Abstract
Aim
To evaluate the predictive factors for visual outcome after intravitreal triamcinolone acetonide injection to treat refractory diabetic macular oedema (DME).
Methods
A retrospective chart review of patients with DME who met the following inclusion criteria was performed: clinically significant diabetic macular oedema, receipt of a 4 mg/0.1 ml intravitreal triamcinolone acetonide injection and an optical coherence tomography (OCT) of the macula performed up to 10 days before injection. All patients received a full ophthalmic examination including best‐corrected Snellen visual acuity (VA). The main outcome measure was the mean change in vision 3 months after injection.
Results
Data from 73 eyes of 59 patients were analysed. After a mean follow‐up of 324 days, the mean change in vision was −0.075 logarithm of minimum angle of resolution (logMAR) units, with 27.3% improving ⩾3 lines, 6.8% declining ⩾3 lines and 60.2% remaining stable within 1 line of baseline vision. Statistical analysis was performed using multivariate generalised estimating equations on the basis of data from 52 eyes of 42 patients. Factors associated with an improvement in vision 3 months after injection were worse baseline VA (−0.27 logMAR units/unit increase in baseline VA, p = 0.002) and presence of subretinal fluid (−0.17 logMAR units, p = 0.06). The presence of cystoid macular oedema negatively affected the visual outcome (0.15 logMAR units, p = 0.03). In addition, the presence of an epiretinal membrane (ERM) was associated with less visual improvement. ERM modified the effect of baseline VA as demonstrated by a significant interaction between these two variables (0.34 logMAR units/unit increase in baseline VA, p = 0.04).
Conclusions
OCT factors and baseline VA can be useful in predicting the outcomes of VA 3 months after intravitreal triamcinolone acetonide injection in patients with refractory DME.
Diabetic retinopathy is one of the four leading causes of visual loss in the US, and is the leading cause of visual loss in working‐aged Americans. Macular oedema is one of the major sources of visual loss in patients with diabetic retinopathy. The Early Treatment Diabetic Retinopathy Study established the benefit of focal macular photocoagulation in the treatment of clinically significant diabetic macular oedema (CSME). In the Early Treatment of Diabetic Retinopathy Study, focal laser photocoagulation was shown to decrease the rate of moderate visual loss (a doubling of the visual angle) by 50% and to decrease the rate of persistent macular oedema by 50% in patients with CSME. Unfortunately, despite laser treatment, only a small subgroup of patients had an improvement in vision. Thus, new treatments for DME have been evaluated.
Recently, there has been renewed interest in the use of triamcinolone acetonide, a potent, relatively insoluble corticosteroid available as suspension (Kenalog‐40, Bristol‐Myers Squibb, Princeton, New Jersey, USA), through either a posterior subtenon (PST) or an intravitreal route (IVTA). In particular, IVTA has been reported to successfully treat macular oedema secondary to diabetic retinopathy,1,2,3,4,5,6,7,8,9,10,11,12,13 as well as to treat other retinal vascular conditions including retinal vein occlusions14,15,16,17 and uveitis.18,19,20,21 The benefits of IVTA must be weighed against the risk of injection including retinal detachment, retinal tears, vitreous haemorrhage and endophthalmitis,22,23,24,25 and the rather frequent steroid‐related adverse events including mostly transitory, but occasionally severe, increases in the intraocular pressure (IOP) and cataract.26,27,28,29,30,31
Given this risk–benefit ratio, we set out to determine factors predictive of a greater benefit from IVTA to help clinicians decide which patients to subject to this invasive procedure. To accomplish this, we used the optical coherence tomography (OCT) scanner to help identify the various baseline patterns of diabetic macular oedema (DME) including cystoid macular oedema (CME), diffuse retinal thickening (DRT), subretinal fluid (SRF) accumulation, posterior hyaloidal traction (PHT) and formation of epiretinal membranes (ERMs). Thus, the purpose of this study was to evaluate the predictive factors for visual outcome after IVTA injection to treat DME, with special consideration for baseline OCT characteristics.
