Abstract
Aim
To determine the prevalence of glaucoma in the Meiktila district of central, rural Myanmar.
Methods
A cross‐sectional, population‐based survey of inhabitants ⩾40 years of age from villages in Meiktila district, Myanmar, was performed; 2481 eligible participants were identified and 2076 participated in the study. The ophthalmic examination included Snellen visual acuity, slit‐lamp examination, tonometry, gonioscopy, dilated stereoscopic fundus examination and full‐threshold perimetry. Glaucoma was classified into clinical subtypes and categorised into three levels according to diagnostic evidence.
Results
Glaucoma was diagnosed in 1997 (80.5%) participants. The prevalence of glaucoma of any category in at least one eye was 4.9% (95% CI 4.1 to 5.7; n = 101). The overall prevalence of primary angle‐closure glaucoma (PACG) was 2.5% (95% CI 1.5 to 3.5) and of primary open‐angle glaucoma (POAG) was 2.0% (95% CI 0.9 to 3.1). PACG accounted for 84% of all blindness due to glaucoma, with the majority due to acute angle‐closure glaucoma (AACG).
Conclusion
The prevalence of glaucoma in the population aged ⩾40 years in rural, central Myanmar was 4.9%. The ratio of PACG to POAG was approximately 1.25:1. PACG has a high visual morbidity and AACG is visually devastating in this community. Screening programmes should be directed at PACG, and further study of the underlying mechanisms of PACG is needed in this population.
Glaucoma is the second most common cause of world blindness, and the majority of those blinded reside in Asia.1,2 Recent studies have provided valuable information about the prevalence and subtypes of glaucoma in certain Asian regions,3,4,5,6,7,8,9,10,11,12 and it has become recognised that angle‐closure glaucoma is more common in people of Asian origin than those with European or African ethnicity5,13,14,15,16; however, the relative rates of open‐angle to closed‐angle glaucoma are region‐dependent within Asia, with the rate of primary angle‐closure glaucoma (PACG) particularly high in Mongolian and Chinese eyes,5,8,17 and variable across India.6,10,11,12,18 In accordance with the World Health Organization's (WHO's) Vision 20/20 initiative, the assessment of the prevalence of glaucoma subtypes is important because it has implications for the optimisation of screening programmes and treatment strategies.19,20,21,22,23
WHO estimates of the prevalence of glaucoma in many Asian regions are crude. Limited WHO data24 and anecdotal evidence suggested high rates of angle‐closure glaucoma in the Union of Myanmar (Myanmar; formerly Burma). Until now, no robust population‐based data have been available on the prevalence and subtypes of glaucoma in Myanmar. Here, we report on the prevalence and subtypes of glaucoma in the inhabitants of the rural, central region of this country.
Methods
Sampling procedure
The Meiktila Eye Study (MES) was a population‐based, cross‐sectional ophthalmic survey of the inhabitants of rural villages in central Myanmar. The principal aims of this project were to estimate the prevalence and causes of visual impairment, and the prevalence and risk factors of ocular disorders, including glaucoma, among persons ⩾40 years of age in this region.
The study was conducted within the Mandalay Division, an area encompassing 34 253 km2 divided into seven second‐order administrative districts of approximately equal size. The township of Meiktila (population approximately 251 000), located at 20°53′N, 95°53′ E, lies centrally in the Meiktila District, and is the only urban region in this entire district. The District is arbitrarily divided by the Ministry of Health (MOH) into six zones served by a centrally located eye hospital in Meiktila.
Participants were selected using a randomised, stratified, cluster sampling process. A sampling frame consisting of a list of all villages in the Meiktila District along with their populations was obtained from the MOH. Villages were arbitrarily stratified as large (population >825) or small (population ⩽825), with small villages in each of the six zones within the Meiktila District constituting six separate strata. For logistical reasons, sampling was restricted to villages within 3 h drive from Meiktila (an area encompassing approximately 80% of the district). All persons aged ⩾40 years from each selected village were eligible for inclusion. The sample size was based on the desired precision of the estimate of blindness (the principal aim of the MES); the assessment of glaucoma prevalence was a secondary objective. Healthcare workers from Meiktila township enumerated the selected villages (and advertised and promoted the survey) before commencement of the survey. Six small villages (one from each zone) and four large villages were enumerated, providing a total sample population of 2481 people.
