Abstract
Aim
To evaluate the diurnal intraocular pressure (IOP) control and safety of bimatoprost versus latanoprost in exfoliative glaucoma (XFG).
Methods
One eye of 129 consecutive patients with XFG (mean (SD) age 66.5 (8.3) years) was included in this prospective, observer‐masked, three‐centre, crossover comparison. After a 4–6 week medicine‐free period patients were randomised to bimatoprost or latanoprost monotherapy for 3 months. Patients were then switched to the opposite treatment for another 3 months. At the end of the washout and the treatment periods diurnal IOP was measured at 0800, 1300, and 1800.
Results
At baseline the IOP (mean (SD)) was 28.0 (4.0), 26.9 (3.6), and 25.9 (3.6) mm Hg, at the three time points, respectively. Both treatments significantly reduced mean diurnal IOP at month 3. Mean diurnal IOP was 26.9 (3.5) mm Hg at baseline, 17.6 (3.3) mm Hg with bimatoprost, and 18.6 (3.6) mm Hg with latanoprost (p<0.0001). Furthermore, lower IOP values were obtained with bimatoprost at all time points (17.9 (3.4), 17.3 (3.3), and 17.6 (3.5) mm Hg, respectively) compared with latanoprost (18.7 (3.6), 18.5 (3.6), and 18.6 (4.1) mm Hg, respectively). The corresponding mean differences (0.8, 1.1, and 1.0 mm Hg, respectively) were all significant (p<0.001 for each comparison). Significantly more patients with XFG obtained a target diurnal IOP <17 mm Hg with bimatoprost than with latanoprost, 55/123 (45%) v 34/123 (28%); (p = 0.001), and significantly fewer patients were non‐responders with bimatoprost than with latanoprost (5 v 13, p = 0.021). More patients reported at least one adverse event with bimatoprost than with latanoprost (58 v 41 at 3 months; p = 0.0003).
Conclusion
This crossover study suggests that better diurnal IOP control is obtained with bimatoprost than with latanoprost in patients with XFG.
Keywords: intraocular pressure, bimatoprost, latanoprost, glaucoma
Exfoliative glaucoma (XFG) is a common, sight‐threatening disease, which develops as a consequence of exfoliation syndrome.1 Clinical characteristics, course, and prognosis of XFG are different from those in primary open angle glaucoma (POAG).1,2,3,4,5 The subtlety of clinical signs results in the diagnosis of XFG being often overlooked,1,2,3 sometimes resulting in less than ideal management. XFG is a severe type of glaucoma with a higher mean, peak, and fluctuation of untreated 24‐hour intraocular pressure (IOP) than POAG.6,7,8
The initial approach to the medical treatment of a patient with XFG is currently similar to that followed in POAG.2,6 It includes topical prostaglandin F 2α analogues (bimatoprost, latanoprost, and travoprost), or topical β blockers (for example, timolol maleate). However, this therapeutic approach has not been refined specifically for XFG by taking into account the response of this glaucoma to the various drugs. To date there is limited information about the success of the newer drugs specifically in XFG.2,6,9,10,11 A directed treatment for XFG may employ a certain topical drug as the preferred treatment if controlled data indicate a better long term hypotensive response specifically in this type of glaucoma. To compare the IOP‐lowering efficacy and the safety of bimatoprost and latanoprost specifically in XFG we performed a prospective, crossover, observer‐masked, three‐centre investigation.
Material and methods
The research protocol was approved by the Institutional Review Boards for Human Research of the Aristotle University of Thessaloniki, Hacettepe University, and Sam Rothberg Glaucoma Centre, Tel‐Hashomer, Israel, and informed consent was obtained from all participants before they entered the study. Consecutive white patients with XFG were recruited from the glaucoma unit of the “A” University Department of Ophthalmology, AHEPA Hospital, Thessaloniki, Greece; the Department of Ophthalmology, Hacettepe University, Ankara, Turkey; and the Sam Rothberg Glaucoma Centre, Tel‐Hashomer, Israel.
