Abstract
Background
It has been suggested that replicative senescence might be involved in the pathophysiology of age‐related diseases.
Aim
To study the process of senescence in trabecular meshwork (TM) cells.
Methods
Porcine TM tissues were obtained and placed in primary cultures with Dulbecco's modified Eagle's medium/Ham's F‐12 medium. After 2–3 weeks, migrated and proliferated TM cells were trypsinised and cultured in serial passages, and identified with fluorescein‐labelled low‐density lipoprotein (DiI‐Ac‐LDL), a marker of TM cells. Staining for senescence‐related β‐galactosidase activity was performed at population doubling level (PDL) 2, 8 and 16 at pH 6. Terminal restriction fragment (TRF) length was examined by Southern blot analysis using a 32P‐labelled telomere‐specific sequence (TTAGGG)3 at each PDL.
Results
DiI‐Ac‐LDL staining revealed that most (nearly 100%) of the cells in the culture were TM cells, which were flattened in shape and positive for senescence‐related β‐galactosidase staining at PDL 16. Reduction of TRF length as a function of population doubling was also shown.
Conclusions
TM cells exhibited characteristics of senescence at PDL 16 in vitro. The results demonstrated that cellular senescence may be related to the pathophysiology of primary open‐angle glaucoma.
Normal diploid cells have a finite proliferative life span and finally enter a non‐dividing state termed senescence.1 Senescent cells are unable to duplicate themselves and are accompanied with altered gene expression, at least when cultured in vitro.2 It has been suggested that replicative senescence might be involved in the pathophysiology of age‐related disorders, such as progeria and Werner's syndrome, as well as related atherothrombotic diseases.3,4,5 Primary open‐angle glaucoma (POAG) is an optic neuropathy associated with abnormally increased intraocular pressure that can lead to blindness, especially in elderly patients, and age‐ and disease‐related losses of trabecular meshwork (TM) cells have been reported in patients with POAG.6,7 It has also been suggested that a loss of TM cells, followed by substitution with extracellular matrix, might contribute to an increased resistance to aqueous outflow in those patients, resulting in an increase in intraocular pressure.8,9 Thus, ageing is thought to have a relationship with the pathophysiology of POAG. Our previous study results showed that aged retinal pigment epithelial (RPE) cells exhibited characteristics of cellular senescence and suggested that senescent RPE cells could be involved in the pathogenesis of age‐related macular degeneration.10 TM cells are derived from the embryonic neural crest,11 and are known to have phagocytic12 and migratory13 abilities similar to RPE cells. Therefore, we speculated that TM cells also exhibit cellular senescence, which may be involved in the pathophysiology of POAG, as it was reported previously that levels of type VI collagen, thrombospondin and fibronectin were increased in aged TM cells.14 In this study, we investigated whether cellular senescence occurred in cultured TM cells.
Materials and methods
Tissue and cell culture
Porcine eyes were transported to our laboratory in ice‐cold saline. The eyeballs were cut and opened at a point 3 mm posterior from the limbus under a dissection microscope. TM tissue and Tenon's connective tissue samples were also obtained, and placed separately in six‐well plates (Falcon, New York, New York, USA) with Dulbecco's modified Eagle's medium/Ham's F‐12 culture medium (Sigma‐Aldrich, St Louis, Missouri, USA) supplemented with 10% fetal bovine serum (HyClone Laboratories, South Logan, Utah, USA), 100 U/ml of penicillin and 100 mg/ml of streptomycin for 4 weeks, to allow the TM cells and fibroblasts to migrate and proliferate. Primary cultures of each sample were maintained in an atmosphere of 5% CO2/95% air at 37°C and supplied with fresh medium once a week. Further, corneal samples were obtained and covered with Hanks's balanced salt solution containing 0.025% trypsin and 0.01% EDTA for 30 min at 37°C to obtain corneal endothelial cells. TM cells obtained from confluent primary cultures were arbitrarily designated as population doubling level (PDL) 0. When the cells became confluent, they were subcultured at a split ratio of 1:4 using Hanks's balanced salt solution (Gibco, Grand Island, New York, USA) containing 0.05% trypsin and 0.01% EDTA; thus, subcultured cells increased by two PDLs at each passage. Cells without tissue were seeded separately in six‐well plates (Falcon), and then incubated under the same conditions and supplied with fresh medium every 3 days.
