Abstract
Background
The remaining retinal neurones or layered structure in the degenerating retina have been the prerequisite for epiretinal or subretinal retinal prostheses.
Aim
To detect the layered structure in the eyes of patients with retinitis pigmentosa by optical coherence tomography.
Methods
In a prospective non‐comparative study, 115 eyes of 58 consecutive patients with retinitis pigmentosa underwent optical coherence tomography to obtain horizontal and vertical retinal cross‐section images at the centre of the macula. The number of high‐reflectance retinal layers, one, two or three layers, was tested to determine whether it correlates with best‐corrected visual acuity.
Results
The best‐corrected visual acuity was significantly better in the eyes in which more retinal layers were detected (p<0.001, Kruskal–Wallis test, p<0.05, Tukey–Kramer test). The best‐corrected visual acuity in the right eye and in the left eye was correlated with each other (p<0.001, Spearman rank correlation test) and decreased with age.
Conclusions
Optical coherence tomography can be used to obtain information regarding the retinal layer structure in patients with retinitis pigmentosa, and may be used as a clinical test to assess the feasibility of retinal prostheses in future.
Retinitis pigmentosa is a hereditary disease caused by genetic abnormalities in a large number of genes expressed in retinal photoreceptor cells and in retinal pigment epithelial cells. A hallmark of the disease is the slowly progressive degeneration of photoreceptor cells, leading to night blindness, visual field constriction and visual acuity reduction. At present, no treatment is available to stop the progression or to regain the lost vision in patients with retinitis pigmentosa.
Retinal prosthesis is one of the promising treatment modalities for retinitis pigmentosa in the near future, and several groups have developed the prototypes.1,2,3,4,5 We have developed photoelectric dye‐coupled polyethylene films as the prototype of retinal prostheses that would be implanted surgically in the subretinal space.6,7,8 The remaining retinal neurones that connect to the brain have been the prerequisite for subretinal photodiode arrays and epiretinal, subretinal or transchoroidal microelectrode arrays developed so far.1,2,3,4,5 It is therefore mandatory to assess the function of the degenerating retina in patients with retinitis pigmentosa to predict the outcome of the treatment.
Optical coherence tomography is a non‐invasive technique to obtain cross‐sectional retinal images by differential near‐infrared light reflection at optical interfaces, and is a well‐used clinical examination in diagnosing retinochoroidal diseases. A new version of optical coherence tomography with spectral/Fourier domain detection techniques can delineate anatomical retinal layers in a microscopic dimension.9 Even with the currently used version of optical coherence tomography, retinal layers can be identified to some extent. In this study, we evaluated the layered structure of the retina in patients with retinitis pigmentosa to test whether optical coherence tomography could be used as a clinical test to predict the visual outcome of retinal prostheses.
Patients and methods
In a prospective non‐comparative clinical study, 115 eyes of 58 consecutive patients with retinitis pigmentosa, seen at Okayama University Hospital, Okayama City, Japan, from October 2005 to August 2006, underwent optical coherence tomography (OCT‐Ophthalmoscope, Nidek, Aichi, Japan). All patients, except for one whose left eye had been enucleated after injury, had both eyes tested. All patients showed non‐recordable electroretinograms and complained of night blindness. Informed consent to the purpose of the study was obtained from each patient, and the study conformed to the Declaration of Helsinki.
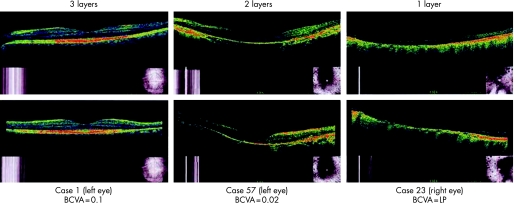
In the examination, horizontal and vertical retinal cross‐sectional images were obtained at the alignment with the centre of the macula (fig 1). The three high‐reflectance layers of the retina around the macula, corresponding to the surface nerve fibre layer, the inner plexiform layer and the outer plexiform layer, were identified independently by two readers blinded to the patients' name and the best‐corrected visual acuity. When the two readers had different interpretations, a discussion was carried out to reach a conclusion.
Figure 1 Horizontal (top) and vertical (bottom) sections of optical coherence tomography aligned at the centre of the macula in three patients with retinitis pigmentosa. Note that three high‐reflectance retinal layers around the macula (left column) and two layers (middle column) are identified in contrast with one surface layer only (right column). BCVA, best‐corrected visual acuity; LP, light perception.
The eyes were classified into three groups, based on the number of retinal layers identified by optical coherence tomography: one retinal surface layer only, two retinal layers and three retinal layers. The data were stratified into the right eyes and the left eyes for statistical analysis. The number of layers detected (one, two or three layers) was tested to determine whether it correlates with best‐corrected visual acuity.