All patients who agreed to participate in the study and met the inclusion and exclusion criteria were enrolled. Inclusion criteria were age between 39 and 85 years; best corrected distance Snellen visual acuity >0.1 in the study eye; early to moderate XFG (glaucomatous disc damage with disc cupping not exceeding 0.7 and/or reproducible glaucomatous visual field loss less than 12.0 dB in the study eye with Humphrey 24‐2 automated perimetry); patient could safely undergo wash out; open anterior chamber angles; untreated baseline IOP between 24 and 38 mm Hg at 1000 hours. Exclusion criteria were evidence of concurrent conjunctivitis, keratitis or uveitis in either eye; active ocular inflammation, history of ocular herpes simplex, or macular oedema; history of inadequate compliance, allergic hypersensitivity, poor tolerance or contraindication to either bimatoprost or latanoprost; intraocular conventional or laser surgery in the study eye; child bearing potential or lactation; previous history of ocular trauma, use of corticosteroids (within 2 months before the enrolment), severe dry eyes, and use of contact lenses.
Procedures
At visit 1 the patients' ophthalmic and systemic history were recorded. Slit lamp biomicroscopy, dilated funduscopy, and automated threshold perimetry were performed, and best‐corrected visual acuity and IOP were measured. Glaucoma drugs were then washed out of qualifying patients with XFG. The washout period was 6 weeks for β blockers, prostaglandin analogues, and fixed combinations; 4 weeks for brimonidine; and 3 weeks for carbonic anhydrase inhibitors.
The baseline visit (visit 2) was performed within 6 weeks from visit 1. At visit 2 IOP was measured at 0800, 1300, and 1800 (±1 hour). For the purposes of this study, mean diurnal IOP and diurnal control represent the average of these three daytime IOP readings. The patients were then randomly assigned to receive bimatoprost or latanoprost once in the evening (2100) for the next 3‐month period (period 1).
At week 3 a safety visit (visit 3) with slit lamp biomicroscopy, morning IOP measurement, and registration of any adverse event was carried out. At the end of period 1 (visit 4) the diurnal IOP was measured and the detailed clinical examination was repeated. Then the patients were switched to the second study drug for period 2, which included a safety visit (visit 5) at week 3, and a final visit with diurnal IOP measurement at month 3 (visit 6). IOP was measured by the same investigators in sitting position using the same calibrated Goldmann tonometer at each site. Non‐responders were defined as those with a mean diurnal IOP decrease of ⩽20% at week 12 for either study period. During the study the investigators were masked to the treatment regimen. Drug labels were removed and the drugs were kept in opaque medicine vials. The patients were aware only of the coloured bottle cap of the study treatment.
Statistics
The primary efficacy variable was the mean diurnal IOP at the end of month 3 for each drug. The secondary efficacy variables were the level of IOP at each time point, the reduction of IOP from untreated baseline for each time point, respectively as well as for the diurnal IOP, the responder rate, and the target IOP obtained. The study had a 90% power to identify a 1.0 mm Hg difference between individual time points and between mean diurnal IOP values, assuming a standard deviation of 3.3 mm Hg between treatments if 118 patients with XFG completed the trial. In patients where both eyes qualified one eye was randomly selected for the study; hence one eye for each patient was analysed for the efficacy analysis. Wilcoxon's signed rank test was used for comparing the IOP values. The responder rate and the target IOP achieved with each drug were evaluated with McNemar's test and Bowker's test. The significance level was set at 5%. Adverse events were evaluated with McNemar's test.