Data collection
Data collection was performed at the end of the rainy season in November 2005. A single survey team conducted the entire study. Each team member was assigned specific tasks and was well trained in the appropriate area. Specific observations were done by 1–2 members, limiting or eliminating interobserver variability. All equipment and personnel were transported to each village, and the data collection was performed on site. A medical and ophthalmic history was obtained from each patient in his or her own language by qualified healthcare workers. Each participant then received a comprehensive vision and eye examination.
Box 1 Diagnostic criteria for glaucoma
Category 1 diagnosis (structural and functional evidence): eyes with a cup:disc ratio (CDR) >97.5th centile for the normal (non‐glaucomatous) population (CDR ⩾0.7 was used on the basis of the data from previous studies in the region), or a CDR ⩾0.6 in the presence of asymmetry ⩾0.3 or a neuroretinal rim width reduced to <0.1 CDR (between 11:00 and 13:00 or between 17:00 and 19:00 h) and a definite visual field defect consistent with glaucoma. Eyes with evidence of previous acute angle‐closure glaucoma (AACG) which had no perception of light (NPL) were also classified as category 1, even if the optic disc was not visualised (“end‐stage” AACG).
Category 2 diagnosis (advanced structural damage with unproved field loss): if the subject could not satisfactorily complete visual field testing, but had a CDR >99.5th centile for the normal (non‐glaucomatous) population (CDR ⩾0.8 was used on the basis of the data from the normal population in this study), glaucoma was diagnosed solely on the basis of structural evidence.
Category 3 diagnosis (optic disc not seen; field test impossible): if it was not possible to examine the optic disc, glaucoma was diagnosed if: (A) the visual acuity (VA) was <3/60 and the intraocular pressure >99.5th centile; or (B) the VA was <3/60 and the eye showed evidence of glaucoma‐filtering surgery.
Visual acuity (VA) was tested unaided, and with a pinhole using a well‐illuminated Snellen chart at 6 m. Intraocular pressure (IOP) was measured with a Goldmann applanation tonometer (Haag‐Streit, Koeniz, Switzerland) and anterior segment examination was performed using a slit lamp. The presence of previous iris ischaemia or pseudoexfoliation was recorded. Gonioscopy was performed by two experienced ophthalmologists using a Sussman goniolens. Static gonioscopy was performed in dim illumination with minimal pressure on the cornea using a short slit beam; each quadrant was graded using the Scheie classification. If >90° of posterior trabecular meshwork (TM) was visible, the pupil was dilated with tropicamide 1% and phenylephrine 2.5%. Eyes with ⩽90° of posterior TM visible were deemed “occludable” and dilated with tropicamide 0.5% only and kept under observation for 4 h; if not possible, they were not dilated. If either eye had evidence of previous acute angle‐closure glaucoma (AACG; see definition below), then neither eye was dilated. Optic disc and retinal examination was performed by two experienced ophthalmologists using a 78 D lens and reference to standard disc images. The vertical cup:disc ratio (CDR) and the presence of focal notching were recorded. The agreement between the two ophthalmologists was good for grading the occludability (κ) = 0.78 and determining the CDR (κ) = 0.72.
Eyes with VA >6/60, and which fulfilled category 1 optic disc criteria (see below), underwent full‐threshold perimetry (C‐20 strategy) using frequency doubling technology (FDT; Zeiss Humphrey Systems, Dublin, California, USA). Tests were considered reliable if there were <20% fixation errors and <33% false‐positive and false‐negative errors. All individuals were naïve to perimetry and received instruction in their own language, followed by a practice in the demonstration mode. If the initial test was unreliable, individuals were given a second attempt. More than one missed point on the pattern deviation was considered abnormal.25
Ethics
The MES was approved by the MOH in Myanmar and had ethical approval from the Royal Adelaide Hospital Ethics Committee. Consent for participation was obtained from the head of each village before commencement of the survey, and written, informed consent, in the participant's own language, was obtained from all willing participants. The study was conducted in accordance with the Declaration of Helsinki.