Results
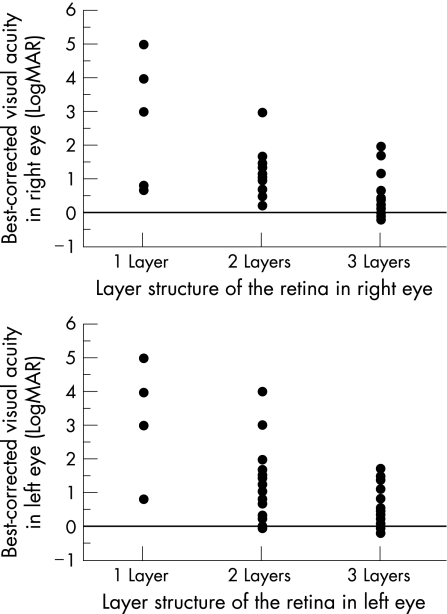
Three retinal layers were identified by optical coherence tomography in 61 eyes, two layers in 43 eyes and one surface layer only in 11 eyes. In patients with retinitis pigmentosa, the best‐corrected visual acuity was significantly better in the eyes in which more retinal layers were detected by optical coherence tomography (p<0.001, Kruskal–Wallis test, p<0.05, Tukey–Kramer test; fig 2).
Figure 2 Relationship of best‐corrected visual acuity with the number of retinal layers identified by optical coherence tomography in the right eyes (top) and in the left eyes (bottom) of patients with retinitis pigmentosa. The more the retinal layers that can be identified, the significantly better the visual acuity (p<0.001, Kruskal–Wallis test, p<0.05, Tukey–Kramer test). LogMAR, logarithm of the minimal angle of resolution. Hand movement, light perception and no light perception are designated as 4, 5 and 6, respectively.
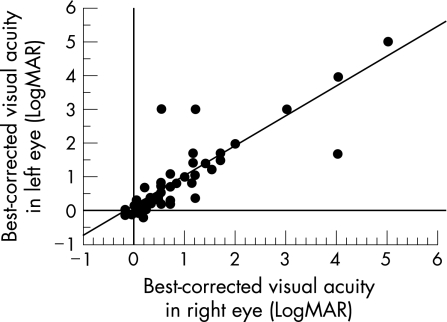
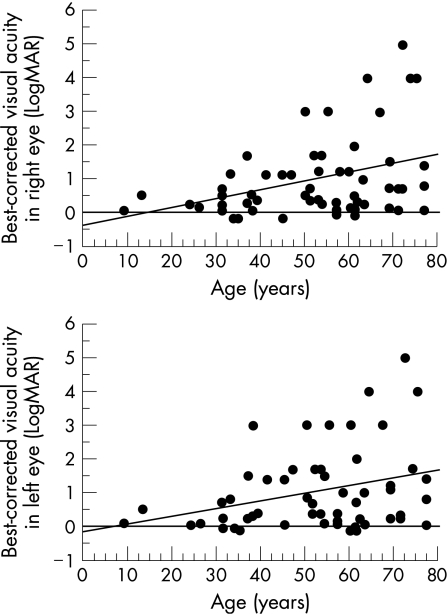
The best‐corrected visual acuity in the right eye and in the left eye was correlated with each other (p<0.001, Spearman's rank correlation test, fig 3), and decreased with age (p = 0.021 and 0.054, respectively, Spearman's rank correlation test; fig 4).
Figure 3 Correlation between best‐corrected visual acuity in the right eye and in the left eye of patients with retinitis pigmentosa (p<0.001, Spearman's rank correlation test). Visual acuity is expressed as a logarithm of the minimal angle of resolution (LogMAR).
Figure 4 Relationship of best‐corrected visual acuity in the right eye (top) and in the left eye (bottom) with the age of patients with retinitis pigmentosa. Visual acuity decreases with age (p = 0.021 in the right eye and p = 0.054 in the left eye, Spearman's rank correlation test). Visual acuity is expressed as a logarithm of the minimal angle of resolution (LogMAR). Hand movement, light perception and no light perception are designated as 4, 5 and 6, respectively.
Discussion
The aim of this study was to test whether optical coherence tomography could be used as a clinical test to assess the function of the degenerating retina in patients with retinitis pigmentosa. The purpose in this setting was to test to what extent retinal neurones, other than photoreceptor cells, remain alive in the degenerating retina of patients with retinitis pigmentosa. As a first step to this goal, we aimed to identify by optical coherence tomography the retinal layered structure in the eyes with retinitis pigmentosa at varying levels of severity and to evaluate the relationship of visual acuity with the layered structure. In this study, we showed that the more the layers of the retina were identified, the better the visual acuity. In other words, visual acuity was correlated with the preserved structure of the retinal layers around the macula.
