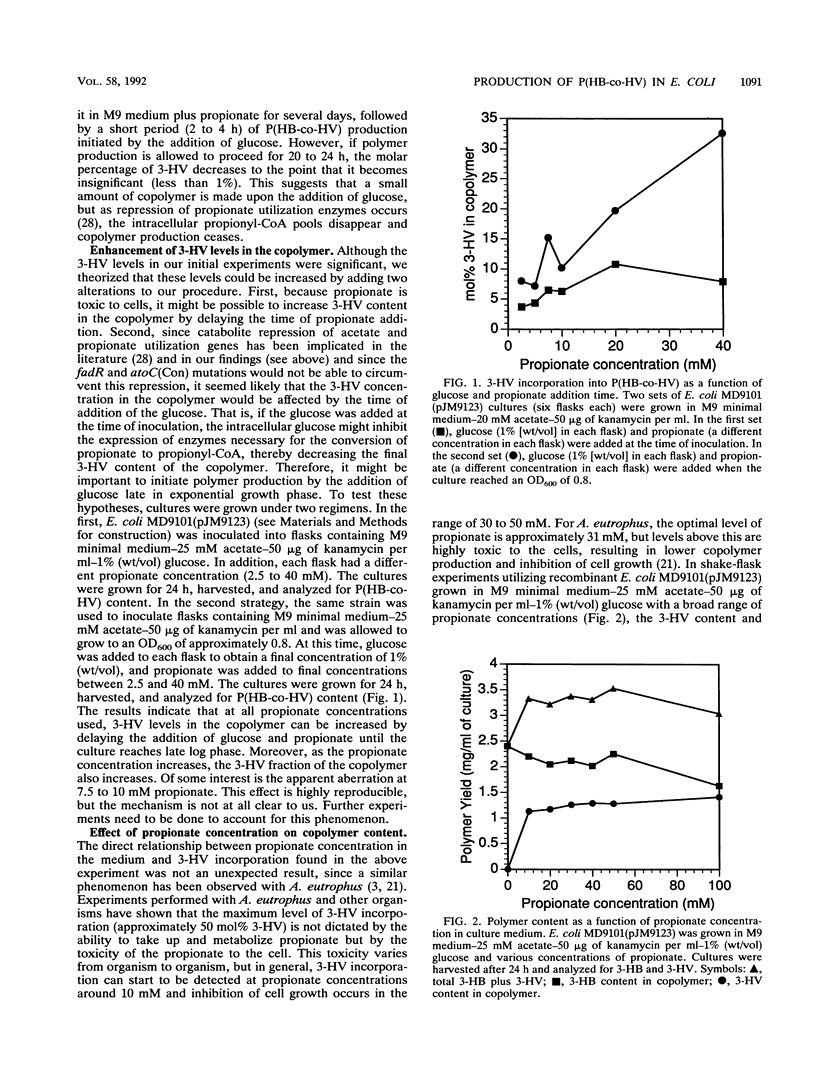
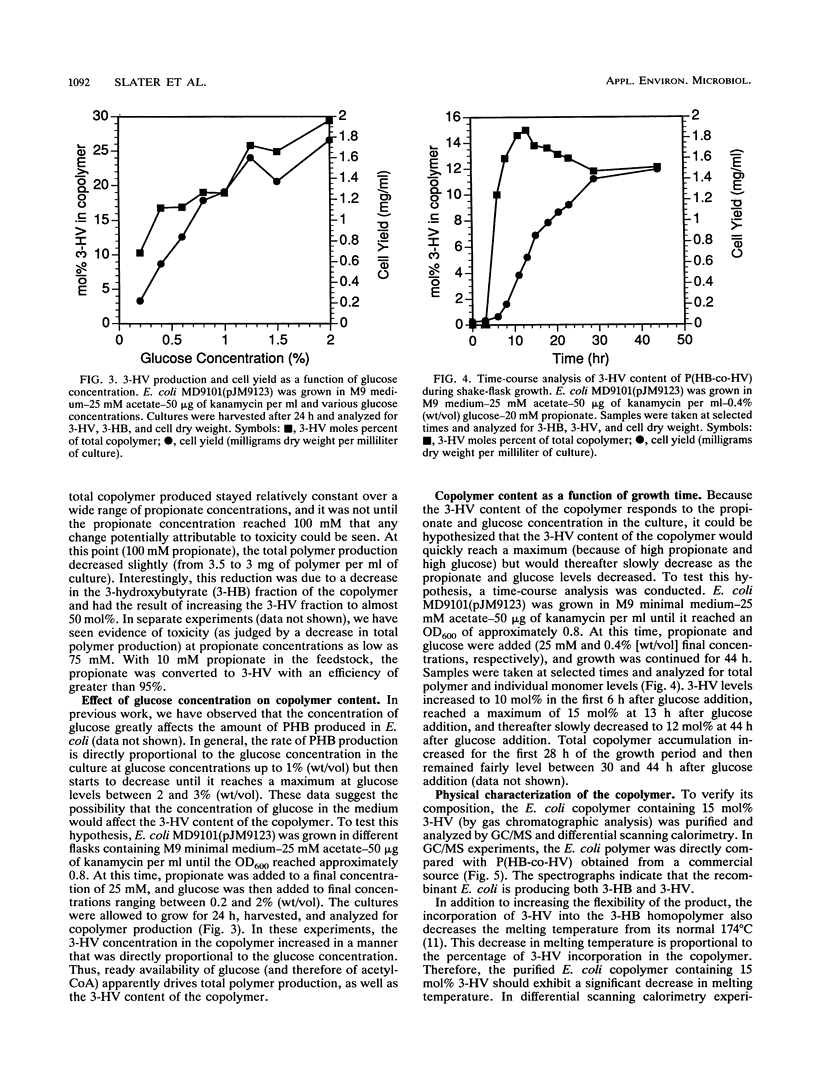
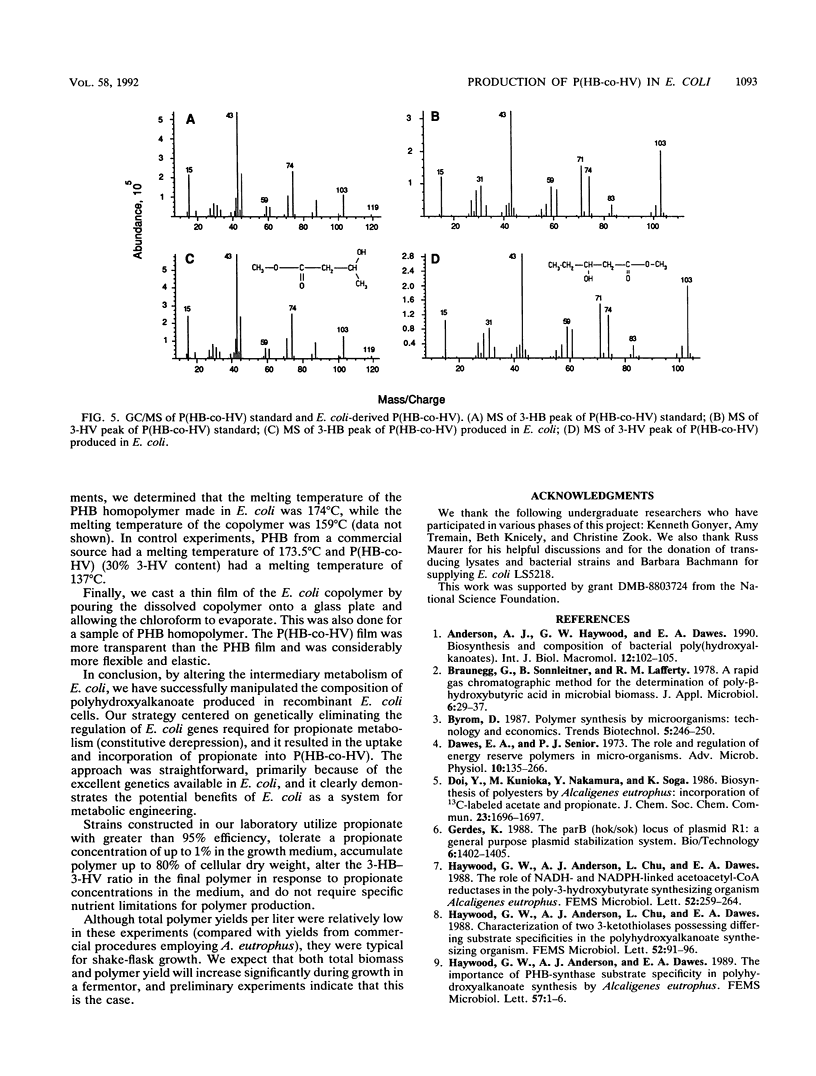
Abstract
An Escherichia coli strain has been constructed that produces the copolymer poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) P(HB-co-HV). This has been accomplished by placing the PHB biosynthetic genes from Alcaligenes eutrophus into an E. coli fadR atoC(Con) mutant and culturing the strain in M9 minimal medium containing glucose and propionate. 3-Hydroxyvalerate incorporation is absolutely dependent on the presence of both glucose and propionate, and 3-hydroxybutyrate-3-hydroxyvalerate ratios in the copolymer can be manipulated by altering the propionate concentration and/or the glucose concentration in the culture. P(HB-co-HV) production can be accomplished by using a wide variety of feeding regimens, but the most efficient is to allow the culture to grow to late log phase in minimal medium containing acetate and then add glucose and propionate to initiate copolymer production. A broad range of propionate concentrations can be used in the culture to stimulate 3-hydroxyvalerate incorporation; however, the most efficient utilization of propionate occurs at concentrations below 10 mM. 3-Hydroxyvalerate molar percentages in the copolymer are relatively constant over the course of growth. The copolymer has been purified and confirmed to be P(HB-co-HV) by gas chromatography/mass spectrometry and differential scanning calorimetry.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Haywood G. W., Dawes E. A. Biosynthesis and composition of bacterial poly(hydroxyalkanoates). Int J Biol Macromol. 