 μm thickness were routinely stained with haematoxylin and eosin. Immunohistochemistry was performed with a panel of antibodies (table 1). To visualise the immunoreactions, we used a standard peroxidase technique (DAB detection kit) in a Ventana ES Immunohistochemistry Instrument (Tucson, Arizona, USA). Optimal antibody dilutions were determined by titration using the positive controls recommended by the manufacturers. The expression pattern was evaluated by two independent investigators.
Table 1 Semiquantitative immunohistochemical characteristics of non‐preserved cultured epithelium and cultured epithelium preserved for 1 week at 23°C (room temperature).
| Specificity | Clone | Source, dilution | Non‐preserved epithelium | 1‐Week storage at 23°C | ||||
|---|---|---|---|---|---|---|---|---|
| B | SB | S | B | SB | S | |||
| p63 | 4A4 | DAKO,* 1/25 | 3 | 2 | 0‐1 | 3 | 3 | 0‐1 |
| Cytokeratin 19 | RCK108 | DAKO,* 1/200 | 2 | 2 | 2 | 2 | 2 | 2 |
| Vimentin | VIM 3B4 | Ventana Medical Systems† | 3 | 2 | 1 | 3 | 2 | 1 |
| Ki67 | MIB‐1 | DAKO,* 1/75 | 1 | 0‐1 | 0 | 0‐1 | 0 | 0 |
| Cytokeratin 3 | AE5 | ImmuQuest,‡ 1/500 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cytokeratin 5 | XM26 | Novocastra Lab.,§ 1/600 | 3 | 3 | 3 | 3 | 3 | 3 |
| Cytokeratin 14 | LL02 | Novocastra Lab.,§ 1/80 | 3 | 3 | 3 | 3 | 3 | 3 |
| Connexin 43 | Polyclonal | Sigma‐Aldrich,¶ 1/500 | 2 | 2 | 1 | 1 | 1 | 0 |
| E‐cadherin | NCH‐38 | Novocastra Lab.,§ 1/25 | 1 | 2 | 0‐1 | 1 | 2 | 0 |
| Integrin β‐1 | 7F10 | Novocastra Lab.,§ 1/10 | 2 | 1 | 0‐1 | 2 | 0 | 0 |
B, basal layer; S, superficial layer; SB, suprabasal layer.
0 = undetectable; 1 = weak positivity; 2 = moderate positivity; 3 = strong positivity.
*Glostrup, Denmark.
†Tucson, Arizona, USA.
‡Cleveland, UK.
§Newcastle, UK.
¶St Louis, Missouri, USA.
Statistical analysis
Data are presented as mean (SD). SPSS V.14.0 was used to assess the cell viability (correlation analysis and t tests for two independent groups). A p value of <0.05 was considered significant.
Results
Viability
A linear relationship was observed between the optical density and the viable cell number in samples from non‐preserved cultured epithelial cells (correlation r = 0.97). The optical density of the non‐preserved epithelia was 0.27 (0.03), whereas that of the preserved epithelia was 0.23 (0.05), giving a viability percentage of 84% (20%). No significant difference was found between the two groups (p = 0.07).
Light microscopy and immunohistochemistry
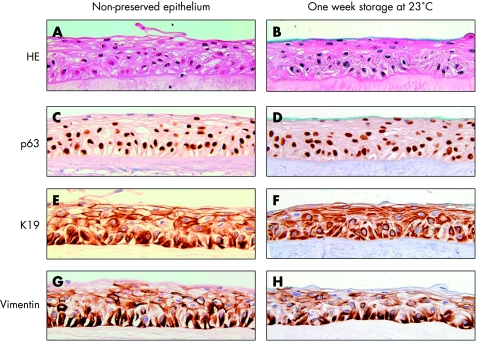
On the whole, the cell borders were maintained, and the nuclei showed no sign of degeneration (fig 2B). The epithelia attached well to the amniotic membrane. Mild intercellular oedema was occasionally observed. No change in staining pattern was revealed for K19, vimentin, K3, K5 and K14. Minimal changes were disclosed for Ki67, p63, Cx43, E‐cadherin and integrin β‐1 (table 1, fig 2).