Methods
After approval by the Cleveland Clinic Institutional Review Board, a retrospective chart review of all patients with IVTA (67 028) and DME (382 83) identified by the International Classification of Diseases‐9 code review of the Cole Eye Institute's (Cleveland, Ohio, USA) clinical database was performed. Inclusion criteria included age >18 years, CSME involving the centre of the fovea >3 months after any prior DME treatment including laser photocoagulation or steroid injection, no history of steroid‐induced ocular hypertension or glaucoma, no contraindication for performing OCT including media opacities, inability to fixate and inability to perform the test, and an OCT scan performed within 10 days of treatment. All patients had a full ophthalmic examination and OCT before a single injection of 4 mg/0.1 ml IVTA. Patients without OCT before IVTA, or whose OCT was available but was performed >10 days before IVTA or did not have “fast macular thickness map” protocol and therefore no retinal thickness/volume tabular output for foveal minimum thickness and foveal average thickness were excluded. Other exclusion criteria included eyes with macular oedema from aetiologies other than diabetes, and any other process that prohibited proper grading of the OCT scans.
All OCT scans were performed through a dilated pupil by an experienced ocular photographer using a Stratus OCT scanner (Humphrey Zeiss, San Leandro, California, USA, software V.4.0). The macula was scanned in the horizontal and vertical meridians using the standard, linear cross‐hair pattern with a scan length of 3 and/or 6 mm centred through the fovea as determined by simultaneous evaluation of the red‐free image on the computer monitor of the OCT scanner. In addition, a fast macular thickness map algorithm centred on the fovea was also performed.
The OCT scans were graded for the presence of specific morphological patterns: presence or absence of retinal thickening, CME, PHT, subretinal fluid and ERM. These various patterns of DME were scored on the basis of their unique appearance on OCT imaging:
DRT was defined as increased retinal thickness (>200 μm) with reduced intraretinal reflectivity and expanded areas of lower reflectivity especially in the outer retinal layers >200 μm in width;
CME was identified by the localisation of intraretinal cystoid‐like spaces that appeared as round or oval areas of low reflectivity with highly reflective septa separating the cystoid‐like cavities
PHT was defined as a highly reflective signal arising from the inner retinal surface and extending towards the optic nerve or peripherally
SRF accumulation/serous retinal detachment was defined as an accumulation of subretinal fluid (which appeared dark) beneath a highly reflective and dome‐like elevation of the detached retina. The identification of the highly reflective posterior border of detached retina distinguished SRF from intraretinal fluid; and
ERM was defined as a highly reflective tissue membrane on the inner retinal surface.
The OCT grader was blinded to the clinical and functional status of the patients while evaluating the OCT scans. The values of foveal minimum thickness and foveal average thickness as calculated by the software were also included in the analysis.
A total of 126 eyes of 94 patients had their clinical data recorded from the charts, including eye, sex, race, age, prior ocular surgeries, prior focal laser photocoagulation, prior PST injections, prior IVTA injections, prior glaucoma, highest past IOP, lens status, best‐corrected Snellen visual acuity (BCVA) and IOP and follow‐up of BCVA, IOP, lens status, any additional steroid injections, other ocular surgeries and complications of injection. Visual acuity (VA) was recorded in Snellen units and converted to logarithm of minimum angle of resolution (logMAR) units for statistical analysis. In all, 42 eyes of 38 patients were excluded for not having OCT scans performed before IVTA and 11 eyes of 7 patients were excluded for not having OCT scans performed using the standard protocol.
To objectively assess visual outcomes, only eyes that had BCVA recorded at 3 months follow‐up after IVTA injection were included in the statistical analysis. Multivariate linear regression analysis was performed using generalised estimating equations accounting for inter‐eye correlations. Statistical modelling allowed the identification of factors independently associated with the change from baseline VA at 3 months after IVTA. In addition to baseline VA and the various patterns of DME, eye, sex, race, age, prior ocular surgeries, prior focal laser photocoagulation, prior PST injections, prior IVTA injections and a history of glaucoma were included in the analysis. A forward‐adding method was used, with factors having p>0.1 being dropped from the statistical model. The analysis included an investigation of interaction between the various patterns of DME and baseline VA. For factors showing significant interactions, the main effect was retained in the model regardless of the level of statistical significance. Factors associated with 3‐month VA with p<0.05 were considered to be significant.