Statistics
Prevalence rates were calculated as ratio estimates using appropriate weights for each of the sampled villages. Bootstrapping was used to overcome the problem of variance estimation in clusters where only the one primary sampling unit (village) was selected. All prevalence estimates were calculated using SAS V.9.1. Villages were randomly selected; hence, point prevalences are unbiased.
Definitions
A three‐tiered system of evidence, as suggested by the International Society for Geographic and Epidemiological Ophthalmology (ISGEO),26 was used to categorise glaucoma (box 1).
Blindness due to glaucoma was defined as an eye with pinhole vision <3/60; fields were not taken into consideration.
Prevalence was calculated on an individual rather than a per eye basis. If at least one eye was diagnosable using the above criteria, the subject was included in the prevalence analysis. In those participants with only one diagnosable eye, if glaucoma was not present in this eye, they were assumed not to have glaucoma in the undiagnosable eye.
Glaucoma was also categorised into three principal clinical subtypes:
PACG was diagnosed if the criteria for category 1–3 were met, ⩽90° of posterior TM was visible with static gonioscopy and no secondary cause for glaucoma was present. PACG was further subdivided into acute and chronic forms. Chronic PACG was diagnosed if the above criteria were met, and acute PACG was diagnosed if the above criteria were met and there was evidence of previous iris ischaemia (defined as the presence of iris whorling or stromal atrophy).27 Historical evidence only of an attack of AACG was considered insufficient for diagnosis.
Primary open‐angle glaucoma (POAG) was diagnosed if the criteria for category 1–3 were met, >90° of posterior TM was visible on static gonioscopy and no secondary cause for glaucoma was present.
Secondary glaucoma was diagnosed if the criteria for category 1–3 were met and a secondary cause was evident. This included pseudoexfoliative and neovascular glaucoma.
Results
A total of 2481 participants were eligible and 2076 were examined (836 men, 1240 women; participation rate 83.7%). The mean age was 56.2 years. Sufficient examination data to diagnose glaucoma (as defined above) in at least one eye were obtained from 1997 participants. There were 110 participants in whom glaucoma was diagnosable in one eye only. The mean IOP and CDR for the normal (non‐glaucomatous) population are shown in tables 1 and 2, respectively. There were seven eyes which had end‐stage AACG and were classified in category 1.
Table 1 Intraocular pressure in normal participants*.
| Right IOP (mm Hg) (95% CI) | Left IOP (mm Hg) (95% CI) | |
|---|---|---|
| Number of measurements | 1952 | 1953 |
| Mean | 14.8 (14.65 to 14.95) | 14.9 (14.75 to 15.05) |
| Median | 14 | 15 |
| 97.5th centile | 21.7 (21.55 to 21.85) | 21.9 (21.75 to 22.05) |
| 99.5th centile | 25.0 (24.85 to 25.15) | 25.4 (25.25 to 25.55) |
IOP, intraocular pressure.
*Subjects had neither structural nor functional evidence of glaucomatous optic neuropathy.
Table 2 Vertical cup:disc ratio in normal participants*.
| Right CDR | Left CDR | |
|---|---|---|
| Number of measurements | 1850 | 1852 |
| Mean | 0.34 | 0.34 |
| Median | 0.3 | 0.3 |
| 97.5th centile | 0.64 | 0.66 |
| 99.5th centile | 0.79 | 0.82 |
CDR, cup:disc ratio.
*Defined as those with available vertical CDR data and excluding eyes with definitive glaucomatous field defect.
The prevalence of glaucoma (allowing for the study design) in any category in at least one eye was 4.9% (95% CI 4.1 to 5.7; n = 101 participants). There were 156 eyes of 101 participants which met the ISGEO three‐tiered evidence‐based classification of glaucoma: 51 eyes were in category 1, 73 were in category 2 (perimetry not performed or unreliable) and 32 were in category 3. Of the 73 eyes in category 2, 46 did not meet the VA criteria (VA >6/60) and the remainder could not perform reliable (as defined above) perimetry by the second attempt. Seven eyes met the structural definitions of criteria 1, but did not meet the VA standard for perimetry or could not perform reliable perimetry, and were deemed non‐glaucomatous. Only three eyes were classified as glaucomatous on the basis of CDR asymmetry.