Results
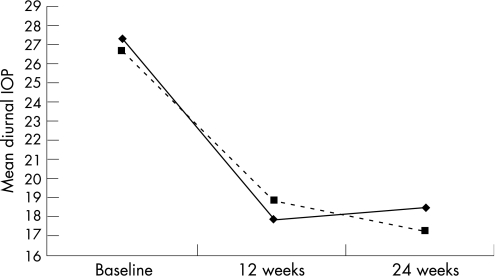
Sixty four patients with XFG were randomised to treatment with bimatoprost in period 1 and then with latanoprost in period 2, whereas 65 patients were randomised to the opposite sequence of treatment. One hundred and twenty three of the 129 enrolled patients with XFG (mean (SD) age 66.5 (8.3) years) completed this 6‐month study. Treatment of two patients was discontinued owing to intolerance to bimatoprost and four patients were lost to follow up. At baseline untreated IOP (mean (SD)) was 28.0 (4.0), 26.9 (3.6), and 25.9 (3.6) mm Hg at 0800, 1300, and 1800 time points, respectively, and the mean diurnal IOP was 26.9 (3.5) mm Hg (table 1). Both bimatoprost and latanoprost decreased IOP significantly compared with the baseline pressure at each time point throughout the study period (p<0.0001 for each comparison) and for the diurnal IOP (35% reduction with bimatoprost v 31% reduction for latanoprost). The IOP decrease was independent of the treatment order for both bimatoprost (p = 0.772) and latanoprost (p = 0.088). Bimatoprost provided significantly greater IOP lowering than latanoprost at each time point and for the diurnal mean IOP (table 1). The mean IOP difference ranged between 0.8 and 1.1 mm Hg (table 1; p<0.001 for each comparison). When latanoprost treatment was followed by bimatoprost treatment (fig 1), the mean diurnal IOP was further reduced by 1.23 (0.27) mm Hg (p<0.0001). With the opposite treatment order, (that is, when patients switched from bimatoprost to latanoprost; fig 1) the IOP increased significantly by 0.69 (0.30) mm Hg (p = 0.042).
Table 1 Intraocular pressure at baseline and at the end of each 3‐month treatment period with latanoprost or bimatoprost.
| Time | Baseline | Latanoprost | Bimatoprost | IOP difference | p Value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Mean | Median | SD | No | Mean | Median | SD | No | Mean | Median | SD | No | Mean | Median | SD | ||
| 0800 | 129 | 28.0 | 26.0 | 4.0 | 125 | 18.7 | 18.0 | 3.6 | 124 | 17.9 | 17.0 | 3.4 | 123 | 0.8 | 1.0 | 2.5 | 0.0009 |
| 1300 | 129 | 26.9 | 26.0 | 3.6 | 125 | 18.5 | 18.0 | 3.6 | 124 | 17.3 | 17.0 | 3.3 | 123 | 1.1 | 1.0 | 2.3 | <0.0001 |
| 1800 | 129 | 25.9 | 25.0 | 3.6 | 125 | 18.6 | 18.0 | 4.1 | 124 | 17.6 | 18.0 | 3.5 | 123 | 1.0 | 1.0 | 3.0 | <0.0001 |
| Diurnal mean | 129 | 26.9 | 25.7 | 3.5 | 125 | 18.6 | 18.0 | 3.6 | 124 | 17.6 | 17.3 | 3.3 | 123 | 1.0 | 1.0 | 2.3 | <0.0001 |
*Wilcoxon signed rank test.
Figure 1 Intraocular pressure reduction with bimatoprost and latanoprost. The changes after the switch are significant (p<0.05): bimatoprost to latanoprost (solid line); latanoprost to bimatoprost (dashed line).
A lower mean diurnal IOP was more frequently obtained with bimatoprost (table 2, p = 0.001). The mean diurnal IOP was <17 mm Hg in 55 patients with XFG (45%) at the end of the bimatoprost treatment period compared with 34 patients with XFG (28%) at the end of the latanoprost period. In contrast, there was a trend for IOP values between 17 and 19 mm Hg and >19 mm Hg to be more common during the latanoprost treatment phase (51 v 40 and 38 v 20 patients, respectively), but this was not significant (p>0.05). Treatment order had no influence on this outcome (p = 0.122). Few patients with XFG responded with a <20% IOP reduction (18/123 (14.6%)), but there were fewer non‐responders with bimatoprost (five (4%) patients) than with latanoprost (13 (10.5%) patients); (p = 0.021).