Results
In all, 73 eyes of 59 patients were included in the study. Baseline demographics included 56.1% right eyes, a male to female ratio of 1:1, 69.8% Caucasians and a mean (SD) age of 64.9 (11.5) years. Prior cataract extraction was performed in 57.5% of patients, pars plana vitrectomy in 10.9% and glaucoma valve implant in 1.3% of patients. History of glaucoma was present in 8.2% of patients and the mean (SD) highest IOP before IVTA was 20.3 (4) mm Hg. Prior focal laser photocoagulation had been performed in 78% of patients, PST in 49.3% (average 1.3 times) and IVTA in 5.4% (one injection each) of patients. The mean baseline BCVA was 20/90 (0.65 (0.77) logMAR units). Posterior subcapsular cataract (PSC) was present in 16.1% of phakic eyes before IVTA.
The frequency of patterns of DME on OCT analysis at baseline before IVTA were CME 57.5%, DRT 47.9%, SRF 20.5% and PHT 6.8%. ERM was present in 21.9% of patients. The mean (SD) foveal minimum thickness was 442.3 (142.5) μm and mean (SD) foveal average thickness was 440.8 (131.1) μm.
During a mean (SD) follow‐up of 323.6 (237.8) days (median 302 days), an average of 1.4 (0.9) IVTA injections were performed. The mean (SD) change in vision was −0.075 (0.349) logMAR units. This corresponds to a mean reduction of 16% in the minimum angle of resolution (eg, an improvement from Snellen VA of 20/100 to 20/84). Improvement of ⩾3 lines occurred in 27.3% of patients, whereas a decline of ⩾3 lines occurred in 6.8% of patients and 60.2% remained stable within 1 line of baseline vision.
Complications from the IVTA were related to the steroid, and no injection‐related complications such as acute postoperative endophthalmitis, retinal tears or retinal detachment were observed. New PSC changes were noted in 22.5% of phakic eyes. Cataract extraction was performed in 38.7% eyes. In 8.2% of cases, there was an increase in IOP of >15 mm Hg from baseline or >32 mm Hg at any time. All cases were controlled with glaucoma drops by the last follow‐up, with none requiring glaucoma laser or surgery. Endophthalmitis was diagnosed in one eye >1 year after injection and, therefore, was considered unrelated to the steroid injection. No other adverse events were recorded.
Statistical modelling allowed the identification of factors associated with the change in VA 3 months after IVTA based on data from 52 eyes of 42 patients. Worse VA at baseline was independently associated with a significantly greater improvement in VA at 3 months. For each one‐unit increase in baseline logMAR VA (eg, 20/200 vs 20/20), an additional improvement in VA of 0.29 logMAR units was observed (p = 0.002, table 1). The presence of CME was significantly associated with a smaller degree of improvement, with a mean of 0.15 logMAR units less improvement than in the absence of CME (p = 0.03). Patients with SRF at baseline showed a greater degree of improvement in VA at 3 months, but the result did not reach significance (−0.17 logMAR units, p = 0.06). The presence of ERM did not have an independent effect on the change in VA, but modified the effect of baseline VA, as indicated by the presence of a statistically significant interaction between these two variables. The magnitude of the effect of ERM was such that it roughly offset the benefit that would otherwise have occurred based on the baseline level of VA. The presence of ERM did not modify the effects of either CME or SRF on the change in VA.