Low‐density lipoprotein staining
Cells were examined for expression of the low‐density lipoprotein (LDL) receptor, which has been shown to be a surface marker of TM cells.15 Cells were grown in 35 mm diameter glass‐bottom dishes (Matsunami Glass, Ind., Osaka, Japan) and incubated for 6 h in 2 ml of medium containing 20 μg of DiI‐labelled acetylated LDL (DiI‐Ac‐LDL, Molecular Probes, Eugene, Oregon, USA). After the dishes were washed three times with phosphate‐buffered saline (pH 7.4), they were processed for fluorescence microscopy examinations. Corneal endothelial cells and fibroblasts obtained from porcine eyes were used as negative controls for DiI‐Ac‐LDL staining. The samples were observed and analysed using a fluorescence microscope (FluoView, Olympus, Tokyo, Japan).
β‐Galactosidase staining
TM cells at PDL 2, 8 and 16 were trypsinised and seeded in 35 mm diameter glass‐bottom dishes. Cells were incubated for 12 h under the same culture conditions as in the above experiment to allow them to adhere to the glass bottom of the dish, and then washed with phosphate‐buffered saline and stained for senescence‐related β‐galactosidase (pH 6) or lysosomal β‐galactosidase (pH 4) activities. Details of the staining procedures have been described previously.10,16 The cells were fixed in 3% formaldehyde for 4 min at room temperature, washed three times with phosphate‐buffered saline, then incubated in 1 ml of a solution containing 5 mM of X‐gal in 40 mM of citric acid–sodium phosphate buffer (pH 6) at 37°C in air for 8 h to develop the blue colour in senescent cells. Control incubations were performed at pH 4 to show the presence of lysosomal β‐galactosidase in all cells. Samples were examined with a bright field microscope (IMT‐2, Olympus) equipped with a computer‐controlled display camera (HC‐1000, Fujix, Tokyo, Japan).
Measurements of mean terminal restriction fragment lengths
Mean terminal restriction fragment (TRF) length was analysed using a method previously described,10,17,18 with a slight modification. Genomic DNA was isolated from TM cells at PDL 2, 8 and 16. Cells were lysed in a lysis buffer (150 mM NaCl, 10 mM Tris–HCl, 10 mM EDTA and 0.1% sodium dodecyl sulphate) containing 0.1 μg/ml of proteinase K (Sigma‐Aldrich). Samples were incubated at 55°C for 30 min and subsequent DNA extraction was performed as described previously.17 Each DNA sample was limit digested with the restriction enzymes Rsa I and Hinf I, to yield terminal restriction fragments containing the telomere and a small amount of sub‐telomeric DNA sequence. Each sample (1 μg) was subjected to electrophoresis on a 0.7% agarose gel in 1× Tris–acetate–EDTA buffer for 200 V‐h, then transferred onto a Hibond‐N+ (Amersham Biosciences, Piscataway, New Jersey, USA) membrane and hybridised with a 32P‐labelled telomere‐specific oligonucleotide of TTAGGG3. After washing with saline sodium citrate containing 0.1% sodium dodecyl sulphate at 42°C for 5 min, the membranes were exposed to x ray films (Fujifilm, Tokyo, Japan).
Results and discussion
TM cell morphology
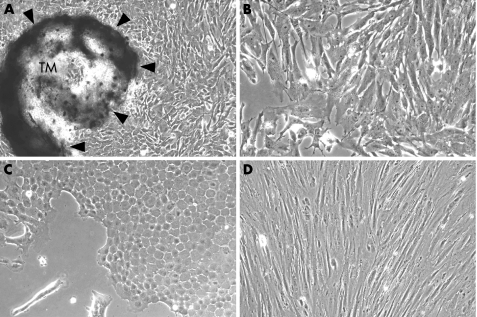
TM cells migrated from TM tissue and proliferated to form colonies around each TM tissue specimen (fig 1A), and showed a cell shape (fig 1B) that was different from fibroblasts and corneal endothelial cells. Corneal endothelial cells showed a round or hexagonal shape, with a cobblestone‐like phenotype (fig 1C), whereas fibroblasts from Tenon's connective tissue showed a spindle‐like shape with a longitudinal axis (fig 1D).
Figure 1 Trabecular meshwork (TM) tissue explant (A arrowheads) and phenotypical differences among trabecular meshwork cells (B), corneal endothelial cells (C) and fibroblasts from Tenon's connective tissue (D). Original magnification ×100 (A), ×400 (B–D).