Until now, optical coherence tomography has been used to measure the central retinal thickness in patients with retinitis pigmentosa.10 In that study, both retinal thinning, because of cell loss, and retinal thickening, because of presumed oedema, seem to be associated with lower visual acuity. The authors of that study concluded that retinal thickness measurements by optical coherence tomography would complement visual acuity measurements as an objective assessment of retinal architecture spanning the fovea. In this study, we showed that the loss of the retinal layered structure was associated with poor visual acuity and that optical coherence tomography could be used as a tool for the objective assessment of the layered structure.
In this study, we also showed that visual acuity in the right eye and in the left eye was correlated with each other and that visual acuity decreased with age. These results are consistent with previous findings.11,12,13,14,15 The patients involved in this study had relatively better levels of visual acuity, and those with lower levels of visual acuity, hand movement or worse, were only in a small number. Patients who become blind as a result of retinitis pigmentosa usually do not seek medical care and also have difficulty in coming to a hospital.
As a treatment option in the near future, retinal prostheses will be implanted surgically in patients with retinitis pigmentosa who have lost vision, and hence, the indication would be visual acuity at levels of hand movement, light perception or no light perception (fig 4). In this study, the number of eyes with such poor levels of visual acuity was too small to conclude that optical coherence tomography is useful in assessing the remaining function of the retina. Furthermore, optical coherence tomography used in this study could not detect any structure except for the surface layer in the eyes with poor levels of visual acuity, hand movement or worse. Utrahigh‐resolution optical coherence tomography using spectral/Fourier domain detection techniques9 would provide more information on the retinal architecture of patients with poor levels of visual acuity.
Footnotes
Competing interests: None declared.
References
- 1.Chow A Y, Chow V Y, Packo K H.et al The artificial silicon retina microchip for the treatment of vision loss from retinitis pigmentosa. Arch Ophthalmol 2004122460–469. [DOI] [PubMed] [Google Scholar]
- 2.Humayun M S, Weiland J D, Fujii G Y.et al Visual perception in a blind subject with a chronic microelectronic retinal prostheses. Vision Res 2003432573–2581. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo JF I I I, Wyatt J, Loewenstein J.et al Perceptual efficacy of electrical stimulation of human retina with a microelectrode array during short‐term surgical trials. Invest Ophthalmol Vis Sci 2003445362–5369. [DOI] [PubMed] [Google Scholar]
- 4.Zrenner E. Will retinal implants restore vision? Science 20022951022–1025. [DOI] [PubMed] [Google Scholar]
- 5.Terasawa Y, Tashiro H, Uehara A.et al The development of a multichannel electrode array for retinal prostheses. J Artif Organs 20069263–266. [DOI] [PubMed] [Google Scholar]
- 6.Matsuo T. A simple method for screening photoelectric dyes towards their use for retinal prostheses. Acta Med Okayama 200357257–260. [DOI] [PubMed] [Google Scholar]
- 7.Uji A, Matsuo T, Ishimaru S.et al Photoelectric dye‐coupled polyethylene film as a prototype of retinal prostheses. Artif Organs 20052953–57. [DOI] [PubMed] [Google Scholar]
- 8.Uji A, Matsuo T, Uchida T.et al Intracellular calcium response and adhesiveness of chick embryonic retinal neurons to photoelectric dye‐coupled polyethylene films as prototypes of retinal prostheses. Artif Organs 200630695–703. [DOI] [PubMed] [Google Scholar]
- 9.Wojtkowski M, Srinivasan V, Fujimoto J G.et al Three‐dimensional retinal imaging with high‐speed ultrahigh‐resolution optical coherence tomography. Ophthalmology 20051121734–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandberg M A, Brockhurst R J, Gaudio A R.et al The association between visual acuity and central retinal thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci 2005463349–3354. [DOI] [PubMed] [Google Scholar]
- 11.Berson E L, Sandberg M A, Rosner B.et al Natural course of retinitis pigmentosa over a three‐year interval. Am J Ophthalmol 198599240–251. [DOI] [PubMed] [Google Scholar]
- 12.Holopigian K, Greenstein V, Seiple W.et al Rates of change differ among measures of visual function in patients with retinitis pigmentosa. Ophthalmology 1996103398–405. [DOI] [PubMed] [Google Scholar]
- 13.Grover S, Fishman G A, Alexander K R.et al Visual acuity impairment in patients with retinitis pigmentosa. Ophthalmology 19961031593–1600. [DOI] [PubMed] [Google Scholar]
- 14.Grover S, Fishman G A, Anderson R J.et al Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology 19991061780–1785. [DOI] [PubMed] [Google Scholar]
- 15.Flynn M F, Fishman G A, Anderson R J.et al Retrospective longitudinal study of visual acuity change in patients with retinitis pigmentosa. Retina 200121639–646. [DOI] [PubMed] [Google Scholar]