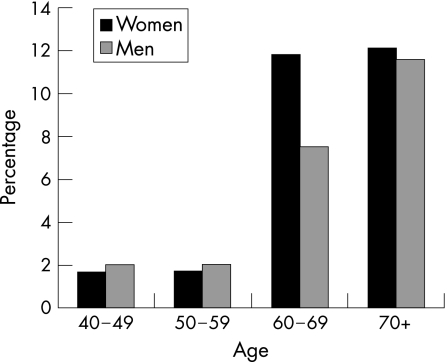
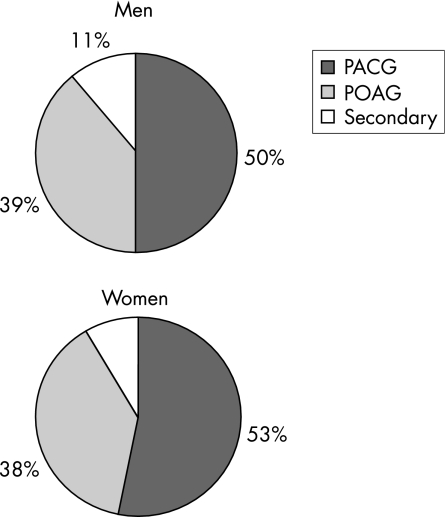
The prevalence of glaucoma increased with age in both men and women (fig 1). Figure 2 shows the distribution of PACG, POAG and secondary glaucoma in men and women. The overall prevalence of PACG was 2.5% (95% CI 1.5 to 3.5) and of POAG was 2.0% (95% CI 0.9 to 3.1). In all, 22 (1.1%) participants had AACG in at least one eye and 30 (1.5%) had CACG. There were 10 (0.5%) participants with secondary glaucoma in at least one eye: 5 eyes with pseudoexfoliative, 3 with uveitic and 2 with neovascular glaucoma.
Figure 1 Prevalence of glaucoma by age and gender.
Figure 2 Distribution of clinical glaucoma subtypes by gender.
There were 32 eyes blinded by PACG, accounting for 84% of all eyes with blindness due to glaucoma. AACG was the cause of blindness in 20 eyes, and CACG in 12 eyes. Eight participants were bilaterally blind due to AACG and three due to CACG. Only three eyes were blind due to POAG and four due to secondary glaucoma.
The use of mydriatics, as per the protocol, produced no adverse events.
Discussion
This study provides the first population‐based data about the prevalence and subtypes of glaucoma in Myanmar. Data relating to the prevalence of “occludable” angles (angle‐closure glaucoma suspects) are not presented in this report. The most striking finding was the high prevalence of PACG in this population. The ratio of PACG to POAG was 1.25:1, which is lower than the 3:1 ratio reported in a Mongolian population,5 but almost twice that reported in Chinese eyes,3,8 and considerably greater than the ratios reported in populations from India and Bangladesh (table 3).6,7 Discerning the relative amounts of PACG to POAG is important because it has profound implications in the optimisation of screening and treatment strategies: a greater prevalence of PACG, coupled with its high visual morbidity, implies that more resources should be directed towards it. A directed screening programme involving Van Herick grading, gonioscopy and laser iridotomy has been highly successful in reducing angle‐closure glaucoma in the Inuit and is undergoing evaluation in Mongolia.19 However, evidence is emerging which suggests that the mechanism of angle closure in certain regions of Asia, including South‐East Asia, may be multifactorial, involving pupillary block and non‐pupillary block components.19,28
Table 3 Prevalence of glaucoma in various Asian populations.