Table 1 Coefficients from multivariate linear generalised estimating equation model of change in best‐corrected visual acuity 3 months after intravitreal triamcinolone acetonide injection.
| Variables | Coefficient | p Value | 95% CI |
|---|---|---|---|
| Baseline BCVA | −0.29 | 0.004 | −0.48 to −0.093 |
| CME | 0.15 | 0.03 | 0.011 to 0.28 |
| SRF | −0.17 | 0.06 | −0.35 to 0.01 |
| ERM | −0.07 | 0.69 | −0.38 to 0.25 |
| ERM/baseline BCVA interaction | 0.34 | 0.04 | 0.01 to 0.67 |
| Constant | 0.001 | 0.99 | −0.20 to 0.20 |
BCVA, best‐corrected visual acuity; CME, cystoid macular oedema; ERM, epiretinal membrane; SRF, subretinal fluid.
On the basis of the results of the statistical model, table 2 illustrates the expected vision 3 months after IVTA for the various patterns of DME on OCT for different baseline VAs and presence of ERM. The visual outcome of patients with DRT was estimated by the effect of baseline VA in the absence of CME and SRF, since DRT was statistically independent of vision change. Table 2 illustrates the pattern of vision change observed in our patient population; it is not intended to be an algorithm to predict visual outcome in other patients. Vision change was calculated according to the following formula: logMAR change = constant coefficient+(baseline logMAR coefficient×baseline logMAR)+CME coefficient (if CME present)+SRF coefficient (if SRF present)+(ERM coefficient+(ERM/VA interaction coefficient×baseline logMAR); if ERM present). For example, in an eye with CME, ERM and baseline VA of 20/80 (0.60 logMAR units): logMAR change = 0.001+(−0.29×0.602)+0.15+(−0.07+(0.34×0.60)) = 0.11. Therefore, the final vision would be (0.60+0.11) = 0.71 logMAR units, which corresponds to a Snellen VA of 20/103.
Table 2 Illustration of expected vision change 3 months after intravitreal triamcinolone acetonide injection for the different patterns of diabetic macular oedema in the presence or absence of epiretinal membrane for different baseline visual acuities.
| Pattern of DME | Baseline Snellen | Baseline logMAR | LogMAR change (−) ERM | LogMAR change (+) ERM | Final Snellen (−) ERM | Final Snellen (+) ERM |
|---|---|---|---|---|---|---|
| SRF | 20/200 | 1 | −0.46 | −0.19 | 20/70 | 20/129 |
| DRT | 20/200 | 1 | −0.29 | −0.02 | 20/103 | 20/191 |
| CME | 20/200 | 1 | −0.14 | 0.13 | 20/145 | 20/270 |
| SRF | 20/80 | 0.60 | −0.34 | −0.21 | 20/36 | 20/49 |
| DRT | 20/80 | 0.60 | −0.04 | −0.04 | 20/54 | 20/73 |
| CME | 20/80 | 0.60 | −0.02 | 0.11 | 20/76 | 20/103 |
| SRF | 20/40 | 0.30 | −0.26 | −0.22 | 20/22 | 20/24 |
| DRT | 20/40 | 0.30 | −0.09 | −0.05 | 20/33 | 20/35 |
| CME | 20/40 | 0.30 | 0.06 | 0.10 | 20/46 | 20/50 |
CME, cystoid macular oedema; DME, diabetic macular oedema; DRT, diffuse macular oedema; ERM, epiretinal membrane; logMAR, logarithm of minimum angle of resolution; SRF, subretinal fluid.
Discussion
There has been considerable interest in the use of IVTA for the management of DME. This study has verified the positive visual effect of IVTA. Similar to other reports, after a median of 302 days, vision improved by ⩾3 lines in 27.3% of patients, whereas it declined by ⩾3 lines lines in only 7%. Complications from the injection were related to the steroid and not to the injection itself, with new PSC changes in 23% of patients and an increase in IOP of >15 mm Hg in 8% of eyes.
