Abstract
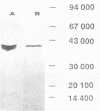
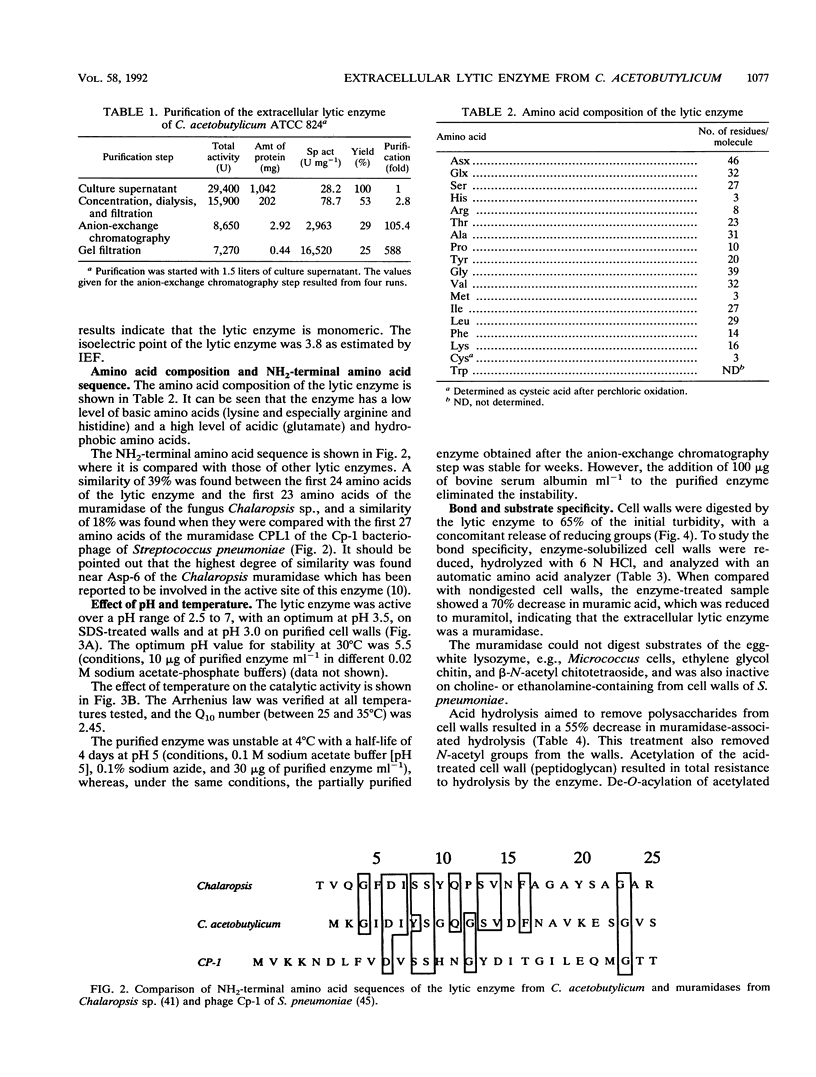
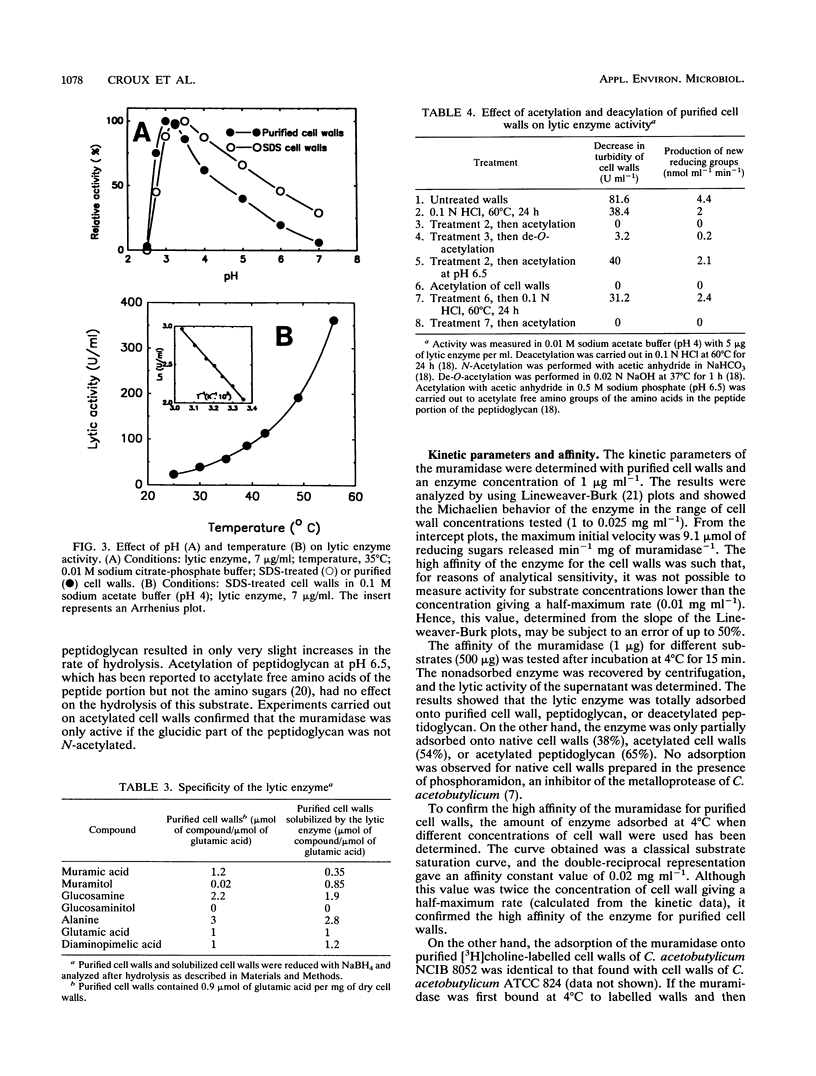
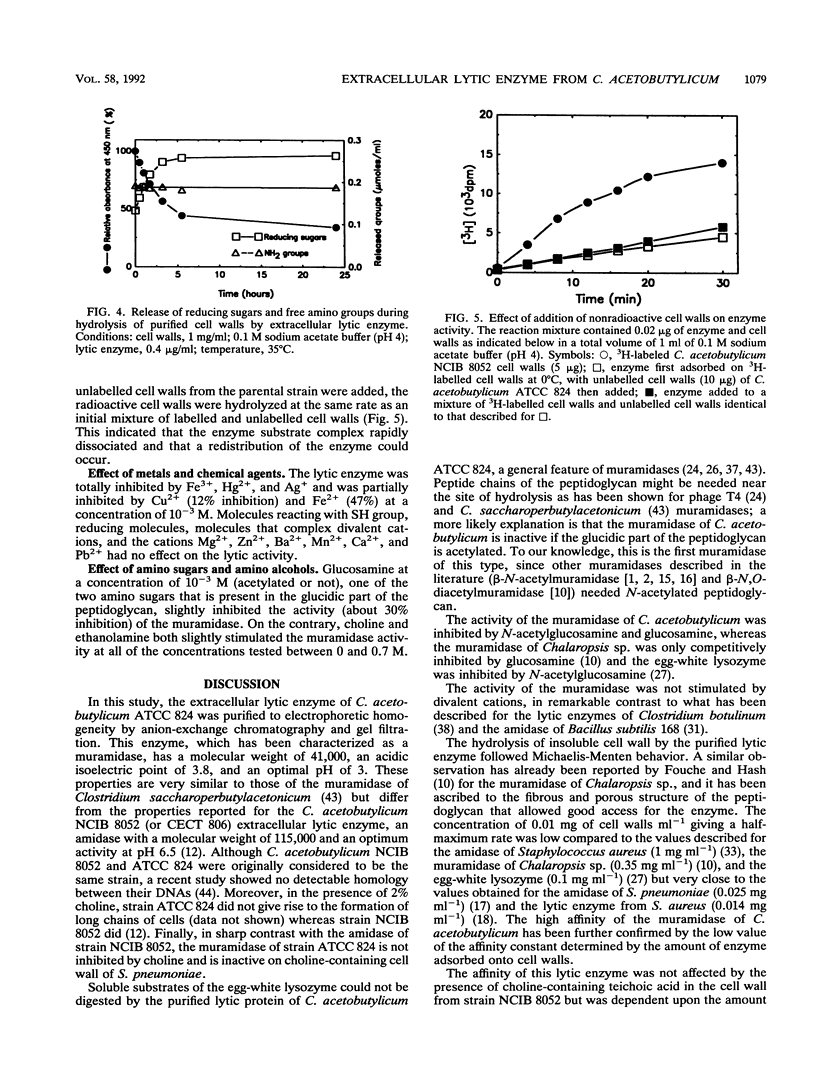
An extracellular enzyme showing lytic activity on non-N-acetylated peptidoglycan has been isolated from Clostridium acetobutylicum ATCC 824. The lytic enzyme was purified to homogeneity by anion-exchange chromatography and gel filtration, with a recovery of 24%. The enzyme was monomeric and had an estimated molecular weight of 41,000 and an isoelectric point of 3.8. It has been characterized as a muramidase whose 23-amino-acid N terminus displayed 39% homology with the N,O-diacetyl muramidase of the fungus Chalaropsis sp. The muramidase hydrolyzed purified cell walls at an optimum pH of 3, with a maximum velocity of 9.1 mumol of reducing sugars released min-1 mg of muramidase-1 and a concentration of cell walls giving a half-maximum rate of 0.01 mg ml-1. Its activity was inhibited by glucosamine, N-acetylglucosamine, Hg2+, Fe3+, and Ag+ but not by choline. The muramidase-peptidoglycan complex rapidly dissociated before total hydrolysis of the chain and randomly reassociated on another peptidoglycan chain. The affinity of the muramidase was affected by the protein content and the acetylation of the cell wall.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Araki Y., Ito E. Effect of N-acyl substitution at glucosamine residues on lysozyme-catalyzed hydrolysis of cell-wall peptidoglycan and its oligosaccharides. Eur J Biochem. 1980 Jun;107(2):547–553. doi: 10.1111/j.1432-1033.1980.tb06062.x. [DOI] [PubMed] [Google Scholar]
- Araki Y., Oba S., Araki S., Ito E. Enzymatic deacetylation of N-acetylglucosamine residues in cell wall peptidoglycan. J Biochem. 1980 Aug;88(2):469–479. doi: 10.1093/oxfordjournals.jbchem.a132994. [DOI] [PubMed] [Google Scholar]