LDL staining
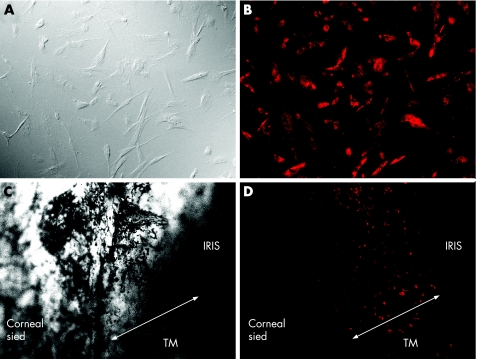
Nearly all the cultured TM cells at PDL 2 showed a cell shape identical (fig 2A) to that seen in the primary culture (fig 1B) and were strongly positive for DiI‐Ac‐LDL staining, a reported previously 15 surface marker of TM cells (fig 2B). LDL staining was also performed with TM tissue (fig 2C) to confirm whether in situ TM cells expressed the LDL receptor, and those cells were also positive for DiI‐Ac‐LDL (fig 2D). By contrast, corneal endothelial cells and fibroblasts from Tenon's connective tissue were negative for LDL staining (data not shown).
Figure 2 Nomarsky view (A,C) and low‐density lipoprotein staining (B,D) of trabecular meshwork cells (A,B) and trabecular meshwork (TM) tissue (C,D). Original magnification ×400 (A,B), ×100 (C,D).
Upregulation of senescence‐related β‐galactosidase in senescent TM cells
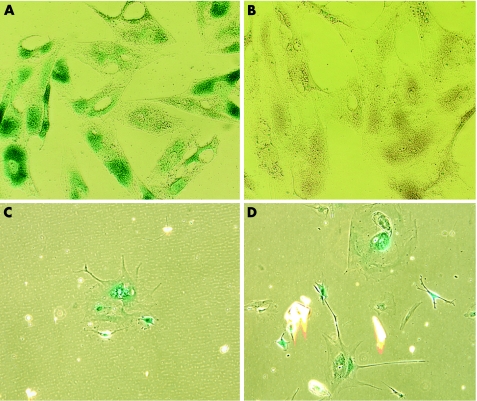
TM cells were flattened in shape at PDL 16 (fig 3C,D) as compared with those at PDL 2 (fig 3A,B). TM cells at PDL 2 were positive only for lysosomal β‐galactosidase staining (pH 4; fig 3A) and not for senescence‐related β‐galactosidase staining (pH 6; fig 3B). On the other hand, senescent TM cells at PDL 16 stained positive for both senescence‐related β‐galactosidase (fig 3D) and lysosomal β‐galactosidase (fig 3C).
Figure 3 Lysosomal (pH 4; A,C) and senescence‐related (pH 6; B,D) β‐galactosidase staining of young (PDL 2; A,B) and senescent (PDL 16; C,D) trabecular meshwork cells. Original magnification ×400.
Reduction of TRF length in senescent TM cells
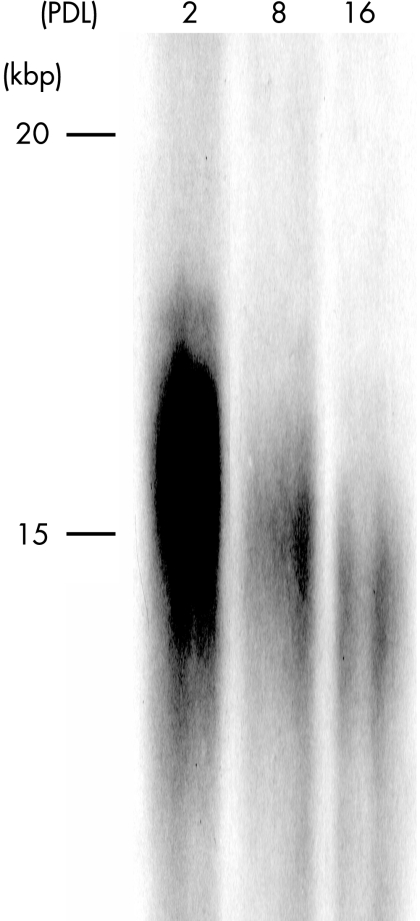
A reduction in TRF length in relation to PDL was shown by genomic Southern blot analysis, as the chromosomal telomeres became shorter with increased numbers of passages (fig 4). TM cells exhibited a mean TRF length of approximately 16, 15 and 14.5 kb at PDL 2, 8 and 16, respectively.
Figure 4 Results of terminal restriction fragment length analysis. Telomere shortening associated with multiple passages of trabecular meshwork cells was seen.
Discussion
Cellular senescence is characterised by telomere loss and altered gene expression,1,2 and senescent cells are unable to duplicate and have altered functional characteristics. Takeda et al19 reported that human skin fibroblasts showed similar modulations in the expression of extracellular matrix components during ageing in in vitro and in vivo experiments, whereas we previously found that cultured human RPE cells exhibited characteristics of cellular senescence.10 Further, Tombran‐Tink et al20 showed that age‐related down regulation of pigment epithelium‐derived factor, a protein possessing neurotrophic and neuronal‐survival activities, occurred in cultured fetal monkey RPE cells.