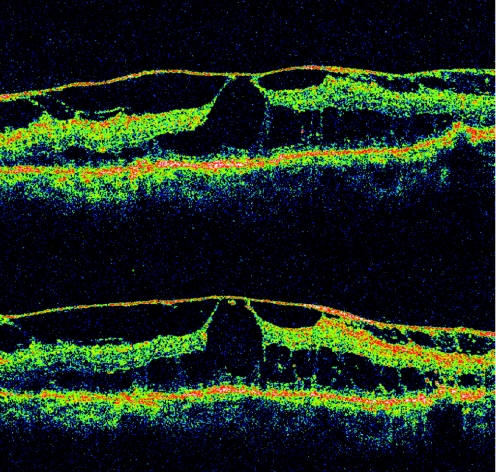
Previous reports evaluating the various patterns of DME on OCT reported an incidence of 88–97% for DRT, described as a “sponge‐like” swelling, 47–55% for CME and 7–15% for SRF.32,33 Our study found a different baseline incidence, with 57.5% of scans demonstrating CME, 47.9% DRT and 20.5% SRF. There are two possible explanations for the discrepancy: first, our analysis only includes patients with DME who took the IVTA injection and not just patients with DME, which would represent a more accurate prevalence for each pattern of DME; second, our data were collected using a newer version of OCT, with higher resolution. CME with small cysts may be mistaken for DRT with lower‐resolution scanners, which can partially explain our increased numbers of CME and decreased numbers of DRT. Another pattern of DME seen in our study is PHT (fig 1), which was present in a minority of eyes submitted to IVTA, and was statistically independent of vision change. PHT has been associated with a shallow, subclinical, macular detachment in previous studies.33,34 It has been suggested that surgery can improve vision in some of these eyes.35,36
Figure 1 Optical coherence tomography scans. In this case, posterior hyaloidal traction, epiretinal membrane and cystoid macular oedema were present with a visual acuity of 20/40 before intravitreal triamcinolone acetonide (IVTA) injection (first scan). There was essentially no change in retinal architecture 1 month after IVTA (second scan). Vision remained 20/40.
One of the factors that significantly influenced vision change 3 months after IVTA was baseline BCVA. Patients with worse baseline vision may be expected to improve more than those with better baseline vision. This may simply be related to the fact that a lower baseline BCVA has more room for improvement than a higher one and it does not imply that IVTA injection should be given only to those with lower baseline visions. This finding was also reported in a previous publication.13
The various baseline OCT patterns of DME had different prognostic significance. For example, our results suggest that SRF has the most positive influence on 3‐month visual outcomes. This is in contrast with a recent publication, which suggested that the presence of subretinal fluid predicted worse postoperative results 1 year after vitrectomy and inner limiting membrane peel for DME.37 Interestingly, contrary to previous suggestions, more visual improvement is expected in cases of DRT than in the presence of CME. In fact, our results suggest that in patients with better baseline VA the detrimental effect of CME leads to a decline in VA at 3 months despite treatment with IVTA.
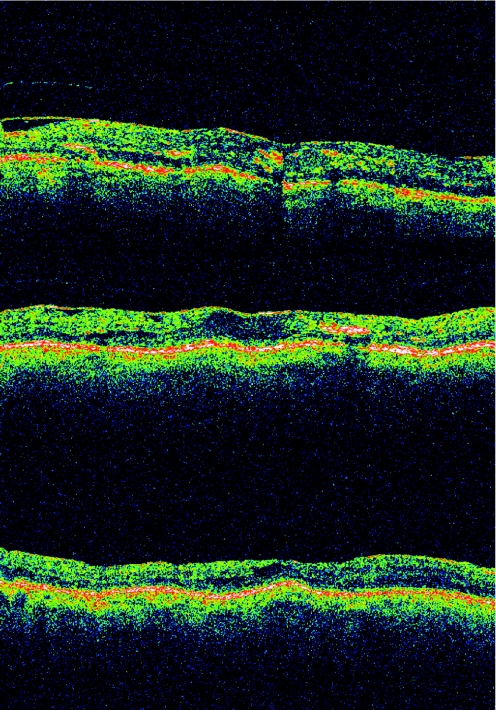
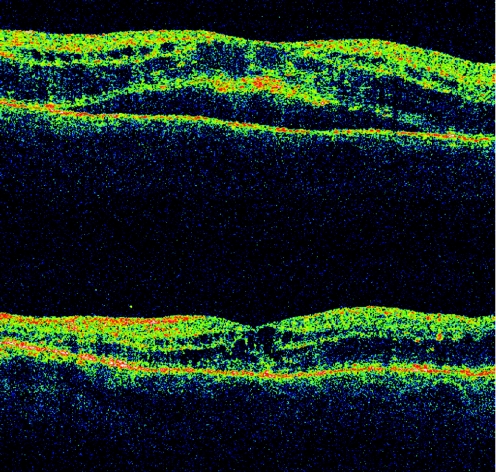
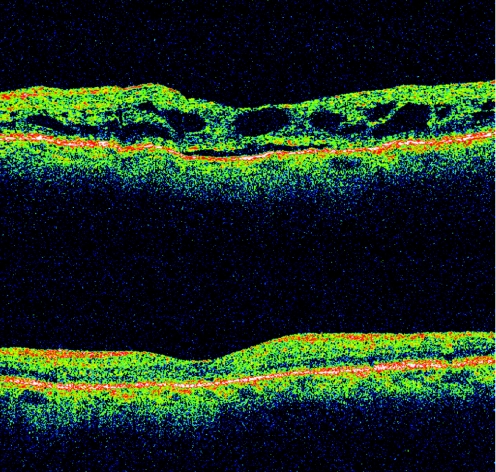