| Author (year) | Location | Number | Age group (years) | Prevalence of PACG | Prevalence of POAG | Ratio POAG:PACG |
|---|---|---|---|---|---|---|
| He et al,3 2006 | Liwan, Guangzhou | 1405 | ⩾50 | 1.5% | 2.1% | 0.71:1 |
| Vijaya et al,4,10 2005, 2006 | Chennai, India | 3924 | ⩾40 | 0.87% | 1.62% | 0.54:1 |
| Raychaudhuri et al,6 2005 | Calcutta, India | 1269 | ⩾50 | 0.72% | 3.6% | 0.2:1 |
| Rahman et al,7 2004 | Dhaka, Bangladesh | 2346 | ⩾35 | 0.4% | 2.5% | 0.16:1 |
| Foster et al,8 2000 | Tanjong Pagar, Singapore | 1232 | 40–79 | 1.13% | 1.78% | 0.63:1 |
| Dandona et al,11,12 2000 | Hyderabad, India | 1399 | ⩾40 | 1.08% | 2.56% | 0.41:1 |
| Present study | Meiktila, Myanmar | 1997 | ⩾40 | 2.5% | 2.0% | 1.25:1 |
PACG, primary angle‐closure glaucoma; POAG, primary open‐angle glaucoma.
Although we recognise that consistency among epidemiological studies is important and have modelled this study, as much as practically possible, on similar studies from this region,3,6,7,8 we chose to slightly modify the ISGEO inclusion criteria: we included eyes with no perception of light (NPL; with evidence of old AACG) in which the optic disc was not seen, irrespective of the IOP, and which did not meet the criteria for classification in the current ISGEO system. In our opinion, these eyes have evidence of severe functional and implied structural optic neuropathy secondary to an old acute IOP increase and warrant classification in category 1. However, even if these eyes were excluded from the analysis, the overall prevalence (4.7%) is minimally affected.
The prevalence of glaucoma in this study is a little higher than prevalence rates reported in other population‐based studies from Asia3,5,6,7,8,9 (table 3). This may partly relate to the use of FDT perimetry, with relatively high sensitivity, and to the inclusion of eyes with old AACG. However, it may simply reflect a particularly high rate of PACG in this population.
Most of those eyes that were not adequately examined for glaucoma, had dense cataracts. Some of these eyes could also have had glaucoma so that the overall estimate for the prevalence of glaucoma (4.9%) may, in fact, be conservative. The 97.5th centile for the CDR in the normal population was approximately 6.5; however, based on recent data from this region a CDR of ⩾0.7 was chosen as the cut‐off for field testing; hence, early glaucoma with concentric cupping could have been missed. It is also likely that many of the participants classified as having PACG actually had combined‐mechanism glaucoma; however, relationships between the amount of angle closure and the IOP, which may arouse suspicion of a combined mechanism, were not taken into consideration in this study.
The optimal method of perimetry for studies of this nature conducted “in the field” is unclear. We chose to use FDT because of its availability, portability, relative impunity to defocus,29 ease of use and recent use in a similar population‐based study in India.4,10 The current study was designed to detect glaucoma based on ISGEO criteria; hence, perimetry was only designated for those participants at least meeting the category 1 structural criteria. An arbitrary VA cut‐off for perimetry was set at presenting Snellen acuity >6/60. Even at this level, almost 63% of eyes in category 2 were not eligible for perimetry, hence the relatively low rate (25%) of reliable perimetric data on the population meeting other diagnostic criteria. Previous similar studies4,10 using FDT had set the VA limit at 6/24; however, given that the FDT sensitivity suffers little from up to 6 D of defocus,29 and the high prevalence of visual impairment in this population, our VA criterion seems reasonable. Arguably, the low rate of reliable perimetric data casts doubt on the prevalence of glaucoma; however, the ISGEO guidelines for the diagnosis of glaucoma are deliberately weighted towards structural changes, because it is well recognised that reliable perimetry in population‐based studies, particularly in the developing world, is difficult.
Although the participation rate was relatively high (83%), we have no robust data about the visual status and ocular health of the non‐participants. Anecdotally (according to the village chiefs), the principal reason for non‐participation was occupation‐related; hence, it is unlikely that any of the non‐participants were glaucoma blind, suggesting that the prevalence of glaucoma in this group would be lower than in the participants. Although accurate data about the gender distribution in the Meiktila district were not available, it is likely that women were over‐represented in this study (59%), a common occurrence among similar studies, possibly reflecting occupation‐related availability.