Table 2 Distribution of IOP target values achieved with latanoprost and bimatoprost monotherapy at the end of each 3‐month period.
| Latanoprost 0.005% | Bimatoprost 0.03% | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean diurnal IOP (mm Hg) | <17 | 17–<19 | 19–<21 | ⩾21 | <17 | 17–<19 | 19–<21 | ⩾21 |
| Number of patients | 34 | 39 | 25 | 27 | 55 | 30 | 20 | 19 |
No serious adverse events were detected in this study. More patients reported at least one adverse event with bimatoprost than with latanoprost (65 v 41 patients at 3 weeks, p = 0.0003, and 58 v 34 patients at 3 months; p = 0.0005). At week 3 the number of adverse events was independent of the treatment sequence (p = 0.46). At the end of the 3‐month treatment period conjunctival hyperaemia and hypertrichosis were more common with bimatoprost than with latanoprost (32 v 9, p = 0.0002 and 14 v 2, p = 0.003, respectively). No significant difference was detected between the two treatments in the incidence of the other adverse events (lid dermatitis, itching, stinging, ocular pain, foreign body sensation, blurred vision, headache, change of iris colour, and periocular pigmentation). The number of adverse events at the end of the trial was independent of the treatment order (p = 0.851).
Discussion
In the current crossover trial we attempted to evaluate latanoprost versus bimatoprost specifically in patients with XFG. To the best of our knowledge, the current trial is the first crossover study assessing diurnal IOP reduction with these two drugs in XFG and the second comparative crossover study of these drugs in glaucoma. A previous, smaller crossover 24‐hour study in POAG12 showed a smaller, but statistically significant mean IOP difference (0.6 mm Hg) between the two drugs. Our current, larger crossover study showed that both treatments provided a statistically significant reduction in IOP from untreated baseline at each individual time point and for the mean diurnal curve (35% reduction with bimatoprost v 31% reduction for latanoprost). Direct comparison of the treatment groups showed a greater reduction with bimatoprost for all time points and for the diurnal curve after 3 months of treatment as well as significantly fewer non‐responders and significantly more eyes reaching a target diurnal IOP of <17 mm Hg.
Of interest in the present crossover study was the incremental IOP lowering when patients with XFG receiving latanoprost were switched to bimatoprost. These patients achieved a further, statistically significant IOP reduction (1.2 mm Hg). In a recent, retrospective analysis of 309 patients, mainly with POAG, switched from latanoprost to bimatoprost a small, but statistically significant, mean reduction of 0.5 mm Hg was also detected.13 These results underline the need for further controlled studies to elucidate the differences in the clinical profile and the mechanism of action of these two popular drugs in XFG and other glaucomas.
The differences in the IOP levels between treatment groups seen in the current crossover study are greater than those previously observed by other investigators in patients with POAG, except for the parallel study by Noecker and coworkers.14 The reason for the greater differences in XFG is not known. Possibly, the higher IOP in XFG allows better separation between the two drugs. It is also conceivable that bimatoprost may be more effective in XFG. More information is needed in the future to elucidate potential differences in ocular absorption between bimatoprost and latanoprost.
This study indicates that the mean diurnal IOP for all time points and for the mean diurnal pressure was statistically lower by 1.0 mm Hg in XFG with bimatoprost than with latanoprost after 3 months of chronic treatment. It is still not clear what diurnal IOP difference is needed to impact long term prognosis in glaucoma. However, the Early Manifest Glaucoma Trial previously established that a 1 mm Hg difference was associated with reduction of glaucoma progression by 10%.15 Consequently, ophthalmologists must decide for their own practice what value constitutes a clinically important IOP difference between two glaucoma drugs.