The presence of an ERM had a significant negative influence on visual outcome 3 months after IVTA. Its presence suggests a worse prognosis in DME. There was almost no change in VA after intravitreal steroid injection in the presence of ERM and DRT, whereas vision actually decreased after IVTA in the presence of ERM and CME for any given baseline VA (figs 2–4). This finding suggests considering a pars plana vitrectomy and membrane peeling in such cases, instead of IVTA injection. Patients with ERM and SRF had a small improvement in vision after IVTA. Using this knowledge a predictive model was produced (table 2). These predictive results apply only to our studied population. In addition, they represent short‐term expectations of a single intravitreal steroid injection, and it has been shown that improvement in VA after IVTA may decrease over time.38
Figure 2 Optical coherence tomography scans. This eye initially presented with diffuse retinal thickening and epiretinal membrane. Its vision was 20/100 before intravitreal triamcinolone acetonide (IVTA) injection (first scan). Both vision and macular anatomy were unchanged 8 months after IVTA (second scan). Pars plana vitrectomy with epiretinal membrane peeling was performed, and after 1 year (third scan) vision had improved to 20/60.
Figure 3 Optical coherence tomography scans. Cystoid macular oedema and subretinal fluid were seen before intravitreal triamcinolone acetonide injection (first scan), whereas only cystoid macular oedema was seen 4 months later (second scan). Vision was 20/80 on both occasions.
Figure 4 Optical coherence tomography (OCT) scans. This is an example of visual and anatomical success in the treatment of diabetic macular oedema with intravitreal triamcinolone acetonide. At presentation (first scan), cystoid macular oedema and subretinal fluid were present and vision was 20/200. Vision had improved to 20/80 and normal foveal contour was observed in the OCT scan 4 months after the injection (second scan).
There are obvious limitations of this study. The most obvious is that this is a retrospective case series. Other limitations include not taking into account the level of macular ischaemia in our analysis. However, it has been shown before that macular ischaemia has a negative effect on the treatment of DME with IVTA.13
In conclusion, OCT findings and baseline VA play a significant role in visual outcomes after intravitreal steroid injections. Patients with lower baseline BCVA and the presence of SRF on OCT positively influence visual change 3 months after IVTA. By contrast, the presence of an ERM negatively influenced visual outcome 3 months after IVTA in every clinical setting studied, and its coexistence with either DRT or CME may be a contraindication to IVTA treatment of DME. These patients would probably benefit from pars plana vitrectomy and membrane peel instead of an intravitreal steroid injection. Additional larger prospective studies would be necessary to substantiate these findings.