1990 Apr;12(2):102–105. doi: 10.1016/0141-8130(90)90060-n. [DOI] [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Kay W. W. Genetic control of the metabolism of propionate by Escherichia coli K12. Biochim Biophys Acta. 1972 May 16;264(3):508–521. doi: 10.1016/0304-4165(72)90014-1. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Role of gene fadR in Escherichia coli acetate metabolism. J Bacteriol. 1981 Oct;148(1):83–90. doi: 10.1128/jb.148.1.83-90.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaki K., Takahashi K., Hasegawa A., Soga Y., Nakazawa M. [A case of Peutz-Jeghers syndrome]. Shigaku. 1986 Apr;73(7):1693–1697. [PubMed] [Google Scholar]
- Peoples O. P., Sinskey A. J. Poly-beta-hydroxybutyrate (PHB) biosynthesis in Alcaligenes eutrophus H16. Identification and characterization of the PHB polymerase gene (phbC). J Biol Chem. 1989 Sep 15;264(26):15298–15303. [PubMed] [Google Scholar]
- Pool R. In Search of the Plastic Potato: Scientists in the emerging field of biopolymer engineering are aiming to produce bacteria and, eventually, food crops that are genetically tailored to yield a whole new breed of plastics. Science. 1989 Sep 15;245(4923):1187–1189. doi: 10.1126/science.245.4923.1187. [DOI] [PubMed] [Google Scholar]
- Ramsay B. A., Lomaliza K., Chavarie C., Dubé B., Bataille P., Ramsay J. A. Production of poly-(beta-hydroxybutyric-co-beta-hydroxyvaleric) acids. Appl Environ Microbiol. 1990 Jul;56(7):2093–2098. doi: 10.1128/aem.56.7.2093-2098.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves H. C., Rabin R., Wegener W. S., Ajl S. J. Fatty acid synthesis and metabolism in microorganisms. Annu Rev Microbiol. 1967;21:225–256. doi: 10.1146/annurev.mi.21.100167.001301. [DOI] [PubMed] [Google Scholar]
- SCHLEGEL H. G., GOTTSCHALK G., VON BARTHA R. Formation and utilization of poly-beta-hydroxybutyric acid by Knallgas bacteria (Hydrogenomonas). Nature. 1961 Jul 29;191:463–465. doi: 10.1038/191463a0. [DOI] [PubMed] [Google Scholar]