- Barber J. M., Robb F. T., Webster J. R., Woods D. R. Bacteriocin production by Clostridium acetobutylicum in an industrial fermentation process. Appl Environ Microbiol. 1979 Mar;37(3):433–437. doi: 10.1128/aem.37.3.433-437.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. F., Dolinger D. L., Schramm V. L., Shockman G. D. The mechanism of soluble peptidoglycan hydrolysis by an autolytic muramidase. A processive exodisaccharidase. J Biol Chem. 1984 Oct 10;259(19):11818–11827. [PubMed] [Google Scholar]
- CANFIELD R. E. THE AMINO ACID SEQUENCE OF EGG WHITE LYSOZYME. J Biol Chem. 1963 Aug;238:2698–2707. [PubMed] [Google Scholar]
- Chan L., Glaser L. Purification of N-acetylmuramic acid-L-alanine amidase from Bacillus megaterium. J Biol Chem. 1972 Sep 10;247(17):5391–5397. [PubMed] [Google Scholar]
- Croux C., Paquet V., Goma G., Soucaille P. Purification and characterization of acidolysin, an acidic metalloprotease produced by Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 1990 Dec;56(12):3634–3642. doi: 10.1128/aem.56.12.3634-3642.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Felch J. W., Inagami T., Hash J. H. The N, O-diacetylmuramidase of Chalaropsis species. V. The complete amino acid sequence. J Biol Chem. 1975 May 25;250(10):3713–3720. [PubMed] [Google Scholar]
- Fouche P. B., Hash J. H. The N,O-diacetylmuramidase of Chalaropsis species. Identificaiton of aspartyl and glutamyl residues in the active site. J Biol Chem. 1978 Oct 10;253(19):6787–6793. [PubMed] [Google Scholar]
- García E., García J. L., García P., Arrarás A., Sánchez-Puelles J. M., López R. Molecular evolution of lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Proc Natl Acad Sci U S A. 1988 Feb;85(3):914–918. doi: 10.1073/pnas.85.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García P., García J. L., García E., Sánchez-Puelles J. M., López R. Modular organization of the lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Gene. 1990 Jan 31;86(1):81–88. doi: 10.1016/0378-1119(90)90116-9. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Araki Y., Ito E. Occurrence of glucosamine residues with free amino groups in cell wall peptidoglycan from bacilli as a factor responsible for resistance to lysozyme. J Bacteriol. 