In this study, TM cells exhibited characteristics of senescence at PDL 16 in vitro, as well as a shorter replicative life span and longer TRF length with senescence, as compared with the RPE cells in our previous report. We consider that these differences might have been because the RPE cells were from an established cell line, whereas the TM cells were from primary cultures. However, the differences may also have been due to cellular differences between human and porcine specimens. Additional experiments are required to understand the pathophysiology of POAG. Nevertheless, if senescent cells are shown to accumulate with age, cellular senescence may play an important role.
Acknowledgements
This work was supported in part by a Grant‐in‐Aid for Young Scientists (B) (number 17791262) from the Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology of Japan, and grants from the Science Research Promotion Fund of the Japan Private School Promotion Foundation.
Abbreviations
PDL - population doubling level
POAG - primary open‐angle glaucoma
LDL - low‐density lipoprotein
TM - trabecular meshwork
TRF - terminal restriction fragment
RPE cells - retinal pigment epithelial cells
Footnotes
Competing interests: None.
References
- 1.Hayflick L. Limited in vitro lifetime of human diploid cell strains. Exp Cell Res 196537614–636. [DOI] [PubMed] [Google Scholar]
- 2.Campisi J. Replicative senescence: an old lives' tale? Cell 199684497–500. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein S. Increased procoagulant activity in cultured fibroblasts from progeria and Werner's syndromes of premature ageing. Nature 1976260711–713. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein S. Studies on age‐related diseases in cultured skin fibroblasts. J Invest Dermatol 19797319–23. [DOI] [PubMed] [Google Scholar]
- 5.Norwood T H, Hoehn H, Salk D.et al Cellular aging in Werner's syndrome: a unique phenotype? J Invest Dermatol 19797392–96. [DOI] [PubMed] [Google Scholar]
- 6.Alvarado J, Murphy C, Polansky J.et al Age‐related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci 198121714–727. [PubMed] [Google Scholar]
- 7.Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open‐angle glaucoma and nonglaucomatous normals. Ophthalmology 198491564–579. [DOI] [PubMed] [Google Scholar]
- 8.Tripathi R C. Pathologic anatomy of the outflow pathway of aqueous humor in chronic simple glaucoma. Exp Eye Res 197725(Suppl)403–407. [DOI] [PubMed] [Google Scholar]
- 9.Lütjen‐Drecoll E, Rittig M, Rauterberg J.et al Immunomicroscopical study of type VI collagen in the trabecular meshwork of normal and glaucomatous eyes. Exp Eye Res 198948139–147. [DOI] [PubMed] [Google Scholar]
- 10.Matsunaga H, Handa J T, Aotaki‐Keen A.et al β‐Galactosidase histochemistry and telomere loss in senescent retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 199940197–202. [PubMed] [Google Scholar]
- 11.Tripathi B J, Tripathi R C. Neural crest origin of human trabecular meshwork and its implications for the pathogenesis of glaucoma. Am J Ophthalmol 1989107583–590. [DOI] [PubMed] [Google Scholar]
- 12.Buller C, Johnson D H, Tschumper R C. Human trabecular meshwork phagocytosis. Invest Ophthalmol Vis Sci 1990312156–2163. [PubMed] [Google Scholar]
- 13.Calthorpe C M, Grierson I. Fibronectin induces migration of bovine trabecular meshwork cells in vitro. Exp Eye Res 19905139–48. [DOI] [PubMed] [Google Scholar]
- 14.Tripathi B J, Li T, Li J.et al Age‐related changes in trabecular cells in vitro. Exp Eye Res 19976457–66. [DOI] [PubMed] [Google Scholar]
- 15.Chang I L, Elner G, Yue Y J.et al Expression of modified low‐density lipoprotein receptors by trabecular meshwork cells. Curr Eye Res 1991101101–1112. [DOI] [PubMed] [Google Scholar]
- 16.Dimri G P, Lee X, Basile G.et al A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 1995929363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allsopp R C, Vaziri H, Patterson C.et al Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA 19928910114–10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okuda K, Bardeguez A, Gardner J P.et al Telomere length in the newborn. Pediatr Res 200252377–381. [DOI] [PubMed] [Google Scholar]
- 19.Takeda K, Gosiewska A, Peterkofsky B. Similar, but not identical, modulation of expression of extracellular matrix components during in vitro and in vivo aging of human skin fibroblasts. J Cell Physiol 1992153450–459. [DOI] [PubMed] [Google Scholar]
- 20.Tombran‐Tink J, Shivaram S M, Chader G J.et al Expression, secretion, and age‐related downregulation of pigment epithelium‐derived factor, a serpin with neurotrophic activity. J Neurosci 1995154992–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]