Figure 2 Sections stained with haematoxylin and eosin (A, B), and immunostaining of p63 (C, D), K19 (E, F) and vimentin (G, H) in non‐preserved cultured epithelial cells (A, C, E and G) and cultured epithelial cells preserved for 1 week at 23°C (B, D, F and H). Original magnification: ×100.
Discussion
Past studies have examined the epithelial proliferative potential of organ cultured corneoscleral rims as a source of limbal epithelial cells.7,8,9 However, no previous reports have examined organ culture preservation of ex vivo expanded limbal epithelial cells. This study shows that cultured limbal epithelial cells can be preserved in organ culture medium at room temperature for 1 week, while maintaining the original layered structure and undifferentiated phenotype.
The initial challenge was to find a suitable carrier for the amniotic membrane. Ultimately, only polyester membrane culture plate inserts met all our requirements. The membranes (1) were able to withstand the tension of the sutures and keep the amniotic membrane distended; (2) were easily released from the culture plate insert; (3) fitted into the glass infusion bottle; and (4) were easy to detach from the amniotic membrane.
Organ culture preservation of donor corneas is currently the most widely used corneal storage method in Europe,10 and the medium supplies the nutrients needed to maintain cellular metabolism in the tissue.11 The present study was conducted at room temperature (23°C), which eliminated the need for heating cabinets and made it easier to distribute the transplants between eye departments. A few reports have been published that consider the influence of room temperature (23–25°C) on corneas stored in culture media such as McCarey–Kaufman medium,12 K‐Sol medium,13 TC 199 medium14 and RPMI 1640 organ culture medium.15 However, in these studies, the corneal endothelium has been the main focus of attention.
The linear relationship observed between the optical density and the cell number is consistent with the results of Kito et al,6 who reported a high correlation (R2 = 0.976). The results of the cell viability assay and the light microscopy examination indicated that the majority of the cultured epithelial cells were viable after preservation. Mild intercellular oedema occurred occasionally, which has previously been reported after organ culture storage.16,17
As there were no data available for direct comparison with our immunohistochemical findings, we compared our data with the results of a study by Joseph et al,8 which investigated limbal explants stored in organ culture medium for 3–4 weeks. The expression of p63, vimentin, Ki67 and Cx43 was close to their results. However, in their study, only a few cells were positive for K19, and K3 was expressed in the superficial layer.
There is no standardised method of culturing limbal epithelial cells. In the present study, we used intact amniotic membrane without 3T3 fibroblast feeder layers or air‐lifting, because previous studies have suggested that this method preserves the characteristics of limbal epithelial stem cells.3,18,19 However, the use of air‐lifting has been reported to provide the epithelial sheet with increased mechanical strength,20 which may be beneficial prior to preservation in organ culture. Further studies are warranted to increase the knowledge of the effects of storage conditions on epithelial biology and morphology. In addition, more work needs to be done to investigate whether limbal epithelial cells can be stored for longer periods, in other tissue culture media, using other cell culture protocols and at other temperatures.
In conclusion, our study demonstrates that organ culture may preserve cultured epithelia for transplantation.
Acknowledgements
We thank Tove Norén at the Department of Pathology, Ullevål University Hospital, Oslo, Leiv Sandvik at the Centre for Clinical Research, Ullevål University Hospital, Oslo, and Hanne Ramstad and Eli Gulliksen at the Center for Eye Research, Department of Ophthalmology, Ullevål University Hospital, Oslo, for their excellent assistance and support.
Footnotes
Funding: This work was supported in part by the Eastern Norway Regional Health Authority, the Norwegian Association of the Blind and Partially Sighted, and the Blindmission IL.
Competing interests: None declared.