In this 3‐month crossover trial no serious adverse events occurred, but a higher incidence of adverse events was seen with bimatoprost treatment. More patients with XFG reported at least one adverse event with bimatoprost than with latanoprost, and the number of adverse events was independent of the treatment order. A higher incidence of conjunctival hyperaemia and eyelash changes was found with bimatoprost. This finding is consistent with previous trials, which showed an approximately 34–45% incidence of conjunctival hyperaemia with bimatoprost and 5–15% with latanoprost.12,14,16,17,18,19,20 Because patients with intolerance to the study drugs were excluded, the real incidence of adverse reactions is likely to be higher than that reported in this study.
The diurnal curve measurements in the present study were limited to three time points from 0800 until 1800. Therefore, the 24‐hour IOP control with these two drugs remains to be elucidated. Previous studies have highlighted the worse 24‐hour IOP characteristics of patients with XFG.21,22 This 3‐month study did not evaluate the long term efficacy and safety of latanoprost and bimatoprost in XFG. The initial and stepwise approach to the treatment of a patient with XFG is currently similar to POAG. Conceivably, however, future medical treatment in XFG may differ from that employed in POAG
Conclusion
The results of this crossover study show that in XFG bimatoprost obtains a significantly lower mean diurnal IOP than latanoprost (1.0 mm Hg), but the incidence of conjunctival hyperaemia is higher with bimatoprost. Bimatoprost may be a future directed treatment of choice in XFG, if data can be provided for a better long term, as well as short term, hypotensive response in patients with XFG.
Acknowledgements
We thank Mikael Astrom, PhD, for assistance with the data analysis.
Abbreviations
IOP - intraocular pressure
POAG - primary open angle glaucoma
XFG - exfoliative glaucoma
Footnotes
Sponsorship: This study was supported in part by an unrestricted grant from Allergan.
Competing interests: AGP Konstas is a consultant of Allergan, Alcon, Pfizer, MSD; M Irkec and S Melamed are consultants of Allergan; G Holló is a consultant of Allergan, Alcon, and Pfizer; M Goldenfeld, S Tsironi, I Durukan: none.
Ethics approval (see also “Methods” section): The research protocol was approved by the Institutional Review Board for Human Research of the Aristotle University of Thessaloniki, Hacettepe University and the Sam Rothberg Glaucoma Centre, Tel‐Hashomer, Israel. Informed consent was obtained from all participants before they entered the study.
References
- 1.Ritch R, Schlötzer‐Schrehardt U, Konstas A G P. Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res 200322253–275. [DOI] [PubMed] [Google Scholar]
- 2.Konstas A G P, Tsironi S, Ritch R. Update on the pathogenesis and management of exfoliation syndrome and exfoliative glaucoma. Compr Ophthalmol Update 20067131–141. [PubMed] [Google Scholar]
- 3.Ritch R, Schlötzer‐Schrehardt U. Exfoliation syndrome. Surv Ophthalmol 200145265–315. [DOI] [PubMed] [Google Scholar]
- 4.Konstas A G P, Stewart W C, Stroman G A. Clinical presentation and initial treatment patterns in patients with exfoliation glaucoma versus primary open‐angle glaucoma. Ophthalmic Surg Lasers 199728111–117. [PubMed] [Google Scholar]
- 5.Konstas A G P, Holló G, Astakhov Y.et al Factors associated with long‐term progression or stability in exfoliation glaucoma. Arch Ophthalmol 200412229–33. [DOI] [PubMed] [Google Scholar]