- Schubert P., Steinbüchel A., Schlegel H. G. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J Bacteriol. 1988 Dec;170(12):5837–5847. doi: 10.1128/jb.170.12.5837-5847.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater S. C., Voige W. H., Dennis D. E. Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-beta-hydroxybutyrate biosynthetic pathway. J Bacteriol. 1988 Oct;170(10):4431–4436. doi: 10.1128/jb.170.10.4431-4436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt S. K., Ginsburgh C. L., Nunn W. D. Isolation and genetic characterization of Escherichia coli mutants defective in propionate metabolism. J Bacteriol. 1981 Jun;146(3):1166–1169. doi: 10.1128/jb.146.3.1166-1169.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbüchel A., Schlegel H. G. Physiology and molecular genetics of poly(beta-hydroxy-alkanoic acid) synthesis in Alcaligenes eutrophus. Mol Microbiol. 1991 Mar;5(3):535–542. doi: 10.1111/j.1365-2958.1991.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Weeks G., Shapiro M., Burns R. O., Wakil S. J. Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. J Bacteriol. 1969 Feb;97(2):827–836. doi: 10.1128/jb.97.2.827-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener W. S., Reeves H. C., Ajl S. J. Propionate metabolism. II. Factors regulating adaptation of Escherichia coli to propionate. Arch Biochem Biophys. 1968 Jan;123(1):55–61. doi: 10.1016/0003-9861(68)90102-1. [DOI] [PubMed] [Google Scholar]
- Wegener W. S., Reeves H. C., Ajl S. J. Propionate oxidation in Escherichia coli. Arch Biochem Biophys. 1967 Aug;121(2):440–442. doi: 10.1016/0003-9861(67)90098-7. [DOI] [PubMed] [Google Scholar]