References
- 1.Pellegrini G, Traverso C E, Franzi A T.et al Long‐term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997349990–993. [DOI] [PubMed] [Google Scholar]
- 2.Lee S H, Tseng S C. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol 1997123303–312. [DOI] [PubMed] [Google Scholar]
- 3.Meller D, Pires R T, Tseng S C. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol 200286463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hershey F B, Cruickshank C N, Mullins L I. The quantitative reduction of 2,3,5‐triphenyl tetrazolium chloride by skin in vitro. J Histochem Cytochem 19586191–196. [DOI] [PubMed] [Google Scholar]
- 5.Bravo D, Rigley T H, Gibran N.et al Effect of storage and preservation methods on viability in transplantable human skin allografts. Burns 200026367–378. [DOI] [PubMed] [Google Scholar]
- 6.Kito K, Kagami H, Kobayashi C.et al Effects of cryopreservation on histology and viability of cultured corneal epithelial cell sheets in rabbit. Cornea 200524735–741. [DOI] [PubMed] [Google Scholar]
- 7.James S E, Rowe A, Ilari L.et al The potential for eye bank limbal rings to generate cultured corneal epithelial allografts. Cornea 200120488–494. [DOI] [PubMed] [Google Scholar]
- 8.Joseph A, Powell‐Richards A O, Shanmuganathan V A.et al Epithelial cell characteristics of cultured human limbal explants. Br J Ophthalmol 200488393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanmuganathan V A, Rotchford A P, Tullo A B.et al Epithelial proliferative potential of organ cultured corneoscleral rims; implications for allo‐limbal transplantation and eye banking. Br J Ophthalmol 20069055–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armitage W J, Easty D L. Factors influencing the suitability of organ‐cultured corneas for transplantation. Invest Ophthalmol Vis Sci 19973816–24. [PubMed] [Google Scholar]
- 11.Summerlin W T, Miller G E, Harris J E.et al The organ‐cultured cornea: an in vitro study. Invest Ophthalmol 197312176–180. [PubMed] [Google Scholar]
- 12.Sachs U, Goldman K, Valenti J.et al Corneal storage at room temperature. Arch Ophthalmol 1978961075–1077. [DOI] [PubMed] [Google Scholar]
- 13.Tamaki K, Varnell E D, Kaufman H E. K‐Sol corneal preservation at room temperature. Br J Ophthalmol 198872370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reim M, Hesse R, Pietruschka G. The metabolism of organ cultures of cornea in TC 199 with added dextran 500 or hydroxyethyl starch 450. Klin Monatsbl Augenheilkd 199019676–80. [DOI] [PubMed] [Google Scholar]
- 15.Sandboe F D, Medin W, Froslie K F. Influence of temperature on corneas stored in culture medium. A comparative study using functional and morphological methods. Acta Ophthalmol Scand 20038154–59. [DOI] [PubMed] [Google Scholar]
- 16.Borderie V M, Kantelip B M, Delbosc B Y.et al Morphology, histology, and ultrastructure of human C31 organ‐cultured corneas. Cornea 199514300–310. [DOI] [PubMed] [Google Scholar]
- 17.Van Horn D L, Doughman D J, Harris J E.et al Ultrastructure of human organ‐cultured cornea. II. Stroma and epithelium. Arch Ophthalmol 197593275–277. [DOI] [PubMed] [Google Scholar]
- 18.Grueterich M, Espana E, Tseng S C. Connexin 43 expression and proliferation of human limbal epithelium on intact and denuded amniotic membrane. Invest Ophthalmol Vis Sci 20024363–71. [PubMed] [Google Scholar]
- 19.Hernandez Galindo E E, Theiss C, Steuhl K P.et al Gap junctional communication in microinjected human limbal and peripheral corneal epithelial cells cultured on intact amniotic membrane. Exp Eye Res 200376303–314. [DOI] [PubMed] [Google Scholar]
- 20.Ban Y, Cooper L J, Fullwood N J.et al Comparison of ultrastructure, tight junction‐related protein expression and barrier function of human corneal epithelial cells cultivated on amniotic membrane with and without air‐lifting. Exp Eye Res 200376735–743. [DOI] [PubMed] [Google Scholar]