1973 Feb;113(2):592–598. doi: 10.1128/jb.113.2.592-598.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff E., Silverman C. S. Lysis of Staphylococcus aureus cell walls by a soluble staphylococcal enzyme. J Bacteriol. 1968 Jan;95(1):99–106. doi: 10.1128/jb.95.1.99-106.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Purification of the pneumococcal N-acetylmuramyl-L-alanine amidase to biochemical homogeneity. J Biol Chem. 1976 Jul 25;251(14):4199–4207. [PubMed] [Google Scholar]
- Kamei K., Hara S., Ikenaka T., Murao S. Amino acid sequence of a lysozyme (B-enzyme) from Bacillus subtilis YT-25. J Biochem. 1988 Nov;104(5):832–836. doi: 10.1093/oxfordjournals.jbchem.a122558. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Shockman G. D. Purification and some properties of the endogenous, autolytic N-acetylmuramoylhydrolase of Streptococcus faecium, a bacterial glycoenzyme. J Biol Chem. 1983 Aug 10;258(15):9514–9521. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin H. H., Kemper S. Endo-N-acetyl-glucosaminidase from Clostridium perfringens, lytic for cell wall murein of gram-negative bacteria. J Bacteriol. 1970 May;102(2):347–350. doi: 10.1128/jb.102.2.347-350.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Kleppe G., Jensen H. B. Studies on the specificity of action of bacteriophage T4 lysozyme. Eur J Biochem. 1975 Jul 1;55(2):369–3-3. doi: 10.1111/j.1432-1033.1975.tb02171.x. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Pollitt S., Zalkin H. Role of primary structure and disulfide bond formation in beta-lactamase secretion. J Bacteriol. 1983 Jan;153(1):27–32. doi: 10.1128/jb.153.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Taylor C., Rayter S., Ward J. B. Purification and properties of autolytic endo-beta-N-acetylglucosaminidase and the N-acetylmuramyl-L-alanine amidase from Bacillus subtilis strain 168. J Gen Microbiol. 1984 Sep;130(9):2395–2402. doi: 10.1099/00221287-130-9-2395. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Begg G. S., Dorow D. S., Morgan F. J. Complete amino acid sequence of the goose-type lysozyme from the egg white of the black swan. Biochemistry. 1980 Apr 29;19(9):1814–1819. doi: 10.1021/bi00550a013. [DOI] [PubMed] [Google Scholar]
- Singer H. J., Wise E. M., Jr, Park J. T. Properties and purification of N-acetylmuramyl-L-alanine amidase from Staphylococcus aureus H. J Bacteriol. 1972 Nov;112(2):932–939. doi: 10.1128/jb.112.2.932-939.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi K., Kawata T., Hisatsune K. Autolytic enzyme system of Clostridium botulinum. II. Mode of action of autolytic enzymes in Clostridium botulinum type A. Jpn J Microbiol. 1971 Mar;15(2):131–141. doi: 10.1111/j.1348-0421.1971.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Thompson J. S., Shockman G. D. A modification of the Park and Johnson reducing sugar determination suitable for the assay of insoluble materials: its application to bacterial cell walls. Anal Biochem. 1968 Feb;22(2):260–268. doi: 10.1016/0003-2697(68)90315-1. [DOI] [PubMed] [Google Scholar]
- Tsugita A., Inouye M. Complete primary structure of phage lysozyme from Escherichia coli T4. J Mol Biol. 1968 Oct 14;37(1):201–212. doi: 10.1016/0022-2836(68)90083-1. [DOI] [PubMed] [Google Scholar]
- Webster J. R., Reid S. J., Jones D. T., Woods D. R. Purification and Characterization of an Autolysin from Clostridium acetobutylicum. Appl Environ Microbiol. 1981 Feb;41(2):371–374. doi: 10.1128/aem.41.2.371-374.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Ward J. B. Characterization of the autolytic enzymes of Clostridium perfringens. J Gen Microbiol. 1979 Oct;114(2):349–354. doi: 10.1099/00221287-114-2-349. [DOI] [PubMed] [Google Scholar]
- Young M., Minton N. P., Staudenbauer W. L. Recent advances in the genetics of the clostridia. FEMS Microbiol Rev. 1989 Dec;5(4):301–325. doi: 10.1111/j.1574-6968.1989.tb03402.x. [DOI] [PubMed] [Google Scholar]