The Meiktila District was chosen for logistical reasons, not randomly, and may not be representative of neighbouring regions within the Mandalay Division of central Myanmar; however, we have no reason to believe that this is the case.
In conclusion, the prevalence of glaucoma in the population ⩾40 years of age in the Meiktila District of rural, central Myanmar is 4.9%. The ratio of PACG to POAG is approximately 1.25:1. Given the high visual morbidity of PACG, screening programmes should be directed at this disease and further study of the underlying mechanisms of PACG in this population is needed.
Acknowledgements
We thank the Myanmar Ministry of Health for their invaluable assistance with this survey. This study was possible only due to the support of the staff at the Yangon Eye Hospital and Meiktila Eye Hospital. Expert assistance was provided by Drs Tun Aung Kyaw and Nyunt Maung from the Trachoma Control and Prevention of Blindness Program. Thanks to Paul Foster for generous and expert advice concerning the methodology and technical aspects. Australian Ambassador to Yangon, Bob Davis, provided valuable assistance and donated a slit lamp to the Meiktila Eye Hospital. We thank Alcon Australia for the loan of equipment. The sole financial support was provided by a generous grant from Pfizer Ophthalmic. We thank the people who participated in this study and who welcomed us so kindly into their villages.
Abbreviations
AACG - acute angle‐closure glaucoma
CDR - cup/disc ratio
FDT - frequency doubling technology
IOP - intraocular pressure
ISGEO - International Society for Geographic and Epidemiological Ophthalmology
MES - Meiktila Eye Study
MOH - Ministry of Health
PACG - primary angle‐closure glaucoma
POAG - primary open‐angle glaucoma
TM - trabecular meshwork
VA - visual acuity
WHO - World Health Organization
Footnotes
Funding: The survey was funded by a grant from Pfizer Ophthalmic. The design of the survey, its execution, analysis, interpretation and publication were carried out independently by the authors.
Competing interests: None.
References
- 1.Quigley H A, Broman A T. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 200690262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya'ale D.et al Global data on visual impairment in the year 2002. Bull World Health Organ 200482844–851. [PMC free article] [PubMed] [Google Scholar]
- 3.He M, Foster P J, Ge J.et al Prevalence and clinical characteristics of glaucoma in adult Chinese: a population‐based study in Liwan district, Guangzhou. Invest Ophthalmol Vis Sci 2006472782–2788. [DOI] [PubMed] [Google Scholar]
- 4.Vijaya L, George R, Arvind H.et al Prevalence of angle‐closure disease in a rural southern Indian population. Arch Ophthalmol 2006124403–409. [DOI] [PubMed] [Google Scholar]
- 5.Foster P J, Baasanhu J, Alsbirk P H.et al Glaucoma in Mongolia. A population‐based survey in Hovsgol province, northern Mongolia. Arch Ophthalmol 19961141235–1241. [DOI] [PubMed] [Google Scholar]
- 6.Raychaudhuri A, Lahiri S K, Bandyopadhyay M.et al A population based survey of the prevalence and types of glaucoma in rural West Bengal: the West Bengal Glaucoma Study. Br J Ophthalmol 2005891559–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman M M, Rahman N, Foster P J.et al The prevalence of glaucoma in Bangladesh: a population based survey in Dhaka division. Br J Ophthalmol 2004881493–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster P J, Oen F T, Machin D.et al The prevalence of glaucoma in Chinese residents of Singapore: a cross‐sectional population survey of the Tanjong Pagar district. Arch Ophthalmol 20001181105–1111. [DOI] [PubMed] [Google Scholar]