Abbreviations
BCVA - best‐corrected Snellen visual acuity
CSME - clinically significant diabetic macular oedema
DME - diabetic macular oedema
DRT - diffuse retinal thickening
ERM - epiretinal membrane
IOP - intraocular pressure
IVTA - intravitreal triamcinolone acetonide
logMAR - logarithm of minimum angle of resolution
OCT - optical coherence tomography
PHT - posterior hyaloidal traction
PST - posterior subtenon
SRF - subretinal fluid
VA - visual acuity
Footnotes
Competing interests: None declared.
References
- 1.Jonas J B, Sofker A. Intraocular injection of crystalline cortisone as adjunctive treatment of diabetic macular edema. Am J Ophthalmol 2001132425–427. [DOI] [PubMed] [Google Scholar]
- 2.Martidis A, Duker J S, Greenberg P B.et al Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology 2002109920–927. [DOI] [PubMed] [Google Scholar]
- 3.Jonas J B, Kreissig I, Sofker A.et al Intravitreal triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol 200312157–61. [PubMed] [Google Scholar]
- 4.Ip M S. Intravitreal injection of triamcinolone: an emerging treatment for diabetic macular edema. Diabetes Care 2004271794–1797. [DOI] [PubMed] [Google Scholar]
- 5.Karacorlu M, Ozdemir H, Karacorlu S.et al Intravitreal triamcinolone as a primary therapy in diabetic macular oedema. Eye 200519382–386. [DOI] [PubMed] [Google Scholar]
- 6.Ciardella A P, Klancnik J, Schiff W.et al Intravitreal triamcinolone for the treatment of refractory diabetic macular oedema with hard exudates: an optical coherence tomography study. Br J Ophthalmol 2004881131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutter F K, Simpson J M, Gillies M C. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three‐month efficacy and safety results of a prospective, randomized, double‐masked, placebo‐controlled clinical trial. Ophthalmology 20041112044–2049. [DOI] [PubMed] [Google Scholar]
- 8.Krepler K, Wagner J, Sacu S.et al The effect of intravitreal triamcinolone on diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 2005243478–481. [DOI] [PubMed] [Google Scholar]
- 9.Ozkiris A, Evereklioglu C, Erkilic K.et al Intravitreal triamcinolone acetonide injection as a primary treatment for diabetic macular edema. Eur J Ophthalmol 200414543–549. [PubMed] [Google Scholar]
- 10.Bakri S J, Beer P M. Intravitreal triamcinolone injection for diabetic macular edema: a clinical and fluorescein angiographic case series. Can J Ophthalmol 200439755–760. [DOI] [PubMed] [Google Scholar]
- 11.Chieh J J, Roth D B, Liu M.et al Intravitreal triamcinolone acetonide for diabetic macular edema. Retina 200525828–834. [DOI] [PubMed] [Google Scholar]
- 12.Patelli F, Fasolino G, Radice P.et al Time course of changes in retinal thickness and visual acuity after intravitreal triamcinolone acetonide for diffuse diabetic edema with and without previous macular laser treatment. Retina 200525840–845. [DOI] [PubMed] [Google Scholar]
- 13.Jonas J B, Martus P, Degenring R F.et al Predictive factors for visual acuity after intravitreal triamcinolone treatment for diabetic macular edema. Arch Ophthalmol 20051231338–1343. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg P B, Martidis A, Rogers A H.et al Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Br J Ophthalmol 200286247–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonas J B, Kreissig I, Degenring R F. Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 2002240782–783. [DOI] [PubMed] [Google Scholar]
- 16.Tewari H K, Sony P, Chawla R.et al Prospective evaluation of intravitreal triamcinolone acetonide injection in macular edema associated with retinal vascular disorders. Eur J Ophthalmol 200515619–626. [DOI] [PubMed] [Google Scholar]