- 6.Konstas A G, Mylopoulos N, Karabatsas C H.et al Diurnal intraocular pressure reduction with latanoprost 0.005% compared to timolol maleate 0.5% as monotherapy in subjects with exfoliation glaucoma. Eye 200418893–899. [DOI] [PubMed] [Google Scholar]
- 7.Konstas A G P, Kozobolis V P, Tersis I.et al The efficacy and safety of the timolol/dorzolamide fixed combination vs latanoprost in exfoliation glaucoma. Eye 20031741–46. [DOI] [PubMed] [Google Scholar]
- 8.Konstas A G P, Maltezos A, Bufidis T.et al Twenty‐four hour control of intraocular pressure with dorzolamide and timolol maleate in exfoliation and primary open‐angle glaucoma. Eye 20001473–77. [DOI] [PubMed] [Google Scholar]
- 9.Heijl A, Strahlman E, Sverrisson T.et al A comparison of dorzolamide and timolol in patients with pseudoexfoliation and glaucoma or ocular hypertension. Ophthalmology. 1997;104137–142. [DOI] [PubMed]
- 10.Konstas A G P, Kozobolis V P, Katsimpris I E.et al Efficacy and safety of latanoprost versus travoprost in exfoliative glaucoma patients. Ophthalmology. (in press) [DOI] [PubMed]
- 11.Konstas A G P, Tsironi S, Kozobolis V P. Medical therapy of exfoliative glaucoma. In: In: Konstas AGP, Hollo G, eds. From exfoliation syndrome to exfoliative glaucoma. EGS book. (in press)
- 12.Konstas A G P, Katsimbris J M, Lallos N.et al Latanoprost 0.005% versus bimatoprost 0.03% in primary open‐angle glaucoma patients. Ophthalmology 2005112262–266. [DOI] [PubMed] [Google Scholar]
- 13.Law S K, Song B J, Fang E.et al Feasibility and efficacy of a mass switch from latanoprost to bimatoprost in glaucoma patients in a prepaid health maintenance organization. Ophthalmology 20051122123–2130. [DOI] [PubMed] [Google Scholar]
- 14.Noecker R S, Dirks M S, Choplin N T.et al A six‐month randomized clinical trial comparing the intraocular pressure‐lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol 200313555–63. [DOI] [PubMed] [Google Scholar]
- 15.Heijl A, Leske M C, Bengtsson B.et al Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 20021201268–1279. [DOI] [PubMed] [Google Scholar]
- 16.DuBiner H, Cooke D, Dirks M.et al Efficacy and safety of bimatoprost in patients with elevated intraocular pressure: a 30‐day comparison with latanoprost. Surv Ophthalmol 200145S353–S360. [DOI] [PubMed] [Google Scholar]
- 17.Gandolfi S, Simmons S T, Sturm R.et al Three‐month comparison of bimatoprost and latanoprost in patients with glaucoma and ocular hypertension. Adv Ther 200118110–121. [DOI] [PubMed] [Google Scholar]
- 18.Coleman A L, Lerner F, Bernstein P.et al A 3‐month randomized controlled trial of bimatoprost (Lumigan) versus combined timolol and dorzolamide (Cosopt) in patients with glaucoma or ocular hypertension. Ophthalmology 20031102362–2368. [DOI] [PubMed] [Google Scholar]
- 19.Easthope S E, Perry C M. Topical bimatoprost: a review of its use in open‐angle glaucoma and ocular hypertension. Drugs Aging 200219231–248. [DOI] [PubMed] [Google Scholar]
- 20.Parrish R K, Palmberg P, Sheu W P.et al A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12‐week, randomized, masked‐evaluator multicenter study. Am J Ophthalmol 2003135688–703. [DOI] [PubMed] [Google Scholar]
- 21.Konstas A G P, Matziris D A, Stewart W C. Diurnal intraocular pressure in untreated exfoliation and primary open‐angle glaucoma. Arch Ophthalmol 1997115111–117. [DOI] [PubMed] [Google Scholar]
- 22.Konstas A G, Matziris D A, Gate E A.et al Effect of timolol on the diurnal intraocular pressure in exfoliation and primary open‐angle glaucoma. Arch Ophthalmol 1997115975–979. [DOI] [PubMed] [Google Scholar]