- 9.Bourne R R, Sukudom P, Foster P J.et al Prevalence of glaucoma in Thailand: a population based survey in Rom Klao District, Bangkok. Br J Ophthalmol 2003871069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijaya L, George R, Paul P G.et al Prevalence of open‐angle glaucoma in a rural south Indian population. Invest Ophthalmol Vis Sci 2005464461–4467. [DOI] [PubMed] [Google Scholar]
- 11.Dandona L, Dandona R, Mandal P.et al Angle‐closure glaucoma in an urban population in southern India. The Andhra Pradesh Eye Disease Study. Ophthalmology 20001071710–1716. [DOI] [PubMed] [Google Scholar]
- 12.Dandona L, Dandona R, Srinivas M.et al Open‐angle glaucoma in an urban population in southern India: the Andhra Pradesh Eye Disease Study. Ophthalmology 20001071702–1709. [DOI] [PubMed] [Google Scholar]
- 13.Salmon J F, Mermoud A, Ivey A.et al The prevalence of primary angle closure glaucoma and open angle glaucoma in Mamre, western Cape, South Africa. Arch Ophthalmol 19931111263–1269. [DOI] [PubMed] [Google Scholar]
- 14.Foster P J. The epidemiology of primary angle closure and associated glaucomatous optic neuropathy. Semin Ophthalmol 20021750–58. [DOI] [PubMed] [Google Scholar]
- 15.Bourne R R, Sorensen K E, Klauber A.et al Glaucoma in East Greenlandic Inuit—a population survey in Ittoqqortoormiit (Scoresbysund). Acta Ophthalmol Scand 200179462–467. [DOI] [PubMed] [Google Scholar]
- 16.Chew P T, Aung T. Primary angle‐closure glaucoma in Asia. J Glaucoma 200110S7–S8. [DOI] [PubMed] [Google Scholar]
- 17.Foster P J, Johnson G J. Glaucoma in China: how big is the problem? Br J Ophthalmol 2001851277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thulasiraj R D, Nirmalan P K, Ramakrishnan R.et al Blindness and vision impairment in a rural south Indian population: the Aravind Comprehensive Eye Survey. Ophthalmology 20031101491–1498. [DOI] [PubMed] [Google Scholar]
- 19.Johnson G J, Foster P J. Can we prevent angle‐closure glaucoma? Eye 2005191119–1124. [DOI] [PubMed] [Google Scholar]
- 20.Aung T, Chew P T. Review of recent advancements in the understanding of primary angle‐closure glaucoma. Curr Opin Ophthalmol 20021389–93. [DOI] [PubMed] [Google Scholar]
- 21.Nolan W P, Aung T, Machin D.et al Detection of narrow angles and established angle closure in Chinese residents of Singapore: potential screening tests. Am J Ophthalmol 2006141896–901. [DOI] [PubMed] [Google Scholar]
- 22.Friedman D S, Gazzard G, Foster P.et al Ultrasonographic biomicroscopy, Scheimpflug photography, and novel provocative tests in contralateral eyes of Chinese patients initially seen with acute angle closure. Arch Ophthalmol 2003121633–642. [DOI] [PubMed] [Google Scholar]
- 23.Saw S M, Gazzard G, Friedman D S. Interventions for angle‐closure glaucoma: an evidence‐based update. Ophthalmology 20031101869–1878. [DOI] [PubMed] [Google Scholar]
- 24.Alsbirk P H.Prevention and control of visual impairment and blindness (with special reference to glaucoma) in Burma. Consultant Report. World Health Organization, Southeast Asia Region/Ophthalmology 1984
- 25.Muskens R P, Heeg G P, Jansonius N M. An evaluation of algorithms designed to classify the results from frequency doubling perimetry. Ophthalmic Physiol Opt 200424498–503. [DOI] [PubMed] [Google Scholar]
- 26.Foster P J, Buhrmann R, Quigley H A.et al The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 200286238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loon S C, Chew P T, Oen F T.et al Iris ischaemic changes and visual outcome after acute primary angle closure. Clin Experiment Ophthalmol 200533473–477. [DOI] [PubMed] [Google Scholar]
- 28.Aung T, Nolan W P, Machin D.et al Anterior chamber depth and the risk of primary angle closure in 2 East Asian populations. Arch Ophthalmol 2005123527–532. [DOI] [PubMed] [Google Scholar]
- 29.Anderson A J, Johnson C A. Frequency‐doubling technology perimetry and optical defocus. Invest Ophthalmol Vis Sci 2003444147–4152. [DOI] [PubMed] [Google Scholar]