- 17.Cekic O, Chang S, Tseng J J.et al Intravitreal triamcinolone treatment for macular edema associated with central retinal vein occlusion and hemiretinal vein occlusion. Retina 200525846–850. [DOI] [PubMed] [Google Scholar]
- 18.Young S, Larkin G, Branley M.et al Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. Clin Experiment Ophthalmol 2001292–6. [DOI] [PubMed] [Google Scholar]
- 19.Antcliff R J, Spalton D J, Stanford M R.et al Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology 2001108765–772. [DOI] [PubMed] [Google Scholar]
- 20.Androudi S, Letko E, Meniconi M.et al Safety and efficacy of triamcinolone acetonide for uveitic macular edema. Ocul Immunol Inflamm 200513205–212. [DOI] [PubMed] [Google Scholar]
- 21.Kok H, Lau C, Maycock N.et al Outcome of intravitreal triamcinolone in uveitis. Ophthalmology 20051121916. [DOI] [PubMed] [Google Scholar]
- 22.Nelson M L, Tennant M T, Silvalingam A.et al Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina 200323686–691. [DOI] [PubMed] [Google Scholar]
- 23.Moshfeghi D M, Kaiser P K, Scott I U.et al Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol 2003136791–796. [DOI] [PubMed] [Google Scholar]
- 24.Parke D W. Intravitreal triamcinolone and endophthalmitis. Am J Ophthalmol 2003136918–919. [DOI] [PubMed] [Google Scholar]
- 25.Jonas J B, Kreissig I, Degenring R F. Endophthalmitis after intravitreal injection of triamcinolone acetonide. Arch Ophthalmol 20031211663–1664. [DOI] [PubMed] [Google Scholar]
- 26.Bakri S J, Beer P M. The effect of intravitreal triamcinolone acetonide in intraocular pressure. Ophthalmic Surg Lasers Imaging 200334386–390. [PubMed] [Google Scholar]
- 27.Gillies M C, Simpson J M, Billson F A.et al Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol 2004122336–340. [DOI] [PubMed] [Google Scholar]
- 28.Kaushik S, Gupta V, Gupta A.et al Intractable glaucoma following intravitreal triamcinolone in central retinal vein occlusion. Am J Ophthalmol 2004137758–760. [DOI] [PubMed] [Google Scholar]
- 29.Detry‐Morel M, Escarmelle A, Hermans I. Refractory ocular hypertension secondary to intravitreal injection of triamcinolone acetonide. Bull Soc Belge Ophtalmol 200429245–51. [PubMed] [Google Scholar]
- 30.Singh I P, Ahmad S I, Yeh D.et al Early rapid rise in intraocular pressure after intravitreal triamcinolone acetonide injection. Am J Ophthalmol 2004138286–287. [DOI] [PubMed] [Google Scholar]
- 31.Smithen L M, Ober M D, Maranan L.et al Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol 2004138740–743. [DOI] [PubMed] [Google Scholar]
- 32.Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol 1999127688–693. [DOI] [PubMed] [Google Scholar]
- 33.Kim B Y, Smith S D, Kaiser P K. Optical coherence tomographic patterns of diabetic macular edema. Am J Ophthalmol 2006142405–412. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser P K, Riemann C D, Sears J E.et al Macular traction detachment and diabetic macular edema associated with posterior hyaloidal traction. Am J Ophthalmol 200113144–49. [DOI] [PubMed] [Google Scholar]
- 35.Lewis H, Abrams G W, Blumenkranz M S.et al Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology 199299753–759. [DOI] [PubMed] [Google Scholar]
- 36.Harbour J W, Smiddy W E, Flynn H W., Jret al Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol 1996121405–413. [DOI] [PubMed] [Google Scholar]
- 37.Shah S P, Patel M, Thomas D.et al Factors predicting outcome of vitrectomy for diabetic macular oedema: results of a prospective study. Br J Ophthalmol 20069033–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonas J B, Spandau U H, Kamppeter B A.et al Follow‐up after intravitreal triamcinolone acetonide for diabetic macular edema. Eur J Ophthalmol 200616566–572. [DOI] [PubMed] [Google Scholar]